Additive Effects of Omega-3 Fatty Acids and Thiazolidinediones in Mice Fed a High-Fat Diet: Triacylglycerol/Fatty Acid Cycling in Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Dietary Interventions
2.2. Biochemical Analysis of Plasma and Tissue Samples
2.3. Glucose Homeostasis
2.4. Histological and Immunohistological Analysis of eWAT
2.5. Real-time Quantitative PCR (RT-qPCR)
2.6. In Vivo Evaluation of TAG synthesis and DNL-derived FA in eWAT
2.7. Statistical Analysis
3. Results
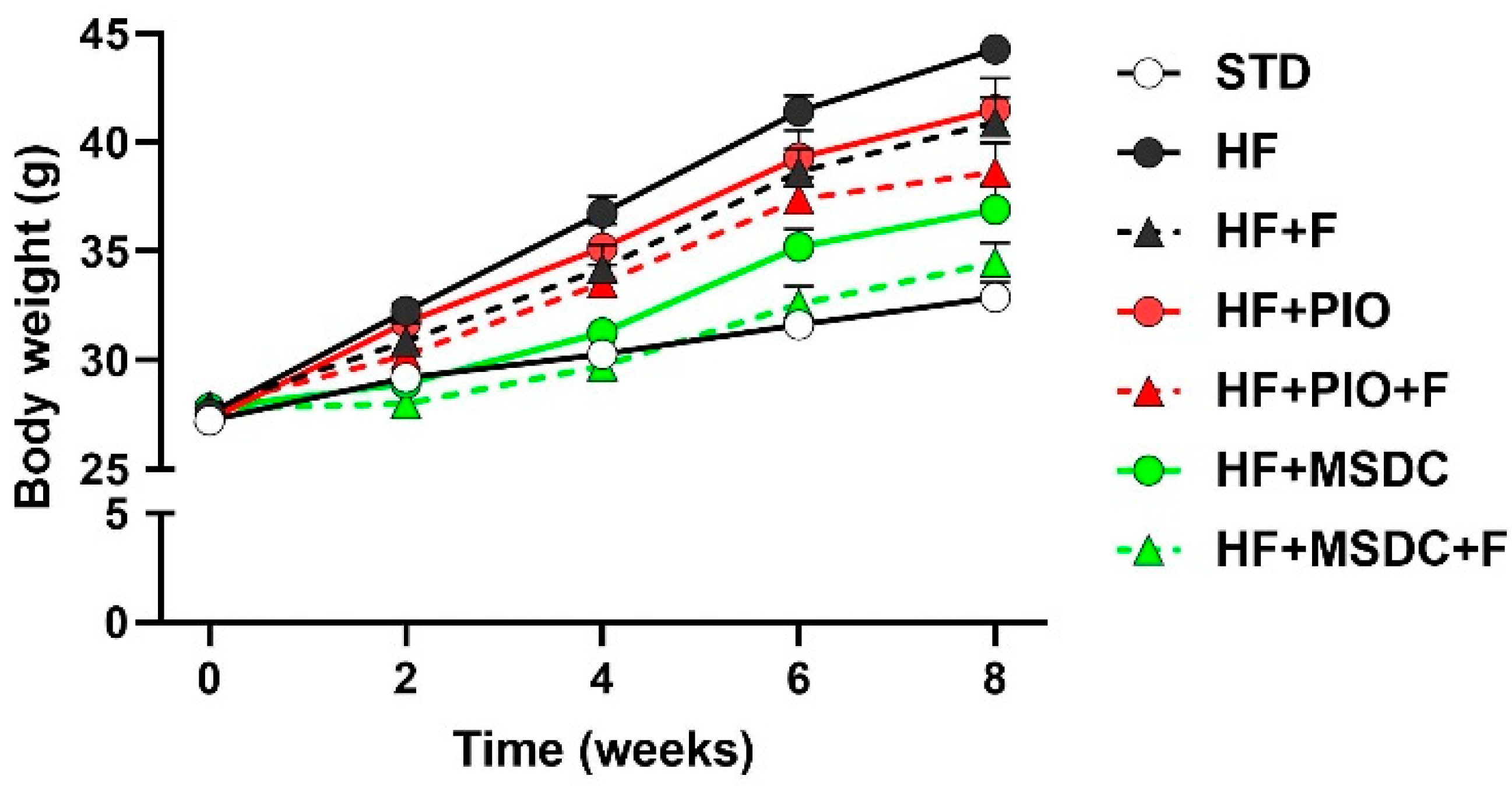
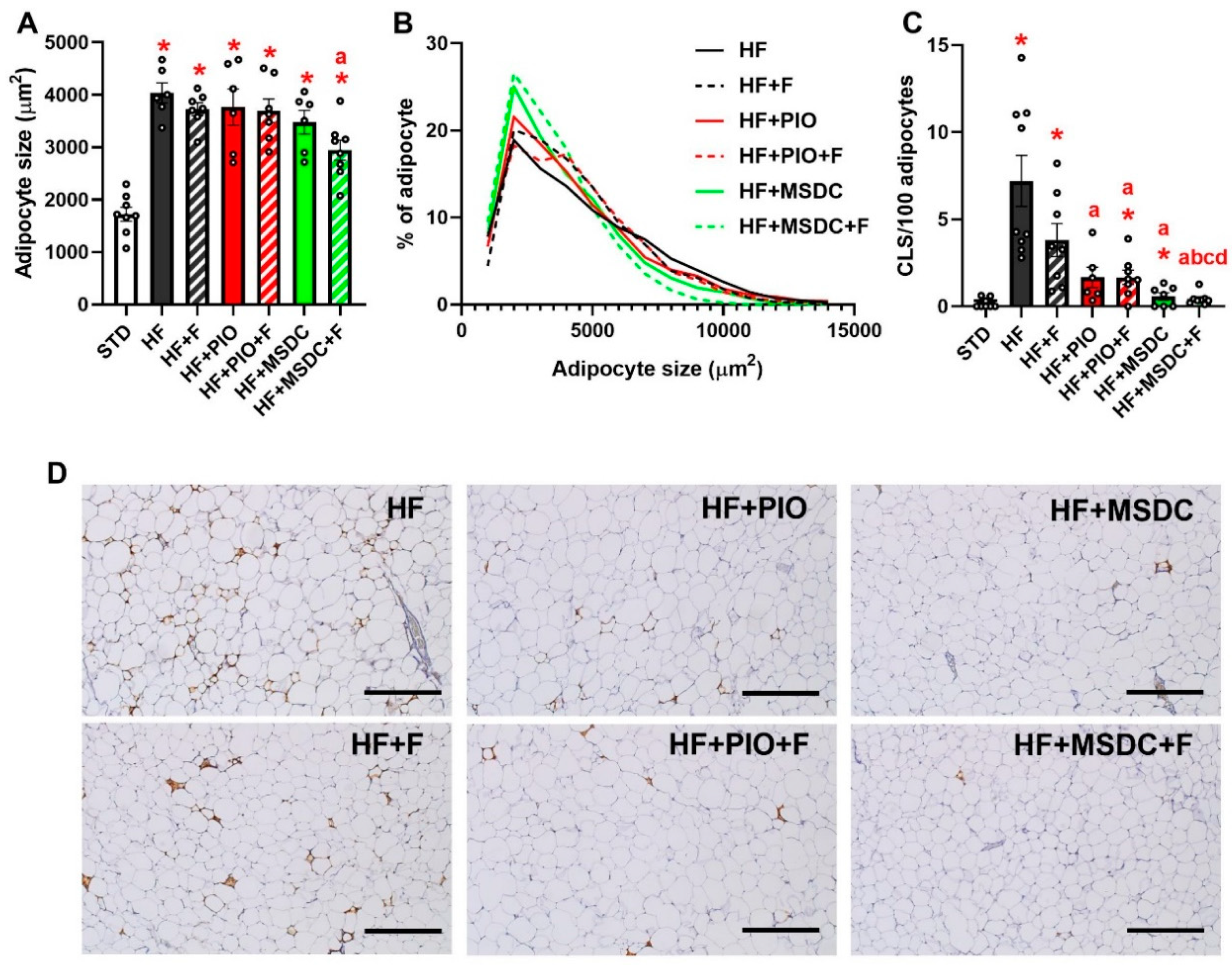
3.1. Parameters of Energy Balance, Adiposity and Lipid Metabolism Markers
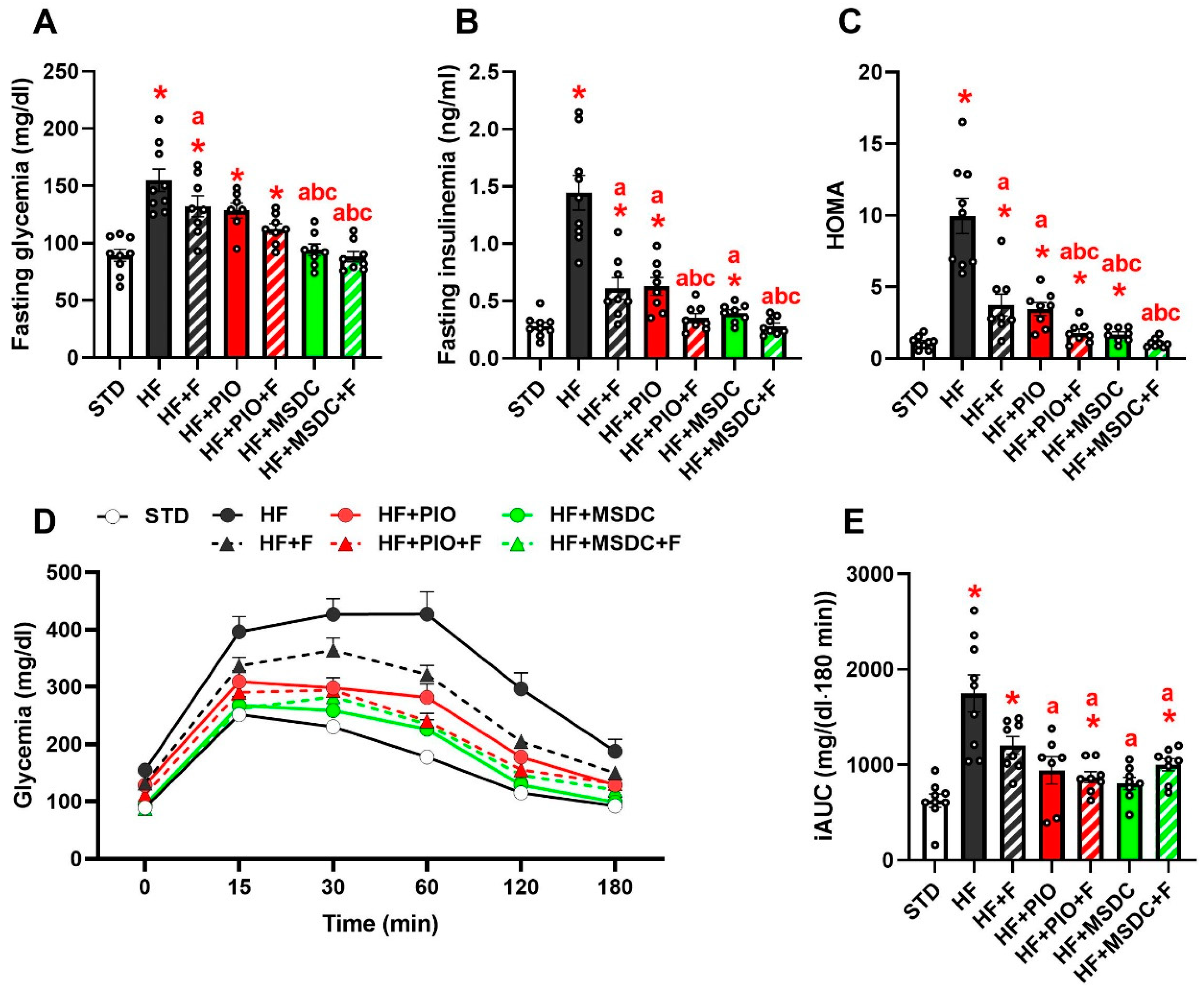
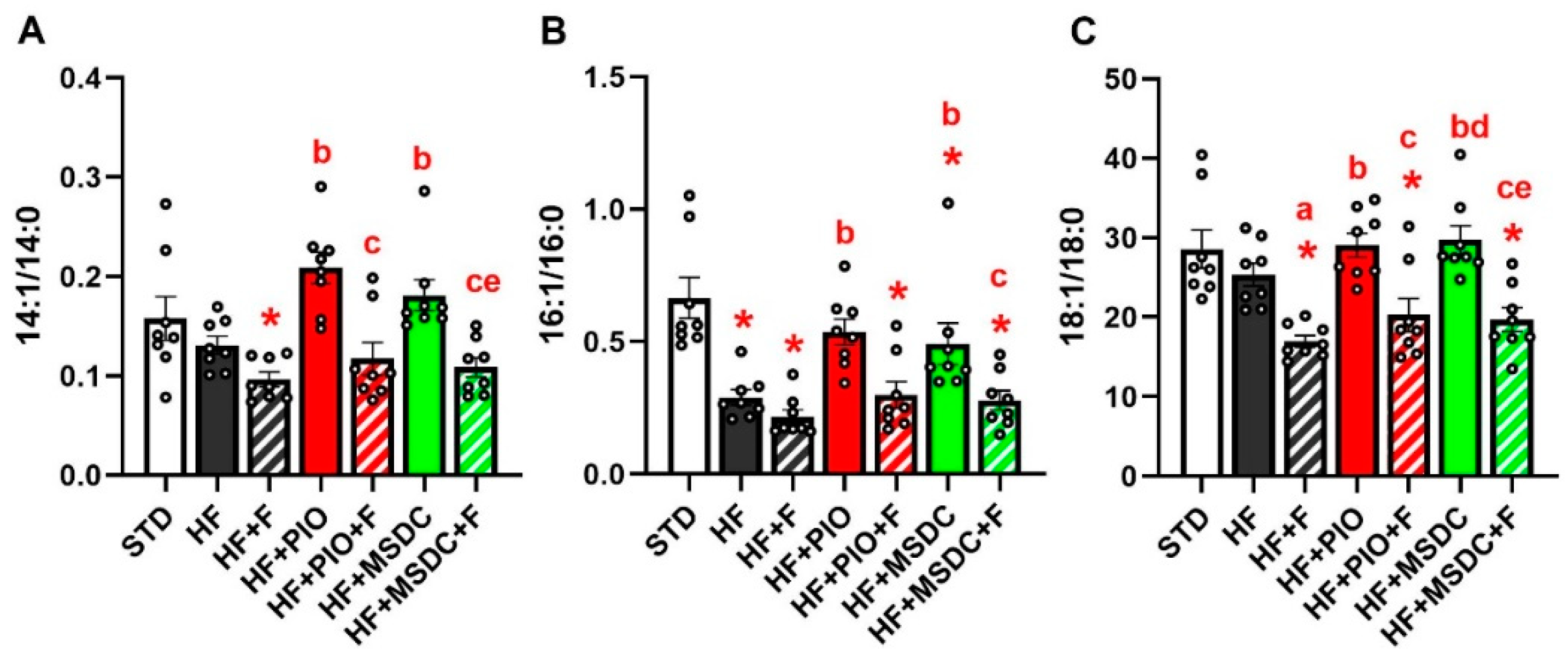
3.2. Lipid and Glucose Homeostasis
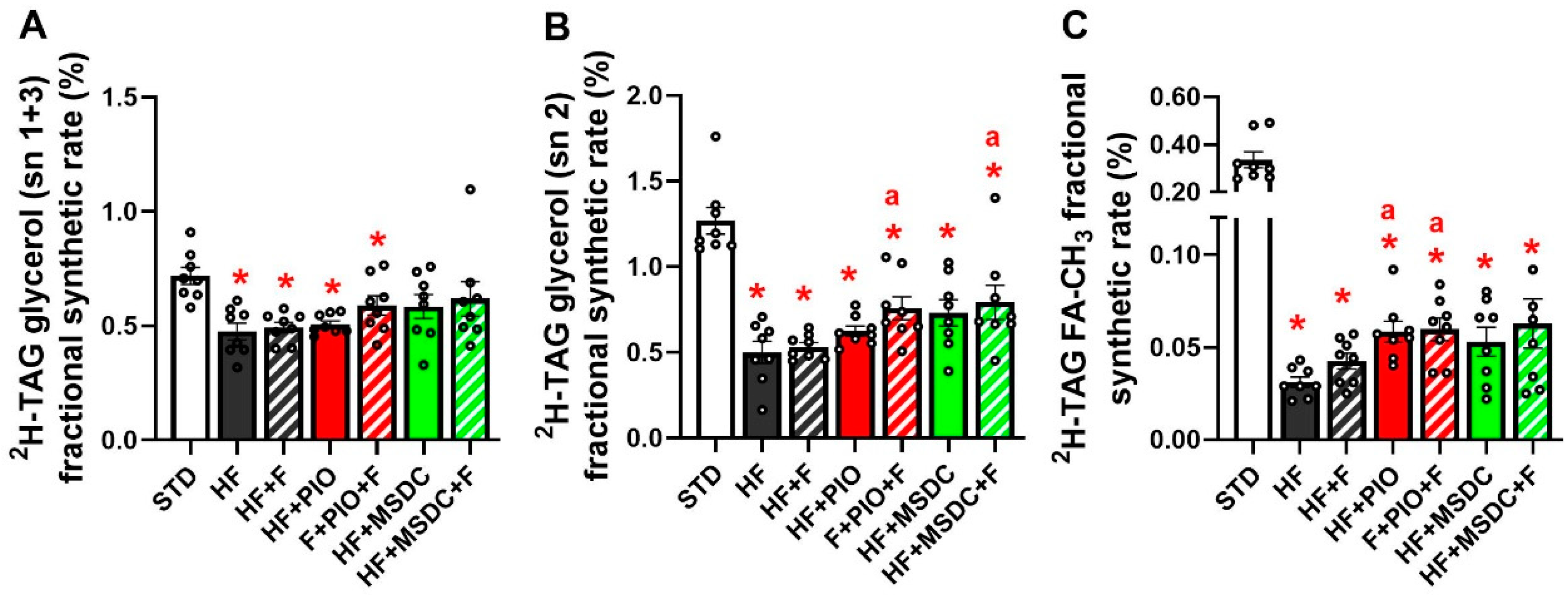
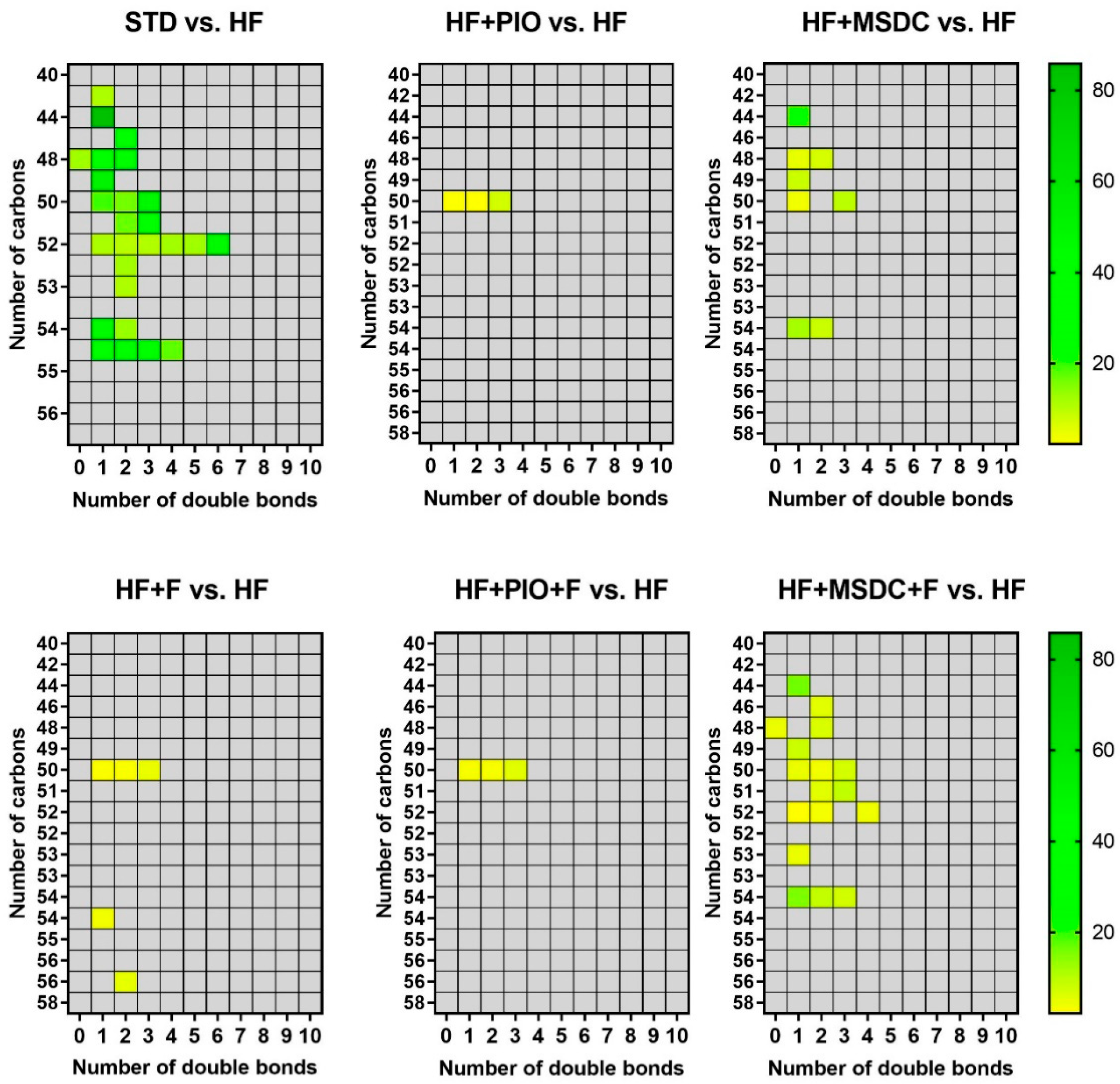
3.3. In Vivo Evaluation of TAG/FA Cycling Activity in eWAT
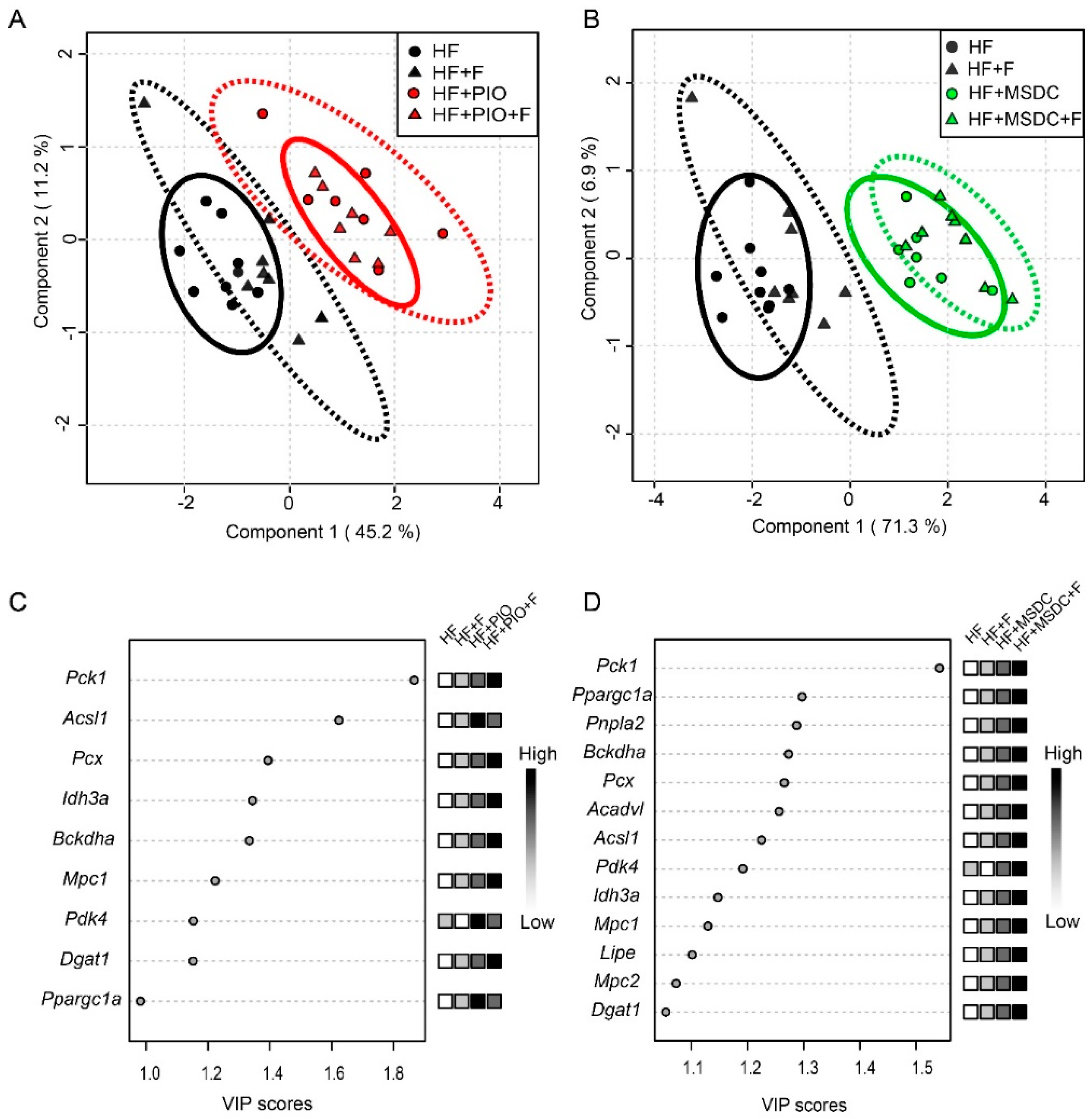
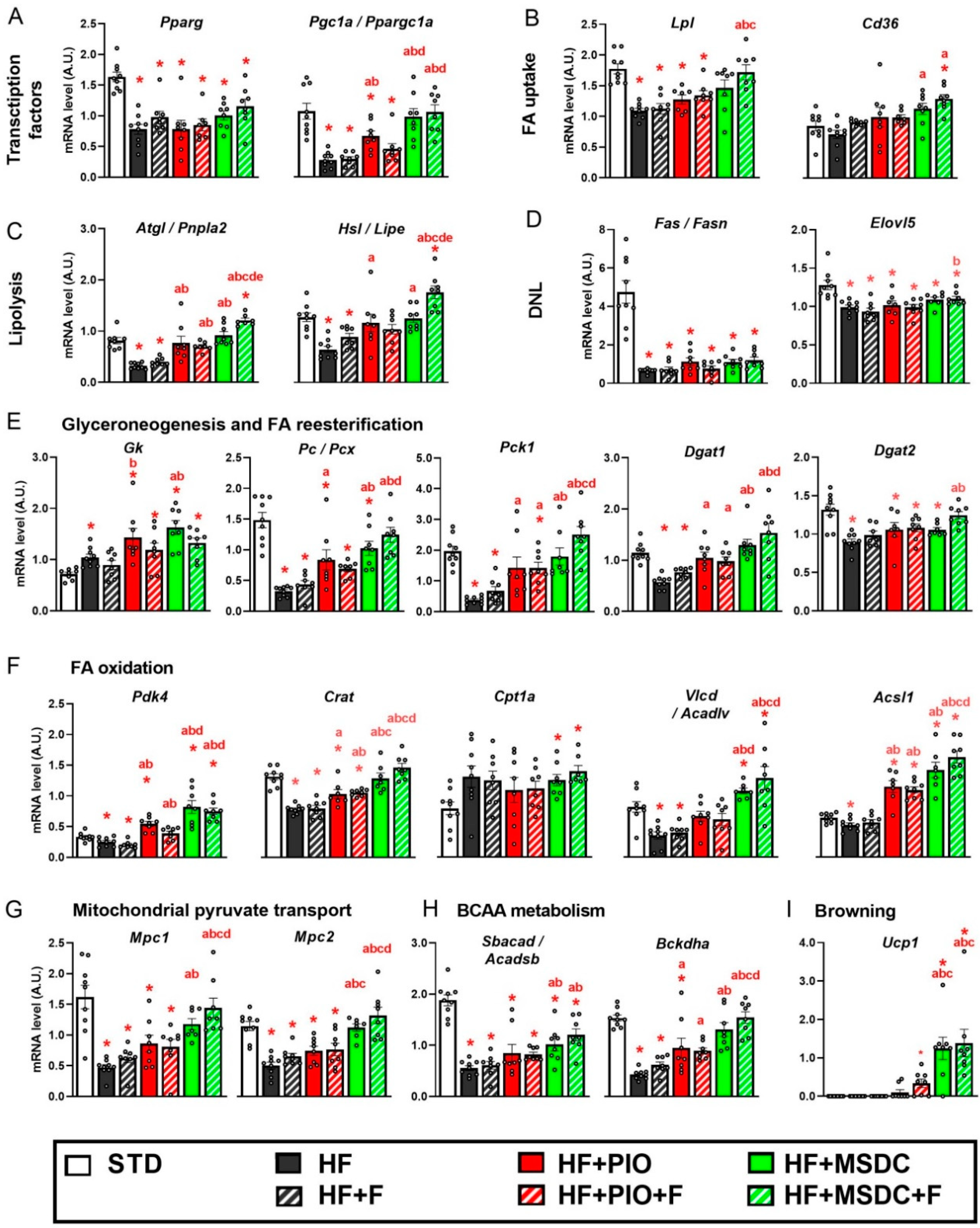
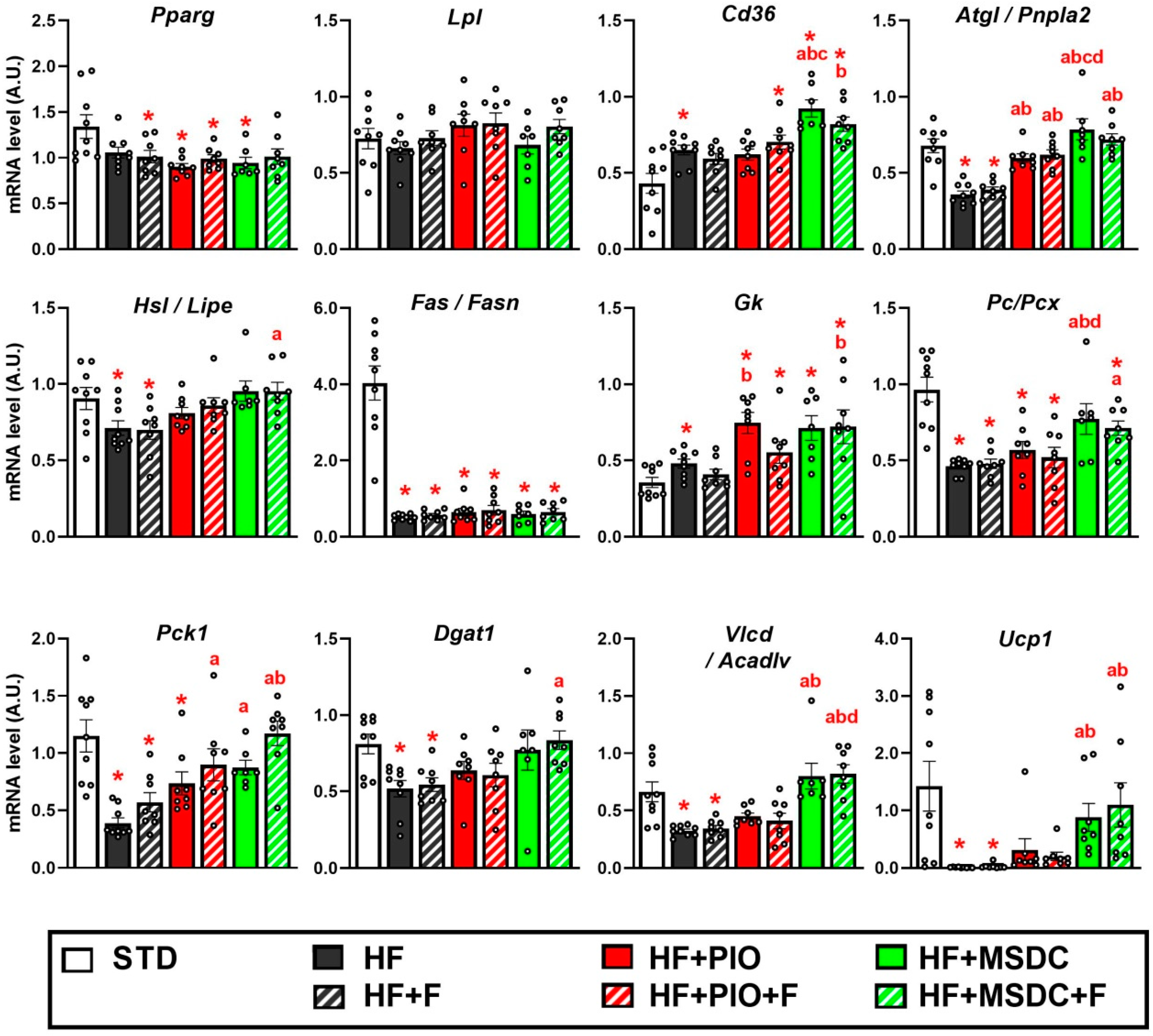
3.4. Gene Expression in Adipose Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flachs, P.; Rossmeisl, M.; Kuda, O.; Kopecky, J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: A key to lean phenotype. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 986–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoodi, M.; Kuda, O.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Lipid signaling in adipose tissue: Connecting inflammation & metabolism. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 503–518. [Google Scholar] [CrossRef]
- Kuda, O.; Rossmeisl, M.; Kopecky, J. Omega-3 fatty acids and adipose tissue biology. Mol. Asp. Med. 2018, 64, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.; de Oliveira Otto, M.C.; Lemaitre, R.N.; McKnight, B.; Song, X.; King, I.B.; Chaves, P.H.; Odden, M.C.; Newman, A.B.; Siscovick, D.S.; et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: Prospective cohort study. BMJ 2018, 363, k4067. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2018, 380, 23–32. [Google Scholar] [CrossRef]
- Flachs, P.; Ruhl, R.; Hensler, M.; Janovska, P.; Zouhar, P.; Kus, V.; Macek, J.Z.; Papp, E.; Kuda, O.; Svobodova, M.; et al. Synergistic induction of lipid catabolism and anti-inflammatory lipids in white fat of dietary obese mice in response to calorie restriction and n-3 fatty acids. Diabetologia 2011, 54, 2626–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossmeisl, M.; Medrikova, D.; van Schothorst, E.M.; Pavlisova, J.; Kuda, O.; Hensler, M.; Bardova, K.; Flachs, P.; Stankova, B.; Vecka, M.; et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2014, 1841, 267–278. [Google Scholar] [CrossRef] [PubMed]
- de Castro, G.S.; Calder, P.C. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin. Nutr. 2018, 37, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Flachs, P.; Mohamed-Ali, V.; Horakova, O.; Rossmeisl, M.; Hosseinzadeh-Attar, M.J.; Hensler, M.; Ruzickova, J.; Kopecky, J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed high-fat diet. Diabetologia 2006, 49, 394–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.H.; Cahill, L.E.; Mozaffarian, D. Effect of fish oil on circulating adiponectin: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2013, 98, 2451–2459. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- van Schothorst, E.M.; Flachs, P.; Franssen-van Hal, N.L.; Kuda, O.; Bunschoten, A.; Molthoff, J.; Vink, C.; Hooiveld, G.J.; Kopecky, J.; Keijer, J. Induction of lipid oxidation by polyunsaturated fatty acids of marine origin in small intestine of mice fed a high-fat diet. BMC Genom. 2009, 10, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroupova, P.; van Schothorst, E.M.; Keijer, J.; Bunschoten, A.; Vodicka, M.; Irodenko, I.; Oseeva, M.; Zacek, P.; Kopecky, J.; Rossmeisl, M.; et al. Omega-3 Phospholipids from Krill Oil Enhance Intestinal Fatty Acid Oxidation More Effectively than Omega-3 Triacylglycerols in High-Fat Diet-Fed Obese Mice. Nutrients 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Vecka, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef]
- Adamcova, K.; Horakova, O.; Bardova, K.; Janovska, P.; Brezinova, M.; Kuda, O.; Rossmeisl, M.; Kopecky, J. Reduced Number of Adipose Lineage and Endothelial Cells in Epididymal fat in Response to Omega-3 PUFA in Mice Fed High-Fat Diet. Mar. Drugs 2018, 16, 515. [Google Scholar] [CrossRef] [Green Version]
- Kunesova, M.; Braunerova, R.; Hlavaty, P.; Tvrzicka, E.; Stankova, B.; Skrha, J.; Hilgertova, J.; Hill, M.; Kopecky, J.; Wagenknecht, M.; et al. The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiol. Res. 2006, 55, 63–72. [Google Scholar]
- Mori, T.A.; Bao, D.Q.; Burke, V.; Puddey, I.B.; Watts, G.F.; Beilin, L.J. Dietary fish as a major component of a weight-loss diet: Effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am. J. Clin. Nutr. 1999, 70, 817–825. [Google Scholar] [CrossRef]
- Flachs, P.; Rossmeisl, M.; Kopecky, J. The Effect of n-3 Fatty Acids on Glucose Homeostasis and Insulin Sensivity. Physiol. Res. 2014, 93–118. [Google Scholar] [CrossRef]
- Kuda, O.; Jelenik, T.; Jilkova, Z.; Flachs, P.; Rossmeisl, M.; Hensler, M.; Kazdova, L.; Ogston, N.; Baranowski, M.; Gorski, J.; et al. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia 2009, 52, 941–951. [Google Scholar] [CrossRef] [Green Version]
- Kus, V.; Flachs, P.; Kuda, O.; Bardova, K.; Janovska, P.; Svobodova, M.; Jilkova, Z.M.; Rossmeisl, M.; Wang-Sattler, R.; Yu, Z.; et al. Unmasking Differential Effects of Rosiglitazone and Pioglitazone in the Combination Treatment with n-3 Fatty Acids in Mice Fed a High-Fat Diet. PLoS ONE 2011, 6, e27126–e27127. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Banks, A.S.; Estall, J.L.; Kajimura, S.; Bostrom, P.; Laznik, D.; Ruas, J.L.; Chalmers, M.J.; Kamenecka, T.M.; Bluher, M.; et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 2010, 466, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divakaruni, A.S.; Wiley, S.E.; Rogers, G.W.; Andreyev, A.Y.; Petrosyan, S.; Loviscach, M.; Wall, E.A.; Yadava, N.; Heuck, A.P.; Ferrick, D.A.; et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl. Acad. Sci. USA 2013, 110, 5422–5427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colca, J.R.; McDonald, W.G.; Cavey, G.S.; Cole, S.L.; Holewa, D.D.; Brightwell-Conrad, A.S.; Wolfe, C.L.; Wheeler, J.S.; Coulter, K.R.; Kilkuskie, P.M.; et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)--relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS ONE 2013, 8, e61551. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dougherty, E.J.; Danner, R.L. PPARgamma signaling and emerging opportunities for improved therapeutics. Pharmacol. Res. 2016, 111, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Vigueira, P.A.; Chambers, K.T.; Hall, A.M.; Mitra, M.S.; Qi, N.; McDonald, W.G.; Colca, J.R.; Kletzien, R.F.; Finck, B.N. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor gamma-sparing thiazolidinedione. J. Biol. Chem. 2012, 287, 23537–23548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCommis, K.S.; Hodges, W.T.; Brunt, E.M.; Nalbantoglu, I.; McDonald, W.G.; Holley, C.; Fujiwara, H.; Schaffer, J.E.; Colca, J.R.; Finck, B.N. Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatology 2017, 65, 1543–1556. [Google Scholar] [CrossRef] [Green Version]
- Colca, J.R.; Tanis, S.P.; McDonald, W.G.; Kletzien, R.F. Insulin sensitizers in 2013: New insights for the development of novel therapeutic agents to treat metabolic diseases. Expert. Opin. Investig. Drugs 2014, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Alkhouri, N.; Davison, B.A.; Sanyal, A.; Edwards, C.; Colca, J.R.; Lee, B.H.; Loomba, R.; Cusi, K.; Kolterman, O.; et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J. Hepatol. 2020, 72, 613–626. [Google Scholar] [CrossRef]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, E.A.; Crabtree, B. Substrate cycles: Their metabolic energy and thermic consequences in man. Biochem. Soc. Symp. 1976, 43, 183–205. [Google Scholar]
- Hui, S.; Cowan, A.J.; Zeng, X.; Yang, L.; TeSlaa, T.; Li, X.; Bartman, C.; Zhang, Z.; Jang, C.; Wang, L.; et al. Quantitative Fluxomics of Circulating Metabolites. Cell. Metab. 2020, 32, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Kalderon, B.; Mayorek, N.; Berry, E.; Zevit, N.; Bar-Tana, J. Fatty acid cycling in the fasting rat. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E221–E227. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Adamcova, K.; Zouhar, P.; Marques, C.; Janovska, P.; Viegas, I.; Jones, J.G.; Bardova, K.; Svobodova, M.; Hansikova, J.; et al. Induction of lipogenesis in white fat during cold exposure in mice: Link to lean phenotype. Int. J. Obes. 2017, 41, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Bederman, I.R.; Foy, S.; Chandramouli, V.; Alexander, J.C.; Previs, S.F. Triglyceride synthesis in epididymal adipose tissue: Contribution of glucose and non-glucose carbon sources. J. Biol. Chem. 2009, 284, 6101–6108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadoudal, T.; Distel, E.; Durant, S.; Fouque, F.; Blouin, J.M.; Collinet, M.; Bortoli, S.; Forest, C.; Benelli, C. Pyruvate dehydrogenase kinase 4: Regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes 2008, 57, 2272–2279. [Google Scholar] [CrossRef] [Green Version]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.L.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef] [Green Version]
- Janovska, P.; Flachs, P.; Kazdova, L.; Kopecky, J. Anti-obesity effect of n-3 polyunsaturated fatty acids in mice fed high-fat diet is independent of cold-induced thermogenesis. Physiol. Res. 2013, 62, 153–161. [Google Scholar] [CrossRef]
- Tordjman, J.; Chauvet, G.; Quette, J.; Beale, E.G.; Forest, C.; Antoine, B. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells3. J. Biol. Chem. 2003, 278, 18785–18790. [Google Scholar] [CrossRef] [Green Version]
- Horakova, O.; Medrikova, D.; van Schothorst, E.M.; Bunschoten, A.; Flachs, P.; Kus, V.; Kuda, O.; Bardova, K.; Janovska, P.; Hensler, M.; et al. Preservation of metabolic flexibility in skeletal muscle by a combined use of n-3 PUFA and rosiglitazone in dietary obese mice. PLoS ONE 2012, 7, e43764. [Google Scholar] [CrossRef] [Green Version]
- Veleba, J.; Kopecky, J., Jr.; Janovska, P.; Kuda, O.; Horakova, O.; Malinska, H.; Kazdova, L.; Oliyarnyk, O.; Skop, V.; Trnovska, J.; et al. Combined intervention with pioglitazone and -3 fatty acids in metformin-treated type 2 diabetic patients: Improvement of lipid metabolism. Nutr. Metab. 2015, 12, 52. [Google Scholar] [CrossRef]
- Fukunaga, T.; Zou, W.; Rohatgi, N.; Colca, J.R.; Teitelbaum, S.L. An insulin-sensitizing thiazolidinedione, which minimally activates PPARgamma, does not cause bone loss. J. Bone Miner. Res. 2015, 30, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky, J., Jr.; Kudova, E.; et al. Docosahexaenoic Acid-Derived Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) With Anti-inflammatory Properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [Green Version]
- Paton, C.M.; Ntambi, J.M. Biochemical and Physiological Function of Stearoyl-CoA Desaturase. Am. J. Physiol. Endocrinol. Metab. 2008, 297, E28–E37. [Google Scholar] [CrossRef] [Green Version]
- Kuda, O.; Stankova, B.; Tvrzicka, E.; Hensler, M.; Jelenik, T.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Prominent role of liver in elevated plasma palmitooleate levels in response to rosiglitazone in mice fed high-fat diet. J. Physiol. Pharmacol. 2009, 60, 135–140. [Google Scholar]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef] [Green Version]
- Buresova, J.; Janovska, P.; Kuda, O.; Krizova, J.; der Stelt, I.R.; Keijer, J.; Hansikova, H.; Rossmeisl, M.; Kopecky, J. Postnatal induction of muscle fatty acid oxidation in mice differing in propensity to obesity: A role of pyruvate dehydrogenase. Int. J. Obes. 2020, 44, 235–244. [Google Scholar] [CrossRef]
- Seiler, S.E.; Koves, T.R.; Gooding, J.R.; Wong, K.E.; Stevens, R.D.; Ilkayeva, O.R.; Wittmann, A.H.; DeBalsi, K.L.; Davies, M.N.; Lindeboom, L.; et al. Carnitine Acetyltransferase Mitigates Metabolic Inertia and Muscle Fatigue during Exercise. Cell Metab. 2015, 22, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Bender, T.; Martinou, J.C. The mitochondrial pyruvate carrier in health and disease: To carry or not to carry? Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, G.; Chapman, J.; Ofrecio, J.M.; Wilkes, J.; Resnik, J.L.; Thapar, D.; Subramaniam, S.; Sears, D.D. Multi-tissue, selective PPARgamma modulation of insulin sensitivity and metabolic pathways in obese rats. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E164–E174. [Google Scholar] [CrossRef] [Green Version]
- Duarte, J.A.; Carvalho, F.; Pearson, M.; Horton, J.D.; Browning, J.D.; Jones, J.G.; Burgess, S.C. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J. Lipid Res. 2014, 55, 2541–2553. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, G.; Unal, R.; Pokrovskaya, I.; Yao-Borengasser, A.; Phanavanh, B.; Lecka-Czernik, B.; Rasouli, N.; Kern, P.A. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: Effects of obesity, insulin resistance, and TZD treatment. J. Lipid Res. 2006, 47, 2444–2450. [Google Scholar] [CrossRef] [Green Version]
- Teran-Garcia, M.; Adamson, A.W.; Yu, G.; Rufo, C.; Suchankova, G.; Dreesen, T.D.; Tekle, M.; Clarke, S.D.; Gettys, T.W. Polyunsaturated fatty acid suppression of fatty acid synthase (FASN): Evidence for dietary modulation of NF-Y binding to the Fasn promoter by SREBP-1c. J. Lipid Res. 2006, 47, 2444–2450. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, L.M.; de Groot, P.J.; Hooiveld, G.J.; Koppen, A.; Kalkhoven, E.; Muller, M.; Kersten, S. Effect of synthetic dietary triglycerides: A novel research paradigm for nutrigenomics. PLoS ONE 2008, 3, e1681. [Google Scholar] [CrossRef]
- Pavlisova, J.; Bardova, K.; Stankova, B.; Tvrzicka, E.; Kopecky, J.; Rossmeisl, M. Corn oil versus lard: Metabolic effects of omega-3 fatty acids in mice fed obesogenic diets with different fatty acid composition. Biochimie 2016, 124, 150–162. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2010, 1801, 338–349. [Google Scholar] [CrossRef]
- Ryden, M.; Andersson, D.P.; Bernard, S.; Spalding, K.; Arner, P. Adipocyte triglyceride turnover and lipolysis in lean and overweight subjects. J. Lipid Res. 2013, 54, 2909–2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allister, C.A.; Liu, L.F.; Lamendola, C.A.; Craig, C.M.; Cushman, S.W.; Hellerstein, M.K.; McLaughlin, T.L. In vivo 2H2O administration reveals impaired triglyceride storage in adipose tissue of insulin-resistant humans. J. Lipid Res. 2015, 56, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallgren, P.; Sjostrom, L.; Hedlund, H.; Lundell, L.; Olbe, L. Influence of age, fat cell weight, and obesity on O2 consumption of human adipose tissue. Am. J. Physiol. 1989, 256, E467–E474. [Google Scholar] [CrossRef] [PubMed]
- Bernlohr, D.A. Exercise and mitochondrial function in adipose biology: All roads lead to NO. Diabetes 2014, 63, 2606–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]

| Gene Name | Gene ID | Forward Primer | Reverse Primer |
|---|---|---|---|
| Acsl1 | 14081 | GAAGCCGTGGCCCAGGTGTTTGTC | TTCGCCTTCAGTGTTGGAGTCAGA |
| Actb | 11461 | GAACCCTAAGGCCAACCGTGAAAAGAT | ACCGCTCGTTGCCAATAGTGATG |
| Atgl/Pnpla2 | 66853 | GGCAATCAGCAGGCAGGGTCTTTA | GCCAACGCCACTCACATCTACG |
| Bckdha | 12039 | ACGGCGGGCTGTGGCTGAGAA | GAGATTGGGTGGTCCTGCTTGTCC |
| Cd36 | 12491 | TGATACTATGCCCGCCTCTCC | TTCCCACACTCCTTTCTCCTCTAC |
| Cpt1a | 12894 | GCAGCTCGCACATTACAAGGACAT | AGCCCCCGCCACAGGACACATAGT |
| Crat | 12908 | ACATGGTGGTGGTAGCAAGTTCAA | GGCAAGGGCACCATAGGAGA |
| Cyphb/Ppib | 19035 | GGGAGATGGCACAGGAGGAAAGAG | ACCCAGCCAGGCCCGTAGTG |
| Dgat1 | 13350 | TGGCCAGGACAGGAGTATTTTTGA | CTCGGGCATCGTAGTTGAGCA |
| Dgat2 | 67800 | TGCCCTACTCCAAGCCCATCACC | TCAGTTCACCTCCAGCACCTCAGTCTC |
| Eef1a1 | 13627 | TGACAGCAAAAACGACCCACCAAT | GGGCCATCTTCCAGCTTCTTACCA |
| Eef2 | 13629 | GAAACGCGCAGATGTCCAAAAGTC | GCCGGGCTGCAAGTCTAAGG |
| Elovl5 | 68801 | CCTCTCGGGTGGCTGTTCTTCC | AGGCTTCGGCTCGGCTTGTC |
| Fas/Fasn | 14104 | TGGGTGTGGAAGTTCGTCAG | GTCGTGTCAGTAGCCGAGTC |
| Gk | 14933 | TCGTTCCAGCATTTTCAGGGTTAT | TCAGGCATGGAGGGTTTCACTACT |
| Hsl/Lipe | 16890 | TGCGCCCCACGGAGTCTATGC | CTCGGGGCTGTCTGAAGGCTCTGA |
| Lpl | 16956 | AGCCCCCAGTCGCCTTTCTCCT | TGCTTTGCTGGGGTTTTCTTCATTCA |
| Mpc1 | 55951 | TCATTCAGGGAGGACGACTTATC | TGTTTTCCCTTCAGCACGACTAC |
| Mpc2 | 70456 | CTCCCACCCTGCTGCTGTCG | GGCCTGCCGGGTGGTTGTA |
| Pc/Pcx | 18563 | CCCCTGGATAGCCTTAATACTCGT | TGGCCCTTCACATCCTTCAAA |
| Pck1 | 18534 | GGCAGCATGGGGTGTTTGTAGGA | TTTGCCGAAGTTGTAGCCGAAGAAG |
| Pdk4 | 27273 | GGCTTGCCAATTTCTCGTCTCTA | TTCGCCAGGTTCTTCGGTTCC |
| Pgc1a/Ppargc1a | 19017 | CCCAAAGGATGCGCTCTCGTT | TGCGGTGTCTGTAGTGGCTTGATT |
| Pparg | 19016 | GCCTTGCTGTGGGGATGTCTC | CTCGCCTTGGCTTTGGTCAG |
| Sbacad/Acadsb | 66885 | GCATCTGAGGTCGCTGGGCTAAC | CGATGTGCTTGGCGATGGTGT |
| Ucp1 | 22227 | CACGGGGACCTACAATGCTTACAG | CACGGGGACCTACAATGCTTACAG |
| Vlcad/Acadvl | 11370 | CAGGGGTGGAGCGTGTGC | CATTGCCCAGCCCAGTGAGTTCC |
| STD | HF | HF + F | HF + PIO | HF + PIO + F | HF + MSDC | HF + MSDC + F | |
|---|---|---|---|---|---|---|---|
| Energy balance | |||||||
| Body weight initial g) | 27.2 ± 0.40 | 27.6 ± 0.40 | 27.9 ± 0.49 | 27.3 ± 0.47 | 27.9 ± 0.48 | 27.8 ± 0.42 | 27.8 ± 0.50 |
| Body weight final (g) | 32.9 ± 0.70 | 44.3 ± 0.64 * | 41.0 ± 1.13 * | 41.5 ± 1.42 * | 38.6 ± 1.35 *,a | 36.9 ± 1.16 *,a | 34.5 ± 0.91 abc |
| Body weight gain (g) | 5.50 ± 0.44 | 16.1 ± 0.63 * | 12.0 ± 0.75 *,a | 13.3 ± 1.17 * | 10.1 ± 1.11 *,a | 8.27 ± 0.94 abc | 6.02 ± 0.58 abcd |
| Cumulative food intake (MJ/animal) | 3.86 ± 0.06 | 4.59 ± 0.11 * | 4.21 ± 0.18 | 4.57 ± 0.25 * | 4.17 ± 0.15 | 4.75 ± 0.20 * | 4.81 ± 0.32 * |
| Tissues | |||||||
| eWAT weight (mg) | 833 ± 73 | 2489 ± 84 * | 1977 ± 88 *,a | 2252 ± 168 * | 1734 ± 142 *,ac | 1511 ± 123 *,ac | 1171 ± 117 *,abcd |
| scWAT weight (mg) | 403 ± 34 | 1398 ± 87 * | 1283 ± 137 * | 1614 ± 288 * | 1191 ± 84 * | 1097 ± 96 * | 936 ± 78 * |
| Liver weight (mg) | 1763 ± 43 | 1793 ± 63 | 1563 ± 58 *,a | 1613 ± 84 * | 1477 ± 58 *,a | 1425 ± 58 *,a | 1418 ± 33 *,a |
| Liver TAG content (mg/g) | 33.2 ± 1.16 | 93.4 ± 8.03 * | 57.0 ± 4.96 *,a | 112 ± 17.6 *,b | 48.6 ± 5.77 *,ac | 67.9 ± 10.0 *c | 42.4 ± 5.58 ac |
| Plasma (random fed state) | |||||||
| NEFA (mmol/L) | 0.50 ± 0.05 | 0.66 ± 0.04 * | 0.38 ± 0.03 *,a | 0.42 ± 0.02 a | 0.34 ± 0.03 *,a | 0.38 ± 0.04 *,a | 0.22 ± 0.02 *,abce |
| TAG (mmol/L) | 1.39 ± 0.06 | 1.79 ± 0.05 * | 1.05 ± 0.09 *,a | 0.99 ± 0.06 *,a | 0.82 ± 0.06 *,a | 0.89 ± 0.06 *,a | 0.54 ± 0.04 *,abce |
| Cholesterol (mmol/L) | 2.81 ± 0.33 | 4.61 ± 0.14 * | 3.40 ± 0.12 a | 3.91 ± 0.10 *,ab | 2.91 ± 0.13 ac | 3.40 ± 0.13 ac | 2.55 ± 0.07 abce |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardova, K.; Funda, J.; Pohl, R.; Cajka, T.; Hensler, M.; Kuda, O.; Janovska, P.; Adamcova, K.; Irodenko, I.; Lenkova, L.; et al. Additive Effects of Omega-3 Fatty Acids and Thiazolidinediones in Mice Fed a High-Fat Diet: Triacylglycerol/Fatty Acid Cycling in Adipose Tissue. Nutrients 2020, 12, 3737. https://doi.org/10.3390/nu12123737
Bardova K, Funda J, Pohl R, Cajka T, Hensler M, Kuda O, Janovska P, Adamcova K, Irodenko I, Lenkova L, et al. Additive Effects of Omega-3 Fatty Acids and Thiazolidinediones in Mice Fed a High-Fat Diet: Triacylglycerol/Fatty Acid Cycling in Adipose Tissue. Nutrients. 2020; 12(12):3737. https://doi.org/10.3390/nu12123737
Chicago/Turabian StyleBardova, Kristina, Jiri Funda, Radek Pohl, Tomas Cajka, Michal Hensler, Ondrej Kuda, Petra Janovska, Katerina Adamcova, Ilaria Irodenko, Lucie Lenkova, and et al. 2020. "Additive Effects of Omega-3 Fatty Acids and Thiazolidinediones in Mice Fed a High-Fat Diet: Triacylglycerol/Fatty Acid Cycling in Adipose Tissue" Nutrients 12, no. 12: 3737. https://doi.org/10.3390/nu12123737
APA StyleBardova, K., Funda, J., Pohl, R., Cajka, T., Hensler, M., Kuda, O., Janovska, P., Adamcova, K., Irodenko, I., Lenkova, L., Zouhar, P., Horakova, O., Flachs, P., Rossmeisl, M., Colca, J., & Kopecky, J. (2020). Additive Effects of Omega-3 Fatty Acids and Thiazolidinediones in Mice Fed a High-Fat Diet: Triacylglycerol/Fatty Acid Cycling in Adipose Tissue. Nutrients, 12(12), 3737. https://doi.org/10.3390/nu12123737






