Effects of Oral Resveratrol Supplementation on Glycogen Replenishment and Mitochondria Biogenesis in Exercised Human Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Participants
2.2. Experimental Procedure
2.3. Resveratrol and Placebo Capsules
2.4. Muscle Biopsy Sampling and Glycogen Analysis
2.5. RNA Extraction and Real-Time RT-PCR
2.6. Gas Sample Collection and Analysis
2.7. Blood Sample Collection and Analysis
2.7.1. Glucose Analysis Method
2.7.2. Insulin Analysis Method
2.7.3. NEFA Analysis Method
2.7.4. Glycerol Analysis Method
2.8. Statistics
3. Results
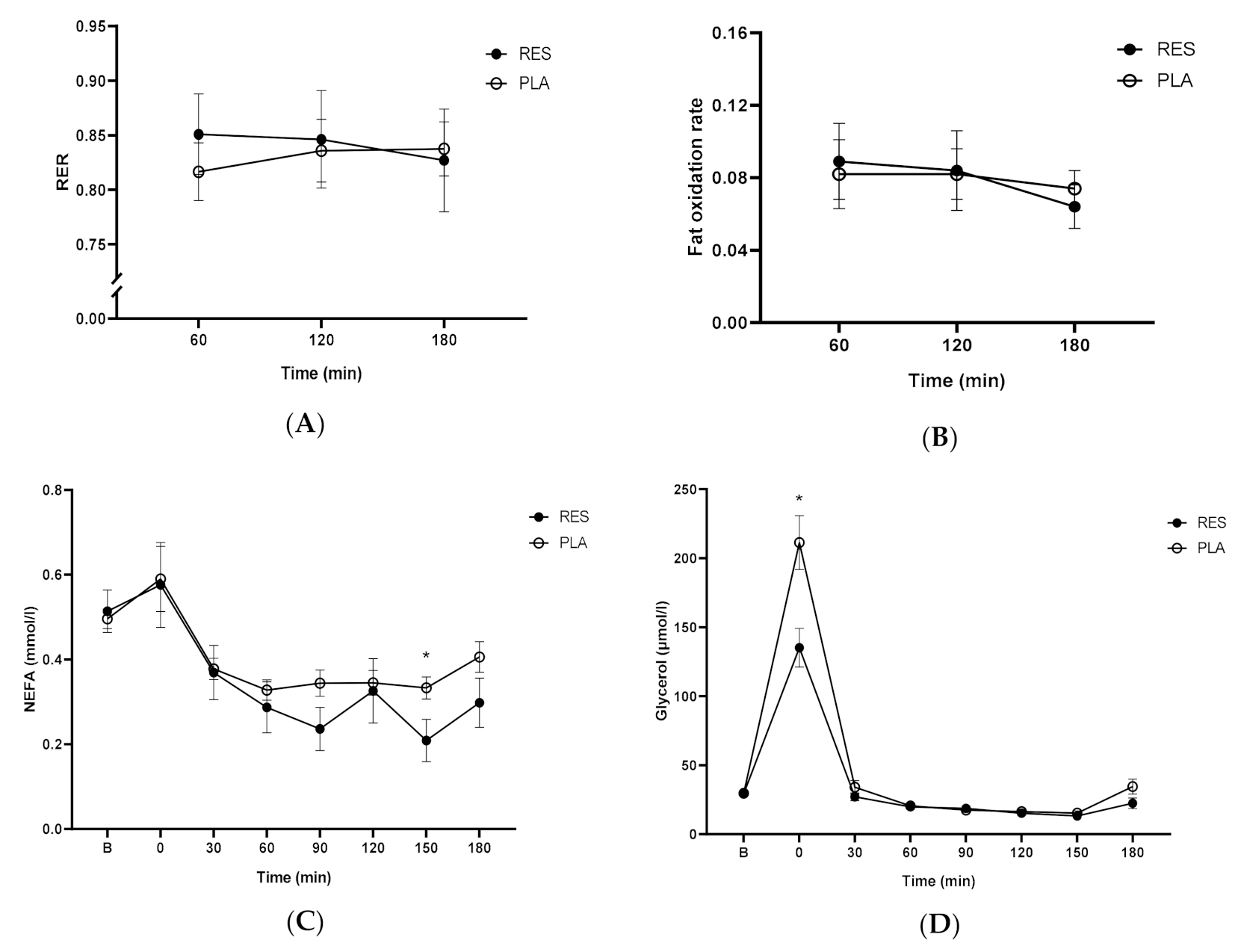
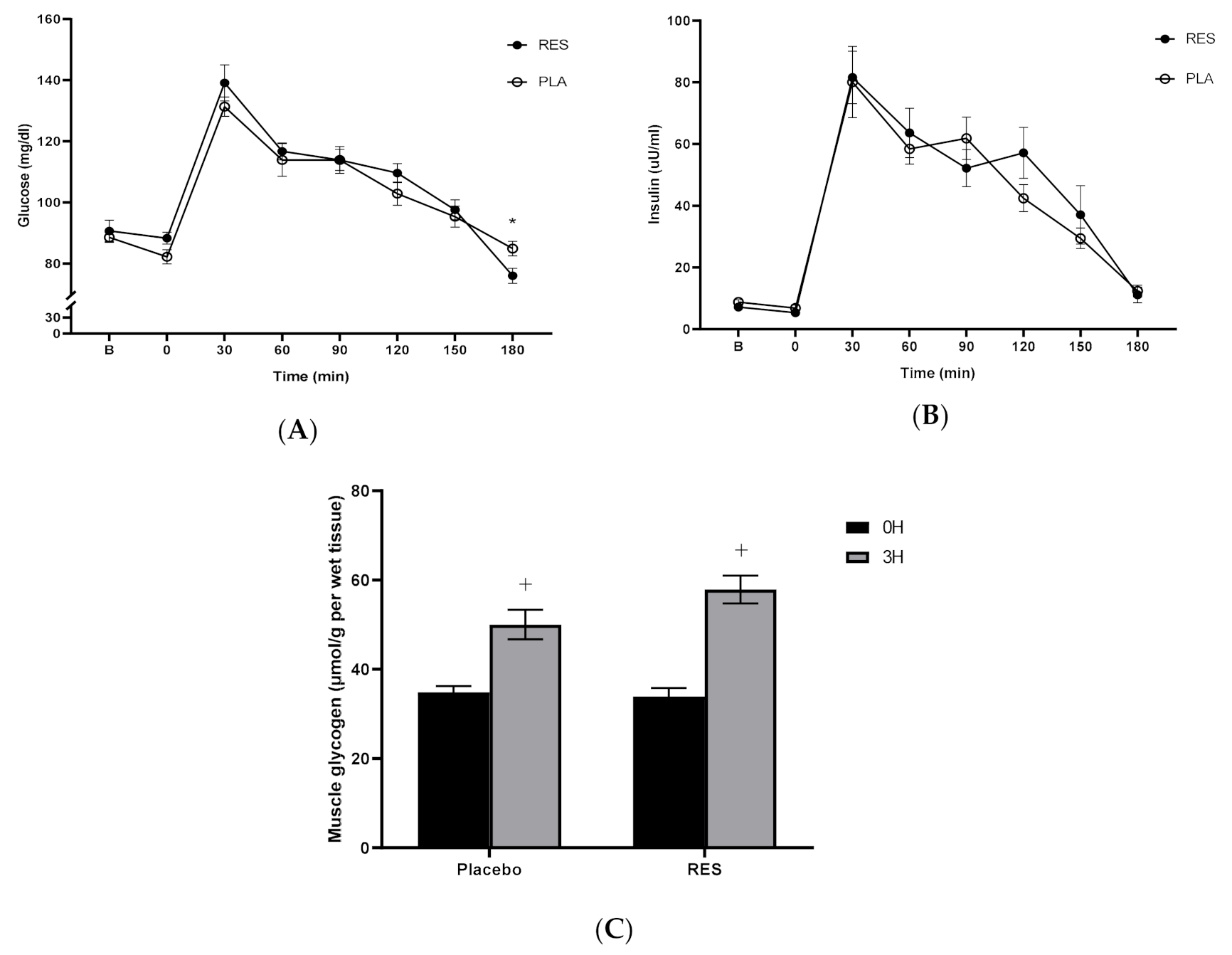
3.1. Resveratrol Was Unable to Alter Fat Metabolism during Cycling Exercise Recovery Period
3.2. Resveratrol Did Not Change Muscle Glycogen Levels Following Exercise
3.3. Resveratrol Was Unable to Upregulate mRNA Expression of Glucose Utilization and Mitochondrial Biogenesis in Skeletal Muscle
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ørtenblad, H.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Morino, T.; Ishii, T.; Kanzaki, M. Cooperative actions of Tbc1d1 and AS160/Tbc1d4 in GLUT4 trafficking activities. J. Biol. Chem. 2018, 294, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Ivy, J.; Kuo, C.H. Regulation of GLUT4 protein and glycogen synthase during muscle glycogen synthesis after exercise. Acta Physiol. 1998, 162, 295–304. [Google Scholar] [CrossRef]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef] [Green Version]
- Bicer, M.; Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K.; Avunduk, M.C. Effect of resveratrol administration on muscle glycogen levels in rats subjected to acute swimming exercise. Cell. Mol. Biol. 2019, 65, 28–31. [Google Scholar] [CrossRef]
- Wilson, J.E. Hexokinases. Reviews of Physiology. Biochem. Pharmacol. 1995, 126, 65–198. [Google Scholar]
- Jessen, N.; An, D.; Lihn, A.S.; Nygren, J.; Hirshman, M.F.; Thorell, A.; Goodyear, L.J. Exercise increases TBC1D1 phosphorylation in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E164–E171. [Google Scholar] [CrossRef] [Green Version]
- Treebak, J.T.; Pehmøller, C.; Kristensen, J.M.; Kjøbsted, R.; Birk, J.B.; Schjerling, P.; Richter, E.A.; Goodyear, L.J.; Wojtaszewski, J.F. Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J. Physiol. 2014, 592, 351–375. [Google Scholar] [CrossRef]
- Jonckheere, A.I.; Smeitink, J.A.; Rodenburg, R.J. Mitochondrial ATP synthase: Architecture, function and pathology. J. Inherit. Metab. Dis. 2012, 35, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Popov, D.; Zinovkin, R.; Karger, E.; Tarasova, O.; Vinogradova, O. Effects of continuous and intermittent aerobic exercise upon mRNA expression of metabolic genes in human skeletal muscle. J. Sports Med. Phys. Fitness 2014, 54, 362–369. [Google Scholar]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Gaamouria, N.; Zouhal, H.; Hammami, M.; Hackney, C.M.; Abderrahman, B.A.; Saeidi, A.; El Hage, R.; Ounis, O.B. Effects of polyphenol (carob) supplementation on body composition and aerobic capacity in taekwondo athletes. Physiol. Behav. 2019, 205, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Neves, A.R.; Lucio, M.; Lima, J.L.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef]
- Švajger, U.; Jeras, M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef]
- Duntas, L.H. Resveratrol and its impact on aging and thyroid function. J. Endocrinol. Investig. 2011, 34, 788–792. [Google Scholar]
- Chang, C.C.; Lin, K.Y.; Peng, K.Y.; Day, Y.J.; Hung, L.M. Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr. J. 2015, 63, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Savouret, J.F.; Widerak, M.; Corvol, M.T.; Rannou, F. Resveratrol, Potential Therapeutic Interest in Joint Disorders: A Critical Narrative Review. Nutrients 2017, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Langfort, J.; Viese, M.; Ploug, T.; Dela, F. Time course of GLUT4 and AMPK protein expression in human skeletal muscle during one month of physical training. Scand. J. Med. Sports 2003, 13, 169–174. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Tsao, J.P.; Liao, S.F.; Cheng, I.S.; Korivi, M.; Hou, C.W.; Kuo, C.H.; Wang, H.F. Oral conjugated linoleic acid supplementation enhanced glycogen resynthesis in exercised human skeletal muscle. J. Sports Sci. 2015, 33, 915–923. [Google Scholar] [CrossRef]
- Wu, C.L.; Nicholas, C.; Williams, C.; Took, A.; Hardy, L. The influence of high-carbohydrate meals with different glycaemic indices on substrate utilisation during subsequent exercise. Br. J. Nutr. 2003, 90, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.C.; Chang, C.W.; Hsu, M.C.; Wu, Y.T. Self-nanoemulsifying drug delivery system for resveratrol: Enhanced oral bioavailability and reduced physical fatigue in rats. Int. J. Mol. Sci. 2017, 18, 1853. [Google Scholar] [CrossRef] [Green Version]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Bergstrom, J. Percutaneous needle biopsy of skeletal ¨ muscle in physiological and clinical research. Scand. J. Clin. Lab. Investig. 1975, 35, 609–616. [Google Scholar] [CrossRef]
- Passonneau, J.V.; Lauderdale, V.R. A comparison of three methods of glycogen measurement in tissues. Anal. Biochem. 1974, 60, 405–412. [Google Scholar] [CrossRef]
- Gibson, U.E.; Heid, C.A.; Williams, P.M. A novel method for real time quantitative RT-PCR. Genome Res. 1996, 6, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Zuniga, J.M.; Housh, T.J.; Camic, C.L.; Bergstrom, H.C.; Traylor, D.A.; Schmidt, R.J.; Johnson, G.O. Metabolic parameters for ramp versus step incremental cycle ergometer tests. Appl. Physiol. Nutr. Metab. 2012, 37, 1110–1117. [Google Scholar] [CrossRef]
- Sato, M.; Maulik, G.; Bagchi, D.; Das, D.K. Myocardial protection by protykin, a novel extract of trans-resveratrol and emodin. Free Radic. Res. 2000, 32, 135–144. [Google Scholar] [CrossRef]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Hou, P.; Zhou, M.; Ren, Q.; Wang, X.; Huang, L.; Hui, S.; Yi, L.; Mi, M. Resveratrol attenuates high-fat diet-induced non-alcoholic steatohepatitis by maintaining gut barrier integrity and inhibiting gut inflammation through regulation of the endocannabinoid system. Clin. Nutr. 2018, 19, 884–894. [Google Scholar] [CrossRef]
- Mercader, J.; Palou, A.; Bonet, M.L. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and RetinolBinding Protein 4 expression in white adipocytes. J. Nutr. Biochem. 2011, 22, 828–834. [Google Scholar] [CrossRef]
- Palsamy, P.; Subramanian, S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Chem. Biol. Interact. 2009, 179, 356–362. [Google Scholar] [CrossRef]
- Randell, R.K.; Hodgson, A.B.; Lotito, D.M.; Jacobs, D.M.; Boon, N.; Mela, D.J.; Jeukendrup, A.E. No effect of 1 or 7 d of green tea extract ingestion on fat oxidation during exercise. Med. Sci. Sports Exerc. 2013, 45, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.H.; Hunt, D.G.; Ding, Z.; Ivy, J.L. Effect of carbohydrate supplementation on postexercise GLUT-4 protein expression in skeletal muscle. J. Appl. Physiol. 1999, 87, 2290–2295. [Google Scholar] [CrossRef]
- Cusi, K.J.; Pratipanawatr, T.; Koval, J.; Printz, R.; Ardehali, H.; Granner, D.K.; Defronzo, R.A.; Mandarino, L.J. Exercise increases hexokinase II mRNA, but not activity in obesity and type 2 diabetes. Metabolism 2001, 50, 602–606. [Google Scholar] [CrossRef]
- Fueger, P.T.; Shearer, J.; Krueger, T.M.; Posey, K.A.; Bracy, D.P.; Heikkinen, S.; Laakso, M.; Rottman, J.N.; Wasserman, D.H. Hexokinase II protein content is a determinant of exercise endurance capacity in the mouse. J. Physiol. 2005, 566, 533–541. [Google Scholar] [CrossRef]
- Chen, S.; Wasserman, D.H.; MacKintosh, C.; Sakamoto, K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 2011, 13, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.Y.; Hsieh, P.S.; Huang, J.P.; Lu, L.S.; Hung, L.M. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 2008, 57, 1814–1823. [Google Scholar] [CrossRef] [Green Version]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, H.; Zhang, Y.; Ji, L.L. Redefining the role of mitochondria in exercise: A dynamic remodeling. Ann. N. Y. Acad. Sci. 2010, 1201, 121–128. [Google Scholar] [CrossRef]
- Feng, Z.; Bai, L.; Yan, J.; Li, Y.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, Y.; Chen, Y.; et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: Regulatory effects of hydroxytyrosol. Free Radic. Biol. Med. 2011, 50, 1437–1446. [Google Scholar] [CrossRef]

| Gene | (Forward) | (Reverse) |
|---|---|---|
| β-actin | 5′-CCTGGCACCCAGCACAAT-3′ | 5′-GCCGATCCACACGGAGTACT-3′ |
| GLUT4 | 5′-CACAGTCTTCACCTTGGTCTCG-3′ | 5′-GTAGCTCATGGCTGGAACTCG-3′ |
| HKII | 5′-TTGTCCGTAACATTCTCATCGATT-3′ | 5′-TGTCTTGAGCCGCTCTGAGAT-3′ |
| TBC1D1 | 5′-GTGTGGGAAAAGATGCTTAGCA-3′ | 5′-GTGATGACGTGGCACACCTT-3′ |
| TBC1D4 | 5′-AGCTCCAGTGAACAGTGCAGTG-3′ | 5′-CACTTAGGGACTCATTGCTGC-3′ |
| PGC-1α | 5′-CGAGGAATATCAGCACGAGAGG-3′ | 5′-CATAAATCACACGGCGCTCTTC-3′ |
| SIRT1 | 5′-TACGACGAAGACGACGACGA-3′ | 5′-CGCCGCCGCCGCCTCTTCC-3′ |
| ERR-α | 5′-TGCCAATTCAGACTCTGTGC-3′ | 5′-CCAGCTTCACCCCATAGAAA-3′ |
| NRF1 | 5′-CTACTCGTGTGGGACAGCAA-3′ | 5′-AGCAGACTCCAGGTCTTCCA-3′ |
| NRF2 | 5′-AAGTGACAAGATGGGCTGCT-3′ | 5′-TGGACCACTGTATGGGATCA-3′ |
| TFAM | 5′-CGCTCCCCCTTCAGTTTTGT-3′ | 5′-CACTCCGCCCTATAAGCATC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Liu, C.-C.; Tsao, J.-P.; Hsu, C.-L.; Cheng, I.-S. Effects of Oral Resveratrol Supplementation on Glycogen Replenishment and Mitochondria Biogenesis in Exercised Human Skeletal Muscle. Nutrients 2020, 12, 3721. https://doi.org/10.3390/nu12123721
Huang C-C, Liu C-C, Tsao J-P, Hsu C-L, Cheng I-S. Effects of Oral Resveratrol Supplementation on Glycogen Replenishment and Mitochondria Biogenesis in Exercised Human Skeletal Muscle. Nutrients. 2020; 12(12):3721. https://doi.org/10.3390/nu12123721
Chicago/Turabian StyleHuang, Chun-Ching, Chia-Chen Liu, Jung-Piao Tsao, Chin-Lin Hsu, and I-Shiung Cheng. 2020. "Effects of Oral Resveratrol Supplementation on Glycogen Replenishment and Mitochondria Biogenesis in Exercised Human Skeletal Muscle" Nutrients 12, no. 12: 3721. https://doi.org/10.3390/nu12123721
APA StyleHuang, C.-C., Liu, C.-C., Tsao, J.-P., Hsu, C.-L., & Cheng, I.-S. (2020). Effects of Oral Resveratrol Supplementation on Glycogen Replenishment and Mitochondria Biogenesis in Exercised Human Skeletal Muscle. Nutrients, 12(12), 3721. https://doi.org/10.3390/nu12123721





