Physiological Impact of Palm Olein or Palm Oil in Infant Formulas: A Review of Clinical Evidence
Abstract
:1. Introduction
2. Palm Fruit Derived Oils as a Source of Saturated Fat in Infant Formulas
3. Physiological Effects of Palm Olein (POL) or Palm Oil (PO) in Infants
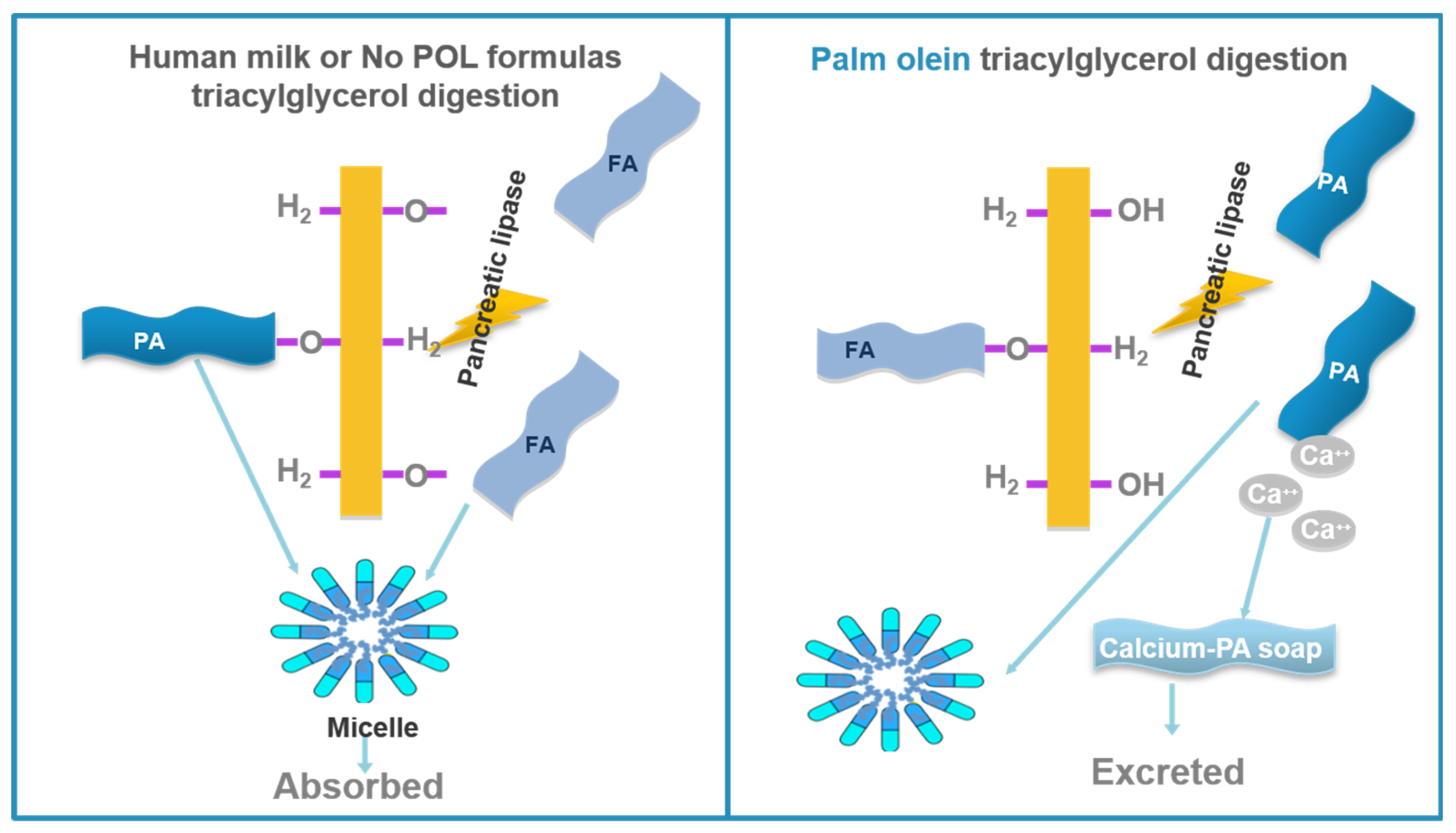
3.1. Calcium and Fat Absorption
3.2. Bone Mineralization
3.3. Stool Consistency
3.4. Growth (Weight Gain)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; DeVine, D.; Trikalinos, T.; Lau, J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep. Technol. Assess. 2007, 153, 1–186. [Google Scholar]
- Demmelmair, H.; Koletzko, B. Lipids in human milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammi-Keefe, C.J.; Jensen, R.G. Lipids in human milk: A review. 2: Composition and fat-soluble vitamins. J. Pediatr. Gastroenterol. Nutr. 1984, 3, 172–198. [Google Scholar] [CrossRef] [PubMed]
- Tomarelli, R.M.; Meyer, B.J.; Weaber, J.R.; Bernhart, F.W. Effect of positional distribution on the absorption of the fatty acids of human milk and infant formulas. J. Nutr. 1968, 95, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.W.; Hockman, E.M.; Dow, M. Palm olein in the fat blend of infant formulas: Effect on the intestinal absorption of calcium and fat, and bone mineralization. J. Am. Coll. Nutr. 2006, 25, 117–122. [Google Scholar] [CrossRef]
- Lasekan, J.B.; Hustead, D.S.; Masor, M.; Murray, R. Impact of palm olein in infant formulas on stool consistency and frequency: A meta-analysis of randomized clinical trials. Food Nutr. Res. 2017, 61, 1330104. [Google Scholar] [CrossRef] [Green Version]
- Bronsky, J.; Campoy, C.; Embleton, N.; Fewtrell, M.; Mis, N.F.; Gerasimidis, K.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Palm Oil and Beta-palmitate in Infant Formula: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 742–760. [Google Scholar] [CrossRef]
- Elson, C.E. Tropical oils-Nutritional and scientific issues. Crit. Rev. Food Sci. Nutr. 1992, 31, 79–102. [Google Scholar] [CrossRef]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar]
- Edem, D.O. Palm oil: Biochemical, physiological, nutritional, hematological, and toxicological aspects: A review. Plant Food Hum. Nutr. 2002, 57, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, B.; Unnithan, U.R.; Choo, Y.M.; Sundram, K. Characteristics of Red Palm Oil, a Carotene- and Vitamin E–rich Refined Oil for Food Uses. Food Nutr. Bull. 2000, 21, 189–194. [Google Scholar] [CrossRef]
- Nelson, S.E.; Frantz, J.A.; Ziegler, E.E. Absorption of fat and calcium by infants fed a milk-based formula containing palm olein. J. Am. Coll. Nutr. 1998, 17, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F.; Pansu, D. Nutritional aspects of calcium absorption. J. Nutr. 1999, 129, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Souza, C.O.; Leite, M.E.Q.; Lasekan, J.; Baggs, G.; Pinho, L.S.; Druzian, J.I.; Ribeiro, T.C.M.; Mattos, A.P.; Menezes-Filho, J.A.; Costa-Ribeiro, H. Milk protein-based formulas containing different oils affect fatty acids balance in term infants: A randomized blinded crossover clinical trial. Lipids Health Dis. 2017, 16, 78. [Google Scholar] [CrossRef] [Green Version]
- Leite, M.E.; Lasekan, J.; Baggs, G.; Ribeiro, T.; Menezes-Filho, J.; Pontes, M.; Druzian, J.; Barreto, D.L.; de Souza, C.O.; Mattos, A.; et al. Calcium and fat metabolic balance, and gastrointestinal tolerance in term infants fed milk-based formulas with and without palm olein and palm kernel oils: A randomized blinded crossover study. BMC Pediatr. 2013, 13, 215. [Google Scholar] [CrossRef] [Green Version]
- Nelson, S.E.; Rogers, R.R.; Frantz, J.A.; Ziegler, E.E. Palm olein in infant formula: Absorption of fat and minerals by normal infants. Am. J. Clin. Nutr. 1996, 64, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, K.M.; Borschel, M.W.; Westcott, J.E.; Richardson, K.S.; Krebs, N.F. Lower calcium absorption in infants fed casein hydrolysate- and soy protein-based infant formulas containing palm olein versus formulas without palm olein. J. Am. Coll. Nutr. 2002, 21, 564–569. [Google Scholar] [CrossRef]
- Hansen, J.; Huston, R.; Ehrenkranz, R.; Bell, E. Impact of palm olein in infant feedings on fat and calcium absorption in growing premature infants. J. Am. Coll. Nutr. 1996, 15, 526. [Google Scholar]
- Abbott Nutrition. Metabolic Balances and Serum Chemistries in Low-Birth-Weihgt Infants Fed Similac 20 with Iron Containing Alternate Fat Blends; Final Report CP-5092; Abbott Nutrition: Columbus, OH, USA, 1982. [Google Scholar]
- Hicks, P.D.; Hawthorne, K.M.; Berseth, C.L.; Marunycz, J.D.; Heubi, J.E.; Abrams, S.A. Total calcium absorption is similar from infant formulas with and without prebiotics and exceeds that in human milk-fed infants. BMC Pediatr. 2012, 12, 118. [Google Scholar] [CrossRef] [Green Version]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Jones, I.E.; Taylor, R.W.; Williams, S.M.; Manning, P.J.; Goulding, A. Four-year gain in bone mineral in girls with and without past forearm fractures: A DXA study. Dual energy X-ray absorptiometry. J. Bone Miner. Res. 2002, 17, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Saggese, G.; Baroncelli, G.I.; Bertelloni, S. Osteoporosis in children and adolescents: Diagnosis, risk factors, and prevention. J. Pediatr. Endocrinol. Metab. 2001, 14, 833–859. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.W.; Hammami, M.; Margeson, D.P.; Nwaesei, C.; Montalto, M.B.; Lasekan, J.B. Reduced bone mineralization in infants fed palm olein-containing formula: A randomized, double-blinded, prospective trial. Pediatrics 2003, 111, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Borschel, M.W.; Groh-Wargo, S.; Brabec, B.A.; Ziegler, E.E.; Litov, R.E.; Abrams, S.A.; Oliver, J.S. Tolerance, bone mineral content, and serum vitamin D concentration of term infants fed partially hydrolyzed whey-based infant formula. Open Nutr. J. 2012, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Specker, B.L.; Beck, A.; Kalkwarf, H.; Ho, M. Randomized trial of varying mineral intake on total body bone mineral accretion during the first year of life. Pediatrics 1997, 99, E12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.J.; Antonson, D.L.; Ferguson, P.W.; Murray, N.D.; Merkel, K.; Moore, T.E. Neonatal and infant feeding: Effect on bone density at 4 years. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 88–93. [Google Scholar] [CrossRef]
- Koo, W. Re: Neonatal and infant feeding: Effect on bone density at 4 years. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 681. [Google Scholar] [CrossRef]
- Quinlan, P.T.; Lockton, S.; Irwin, J.; Lucas, A.L. The relationship between stool hardness and stool composition in breast- and formula-fed infants. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 81–90. [Google Scholar] [CrossRef]
- Lloyd, B.; Halter, R.J.; Kuchan, M.J.; Baggs, G.E.; Ryan, A.S.; Masor, M.L. Formula tolerance in postbreastfed and exclusively formula-fed infants. Pediatrics 1999, 103, E7. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, P.A.; Tressler, R.L.; Mulvaney, A.; Lam, W.; Comer, G.M. Gastrointestinal tolerance of a new infant milk formula in healthy babies: An international study conducted in 17 countries. Nutrition 2002, 18, 484–489. [Google Scholar] [CrossRef]
- Borschel, M.W.; Choe, Y.S.; Kajzer, J.A. Growth of healthy term infants fed partially hydrolyzed whey-based infant formula: A randomized, blinded, controlled trial. Clin. Pediatr. 2014, 53, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Cordano, A.; Diersen-Schade, D.; Guy, D.; Sickles, V.; Harris, C. Impact of Palm Olein (PO) in Infant Feedings on Growth in Normal Term Infants. J. Am. Coll. Nutr. 1996, 15, 526. [Google Scholar]
- Aggett, P.J.; Agostini, C.; Goulet, O.; Hernell, O.; Koletzko, B.; Lafeber, H.L.; Michaelsen, K.F.; Rigo, J.; Weaver, L.T.; European Society of Pediatric Gastroenterology Hepatology and Nutrition; et al. The nutritional and safety assessment of breast milk substitutes and other dietary products for infants: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Koo, W. Palm Oil and Beta-Palmitate in Infant Formula. J. Pediatr. Gastroenterol. Nutr. 2019, 70, e64. [Google Scholar] [CrossRef] [PubMed]
- Bronsky, J.; Campoy, C.; Embleton, N.; Fewtrell, M.; Mis, N.F.; Gerasimidis, K.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Response to Letter to the Editor—Palm Oil and Beta-Palmitate in Infant Formula. J. Pediatr. Gastroenterol. Nutr. 2019, 70, e64. [Google Scholar] [CrossRef]
| Palm Kernel Oil (PKO) | Palm Oil (PO) | Palm Olein (POL) | |
|---|---|---|---|
| Origin | Kernel | Mesocarp | Palm Oil |
| Major Fatty acids (% of total Fatty acids) | Lauric acid (≈50%) Myristic acid (≈16%) | Palmitic acid (≈44%) Oleic acid (≈39%) | Oleic acid (≈43%) Palmitic acid (≈40%) |
| Viscosity | High | Medium | Low |
| First Author, Year [ref.] | Type of Study | Design of Study | Sample Size | Population Age | Duration | Type of Formula | Intervention Formula | Comparison Formula | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | POL | No POL | |||||||||
| Leite et al. 2013 [16] Souza et al. 2017 [15] | DB-RCT crossover | Two periods, each one including a tolerance study feeding two IFs for 14 d, followed by a 4 d metabolic study | 33 ↓ 17 | 16 ↓ 8 | 17 ↓ 9 | 68–159 ± 3 days (Term infants) | Tolerance phase: 14 days Metabolic phase: 4 d | Cow’s milk protein-based powder | POL formula containing 44% * POL, 21.7% PKO and 18.5% canola oil | No POL formula containing 41.4% HO sunflower oil, 29.6% coconut oil and 27.6% soy oil | DHA and ARA are better absorbed from the No POL IF [15]. No POL formula is associated with improved absorption of fat [15,16]. Calcium retention is significantly greater when the No POL formula was fed [15,16]. |
| Ostrom et al. 2002 [18] | 2 Blinded RCT crossover | Two studies, infants were fed 2 CHFs or 2 SPFs for 7 days, followed by a 3 d balance study | 22 10 (CHFs study) 12 (SPFs study) | ND | ND | Mean age: 84 d (POL) 75 d (No POL) 89 d (POL/No POL) (Full-term infants) | Feeding phase: 7 d Balance study: 3 d | CHF: extensively hydrolyzed protein-based. Liquid SPF: soy protein-based liquid | CHF or SPF containing 45% POL, 20% soy, 20% coconut and 15% HO sunflower oils | CHF containing 50% MCT, 40% safflower and 10% soy oils SPF containing 42% HO safflower, 30% coconut and 28% soy oils | POL formulas (CH or SP) were associated with significantly less calcium absorption. CHFs containing POL were associated with significantly less fat absorption. |
| Nelson et al. 1998 [13] | RCT crossover | Feeding POL or HOS formula for 3 days at least before the 3–4 d metabolic balance study | 10 | ND | ND | 22–192 days (9 term infants, 1 preterm) | Feeding phase: ≥ 3 d Metabolic balance study: 3-4 d | Cow’s milk protein-based liquid | POL formula containing 45% POL, 20% soy, 20% coconut and 15% HO sunflower oils | No POL formula containing 42% HO safflower, 30% coconut and 28% soy oils | In every single infant:
|
| Nelson et al. 1996 [17] | RCT crossover | Feeding POL or No POL formula for 7 days at least before a balance study | 11 | ND | ND | 27–137 days (Term infants) | Feeding phase: ≥ 7 d Metabolic balance study: 3–4 d | Cow’s milk protein-based liquid | POL formula containing 53% POL and 47% soy oil | No POL formula containing 60% soy oil and 40% coconut oil | In every single infant absorption of fat was significantly less with POL formula. In most infants, the percentage of calcium absorption was significantly lower. |
| Hansen et al. 1996 [19] | RCT | Infants were fed 3 IFs with different % of PA for 7 days before a 3 d balance study | 30 | ND | ND | ND (Preterm infants) | Feeding phase: 7 d Balance study: 3 d | ND | 3 POL formulas containing 10%, 22% and 27% of PA each | Human milk | Fat and Ca absorption are inversely (not linearly) related to PA content. An IF with appropriate Ca content results in fat an Ca absorption similar to HM regardless of PA position in TG |
| Abbott Nutrition 1982 [20] | Clinical study | Infants were fed a PO experimental IF or one of two control formulas without PO. A 96 h metabolic balance study was carried out | 16 | 9 | 4 (Coconut/Corn) 3 (Coconut/Sunflower) | 5 days (low-birth-weight infants) | Feeding phase: 3 d (full feeds) Metabolic balance study: 96 h | Cow’s milk protein-based powder | PO formula containing 60% PO and 40% sunflower oil | No PO formula containing 60% coconut and 40% corn oils No PO formula containing 60% coconut and 40% sunflower oils | The PO formula provides a poorly absorbed source of fat for low-birth-weight infants, and it is significantly lower than those provided by PO free formulas. The lower mean calcium absorption associated to the PO formula may be related to de poor absorption of fat. |
| First Author, Year [Ref.] | Type of Study | Design of Study | Sample Size | Population Age | Duration | Type of Formula | Intervention Formula | Comparison Formula | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | POL | No POL | |||||||||
| Borschel et al. 2012 [26] | DB-RCT multicentre | Infants were randomly assigned to either POL or No POL formula during 84 days of life. | 89 ↓ 48 | 44 ↓ 24 | 45 ↓ 24 | 0–8 days (Term infants) | 84 days | Partially hydrolyzed whey -based Powder | PHF containing 46% POL, 26% soy oil, 20% coconut oil and 6% HO safflower or sunflower oil | PHF containing 41% HO safflower, 29% soy and 27% coconut oils | BMC is significantly greater in the No POL formula, only when body weight is used as a covariate. |
| Young et al. 2005 [28] | Retrospective | Children fed exclusively HM or IF with or without POL during the 4 first months were recruited. BMC was analyzed by DEXA at 4 years. | 178 | 65 | 56 57 (HM) | 4,5 years | NA | Milk protein-based | IF containing 45% POL, 20% soy oil, 20% coconut oil and 15% HO sunflower oil. | IF containing 40% HO safflower oil, 30% soy oil and 30% coconut oil. | Feeding infants with the PO formula during the first 4 months of life does not have a negative effect on BMC and BMD at 4 years of age. Challenged by Koo [29]. |
| Koo et al. 2003 [25] | DB-RCT | Two groups of infants were fed either PO or No POL formula for the first 6 months | 128 ↓ 102 | 63 ↓ 52 | 65 ↓ 50 | 6 ± 1 days (Term infants) | 6 months | Cow’s milk protein-based | IF containing 45% POL, 20% soy oil, 20% coconut oil and 15% HO sunflower oil | IF containing 40% HO safflower oil, 30% soy oil and 30% coconut oil | The inclusion of PO in IF at levels needed to provide a fatty acid profile similar to that of HM leads to lower bone mineralization. |
| Specker et al. 1997 [27] | Randomized prospective Phase I | Infants were randomized (first 6 months) to a low (POL) or moderate (No POL) mineral formula. Another group was BF, supplemented with low mineral IF. | 101 ↓ 92 | 30 (Low) 31 (HM + Low) | 31 (Mod) | <14 days (Full-term infants) | 6 months | Cow’s milk protein-based | Low mineral IF containing POL and 430 mg/L of Ca | Moderate mineral IF not containing POL and containing 510 mg/L of Ca | The low mineral formula and the human milk fed groups had similar BMC, which was lower than that in the moderate mineral group at 3 and 6 months of age. |
| Randomized prospective Phase II | The same infants were randomized (6 to 12 months of age) again to a moderate (No POL) or high (POL) mineral formula or to whole cow’s milk (CM). | 92 ↓ 87 | 39 (High) | 38 (Mod) 10 (CM) | <7 months (Full-term infants) | 6 months | Cow’s milk protein-based | High mineral IF containing POL and 1350 mg/L of Ca. | Moderate mineral IF not containing POL and containing 510 mg/L of Ca | No significant differences were found in BMC between feeding groups either at 9 or 12 months of age. The effect of mineral intake and fat composition on BM accretion seems not to be long term. | |
| First Author, Year [Ref.] | Type of Study | Design of Study | Sample Size | Population Age | Duration | Type of Formula | Intervention Formula | Comparison Formula | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | POL | No POL | |||||||||
| Borschel et al. 2014 [33] | Blinded RCT multicentre | Infants were randomized to 1 of 2 PHFs (POL/No POL) during the first 4 months of life. | 209 ↓ 177 | 101 ↓ 83 | 108 ↓ 94 | 0–8 days (Term infants) | 119 days (4 months) | Partially hydrolyzed whey -based (with added prebiotic GOS) Powder | PHF containing 46% POL, 26% soy oil, 20% coconut oil, 6% HO safflower or sunflower oil | PHF containing 41% HO safflower, 29% soy and 27% coconut oils. | MRSC is significantly lower when PHFs without POL are fed. |
| Leite et al. 2013 [16] | DB-RCT crossover | Two periods, each one including a tolerance study feeding two formulas for 14 d, followed by a 4-d metabolic study | 33 ↓ 17 | 16 ↓ 8 | 17 ↓ 9 | 68–159 days (Term infants) | Tolerance phase: 14 d Metabolic phase: 4 d | Cow’s milk protein-based Powder | POL formula containing 44% POL, 21,7% PKO and 18,5% canola oil | No POL formula containing 41,4% HO sunflower oil, 29,6% coconut oil and 27,6% soy oil | Infants fed No POL formula had significantly softer stools (3.0 + 0.5; mean ± SD) than those fed POL formula (2.4 ± 0.3). MRSC score: 5 = watery, 4 = loose/mushy, 3 = soft, 2 = formed, 1 = hard (higher is softer) |
| Borschel et al. 2012 [26] | DB-RCT multicenter | Infants were randomly assigned to either a POL or No POL formula during 56 and 84 days of life. | 89 ↓ 75 ↓ 64 | 44 ↓ 37 ↓ 26 | 45 ↓ 38 ↓ 28 | 0–8 days (Term infants) | 56 and 84 days 56 days ↓ 84 days | Partially hydrolyzed whey -based Powder | PHF containing 46% POL, 26% soy oil, 20% coconut oil, 6% HO safflower or sunflower oil | PHF containing 41% HO safflower, 29% soy and 27% coconut oils | MRSC is significantly greater in infants fed PHF with POL. |
| Alarcon et al. 2002 [32] | Open, not blinded multicenter controlled | The study was conducted in 17 countries. Infants were fed 1 of 5 diets: HM, No POL, POL, HM + No POL or HM + POL for 14 days. | 7673 ↓ 6999 | 1013 (POL) 635 (HM + POL) | 2677 (No POL) 979 (HM) 1695 (HM + No POL) | 28–98 days (Term infants) | 14 days | Cow’s milk protein-based | POL formula containing 45% POL, 20% coconut, 20% soy and 15% sunflower oils | No POL formula containing 42% HO safflower oil, 30% coconut oil and 28% soy oil. (This composition may vary by country). | Stools of infants fed No POL formula are significantly softer than those of infants fed POL containing formula. |
| Lloyd et al. 1999 [31] Study 1 | Blinded RCT | Exclusively breastfed infants were randomized to either formula with or without POL. | 82 ↓ 70 | 39 ↓ 35 | 43 ↓ 35 | 4–188 d (No POL) 8–181 d (POL) (Term infants) | BF phase: 3 d Weaning period: ≈ 30 d Exclusively FF period: 14 d | Cow’s milk protein-based | Cow milk based IF containing 45% POL, 20% coconut, 20% soy and 15% HO sunflower oils | Cow milk based IF containing 42% HO safflower, 30% coconut and 28% soy oils | The stools became firmer as infants moved from breastfed to weaning to exclusively formula feeding. Infants fed POL formula had significantly firmer stools in both periods (weaning and exclusively FF) |
| Lloyd et al. 1999 [31] Study 2 | Blinded RCT | Exclusively formula fed infants were randomized to either formula with or without POL. | 87 ↓ 65 | 42 ↓ 33 | 45 ↓ 32 | 12–16 d (No POL) 12–17 d (POL) (Term infants) | Standard IF feeding phase: 7 d Exclusively FF period: 14 d | Cow’s milk protein-based | Cow milk based IF containing 45% POL, 20% coconut, 20% soy and 15% HO sunflower oils | Cow milk based IF containing 42% HO safflower, 30% coconut and 28% soy oils | Infants fed POL formula had significantly higher average stool consistency. |
| First Author, Year [Ref.] | Type of Study | Design of Study | Sample Size | Population Age | Duration | Type of Formula | Intervention Formula | Comparison Formula | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | POL | No POL | |||||||||
| Specker et al. 1997 [27] | Randomized prospective Phase I | Infants were randomized (first 6 months) to a low (POL) or moderate (No POL) mineral formula. Another group was BF, supplemented with low mineral IF. | 101 ↓ 92 | 30 (Low) 31 (HM + Low) | 31 (Mod) | <14 days (Full-term infants) | 6 months | Cow’s milk protein-based | Low mineral IF containing POL and 430 mg/L of Ca | Moderate mineral IF not containing POL and containing 510 mg/L of Ca | The low mineral formula and the human milk fed groups had significantly less weight gain that moderate mineral formula fed group at 6 months of age. |
| Randomized prospective Phase II | These infants were randomized (6 to 12 months of age) again to a moderate (No POL) or high (POL) mineral IF or to whole CM. | 92 ↓ 87 | 39 (High) | 38 (Mod) 10 (CM) | <7 months (Full-term infants) | 6 months | Cow’s milk protein-based | High mineral IF containing POL and 1350 mg/L of Ca. | Moderate mineral IF not containing POL and containing 510 mg/L of Ca | No significant differences were found in weight gain between feeding groups at 12 months of age. The effect of mineral intake and fat composition on weight gain seems to be short term. | |
| Hansen et al. 1996 [34] | RCT | Infants were fed 3 formulas with different % of PA for 7 days before a 3-d balance study | 440 174 (CM) ↓ 131 266 (Soy) ↓ 165 | 40 (45%) 46 (60%) 55 (45%) 46 (60%) | 45 64 | ND (Term infants) | 120 days | ND | Cow milk or Soy based formulas containing 45% or 60% of POL | Cow milk or Soy based formula not containing POL | Differences in growth between 0% and 60% POL formulas are statistically significant but not clinically significant (< 3 g/d). Using a formula with 45% POL to achieve a FA profile similar to HM results in growth equivalent to standard formulas without POL. |
| Abbott Nutrition 1982 [20] | Clinical study | Infants were fed a PO experimental IF or one of two control formulas without PO. Mean weight gain was analyzed | 16 | 9 | 4 (Coconut/Corn) 3 (Coconut/Sunflower) | 5 days (Low-birth-weight infants) | 7 days | Cow’s milk protein-based Powder | PO formula containing 60% PO and 40% sunflower oil | No PO formula containing 60% coconut and 40% corn oils No PO formula containing 60% coconut and 40% sunflower oils | The infants that were fed the PO formula had the lowest (p < 0.05) weight gain of the 3 study groups. |
| Borschel et al. 2014 [33] | Blinded RCT multicenter | Infants were randomized to 1 of 2 PHFs (POL/No POL) during the first 4 months of life. | 209 ↓ 177 | 101 ↓ 83 | 108 ↓ 94 | 0–8 days (Term infants) | 119 days (4 months) | Partially hydrolyzed whey -based (with added prebiotic GOS) Powder | PHF containing 46% POL, 26% soy oil, 20% coconut oil and 6% HO safflower or sunflower oil | PHF containing 41% HO safflower, 29% soy and 27% coconut oils. | No significant differences in growth or weight gain were found between groups. |
| Borschel et al. 2012 [26] | DB-RCT multicenter | Infants were randomly assigned to either POL or No POL formula during 84 days of life. | 89 ↓ 64 | 44 ↓ 26 | 45 ↓ 28 | 0–8 days (Term infants) | 84 days | Partially hydrolyzed whey -based Powder | PHF containing 46% POL, 26% soy oil, 20% coconut oil and 6% HO safflower or sunflower oil | PHF containing 41% HO safflower, 29% soy and 27% coconut oils. | No significant differences in growth or weight gain were found between groups. |
| Koo et al. 2003 [25] | DB-RCT | Two groups of infants were fed either POL or No POL formula for the first 6 months | 128 ↓ 102 | 63 ↓ 52 | 65 ↓ 50 | 6 ± 1 days (Term infants) | 6 months | Cow’s milk protein-based | IF containing 45% POL, 20% soy oil, 20% coconut oil and 15% HO sunflower oil | IF containing 40% HO safflower oil, 30% soy oil and 30% coconut oil | There was no significant difference between study groups in weight, length or head circumference over the course of the study. |
| Lloyd et al. 1999 [31] Study 1 | Blinded RCT | Exclusively breastfed infants were randomized to either formula with or without POL. | 82 ↓ 70 | 39 ↓ 35 | 43 ↓ 35 | 4–188 d (No POL) 8–181 d (POL) (Term infants) | BF phase: 3 d Weaning period: ≈ 30 d Exclusively FF period: 14 d | Cow’s milk protein-based | Cow milk based IF containing 45% POL, 20% coconut, 20% soy and 15% HO sunflower oils | Cow milk based IF containing 42% HO safflower, 30% coconut and 28% soy oils | There were no significant differences in weight gain between feeding groups. |
| Lloyd et al. 1999 [31] Study 2 | Blinded RCT | Exclusively formula fed infants were randomized to either formula with or without POL. | 87 ↓ 65 | 42 ↓ 33 | 45 ↓ 32 | 12–16 d (No POL) 12–17 d (POL) (Term infants) | Standard IF feeding phase: 7 d Exclusively FF period: 14 d | Cow’s milk protein-based | Cow milk based IF containing 45% POL, 20% coconut, 20% soy and 15% HO sunflower oils | Cow milk based IF containing 42% HO safflower, 30% coconut and 28% soy oils | There were no significant differences in weight gain between feeding groups. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padial-Jaudenes, M.; Castanys-Munoz, E.; Ramirez, M.; Lasekan, J. Physiological Impact of Palm Olein or Palm Oil in Infant Formulas: A Review of Clinical Evidence. Nutrients 2020, 12, 3676. https://doi.org/10.3390/nu12123676
Padial-Jaudenes M, Castanys-Munoz E, Ramirez M, Lasekan J. Physiological Impact of Palm Olein or Palm Oil in Infant Formulas: A Review of Clinical Evidence. Nutrients. 2020; 12(12):3676. https://doi.org/10.3390/nu12123676
Chicago/Turabian StylePadial-Jaudenes, Maria, Esther Castanys-Munoz, Maria Ramirez, and John Lasekan. 2020. "Physiological Impact of Palm Olein or Palm Oil in Infant Formulas: A Review of Clinical Evidence" Nutrients 12, no. 12: 3676. https://doi.org/10.3390/nu12123676
APA StylePadial-Jaudenes, M., Castanys-Munoz, E., Ramirez, M., & Lasekan, J. (2020). Physiological Impact of Palm Olein or Palm Oil in Infant Formulas: A Review of Clinical Evidence. Nutrients, 12(12), 3676. https://doi.org/10.3390/nu12123676





