A High Prevalence of Vitamin D Deficiency Observed in an Irish South East Asian Population: A Cross-Sectional Observation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Analysis
2.3. Statistical Analysis
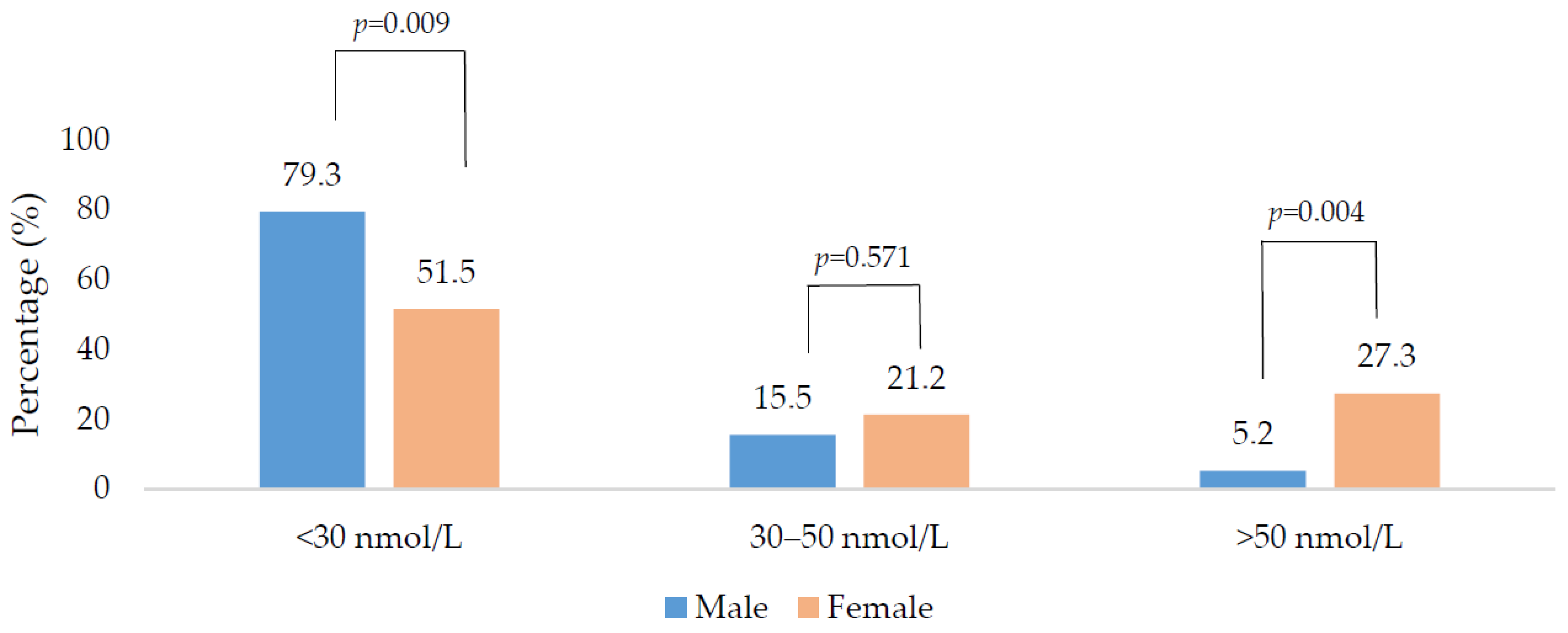
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Institute of Medicine (US). Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njølstad, I.; Løchen, M.L.; März, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 16, e0170791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health; Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scientific Advisory Committee on Nutrition. Vitamin D and Health; Scientific Advisory Committee on Nutrition: London, UK, 2016.
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, E.; O’Halloran, A.M.; Carey, D.; Healy, M.; O’Connor, D.; Moore, P.; Shannon, T.; Molloy, A.M.; Kenny, R.A. The Prevalence of Vitamin D Deficiency and the Determinants of 25(OH)D Concentration in Older Irish Adults: Data From The Irish Longitudinal Study on Ageing (TILDA). J. Gerontol. Biol. Sci. Med. Sci. 2017, 73, 519–525. [Google Scholar] [CrossRef]
- Lips, P.; de Jongh, R.T. Vitamin D deficiency in immigrants. Bone Rep. 2018, 1, 37–41. [Google Scholar] [CrossRef]
- Hakim, O.A.; Hart, K.; McCabe, P.; Berry, J.; Francesca, R.; Rhodes, L.E.; Spyrou, N.; Alfuraih, A.; Lanham-New, S. Vitamin D production in UK Caucasian and South Asian women following UVR exposure. J. Steroid Biochem. Mol. Biol. 2016, 164, 223–229. [Google Scholar] [CrossRef]
- Webb, A.R.; Aseem, S.; Kift, R.C.; Rhodes, L.E.; Farrar, M.D. Target the message: A qualitative study exploring knowledge and cultural attitudes to sunlight and vitamin D in Greater Manchester, UK. Br. J. Dermatol. 2016, 175, 1401–1413. [Google Scholar] [CrossRef]
- O’Neill, C.M.; Kazantzidis, A.; Kiely, M.; Cox, L.; Meadows, S.; Goldberg, G.; Prentice, A.; Kift, R.; Webb, A.R.; Cashman, K.D. A predictive model of serum 25-hydroxyvitamin D in UK white as well as black and Asian minority ethnic population groups for application in food fortification strategy development towards vitamin D deficiency prevention. J. Steroid Biochem. Mol. Biol. 2017, 173, 245–252. [Google Scholar] [CrossRef]
- Farrar, M.D.; Kift, R.; Felton, S.J.; Berry, J.L.; Durkin, M.T.; Allan, D.; Vail, A.; Webb, A.R.; Rhodes, L.E. Recommended summer sunlight exposure amounts fail to produce sufficient vitamin D status in UK adults of South Asian origin. Am. J. Clin. Nutr. 2011, 94, 1219–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darling, A.L.; Blackbourn, D.J.; Ahmadi, K.R.; Lanham-New, S.A. Very high prevalence of 25-hydroxyvitamin D deficiency in 6433 UK South Asian adults: Analysis of the UK Biobank Cohort. Br. J. Nutr. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Central Statistics Office (CSO) Ireland. Census 2016 Profile 8—Irish Travellers, Ethnicity and Religion; Central Statistics Office (CSO): Cork, Ireland, 2016. [Google Scholar]
- Ramachandran, A.; Snehalatha, C.; Ma, R.C. Diabetes in south-east Asia: An update. Diabetes Res. Clin. Pract. 2014, 103, 231–237. [Google Scholar] [CrossRef]
- Chen, Y.; Copeland, W.K.; Vedanthan, R.; Grant, E.; Lee, J.E.; Gu, D.; Gupta, P.C.; Ramadas, K.; Inoue, M.; Tsugane, S.; et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: Pooled analysis of prospective data from the Asia Cohort Consortium. BMJ 2013, 347, f5446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareek, M.; Bangash, M.N.; Pareek, N.; Pan, D.; Sze, S.; Minhas, J.S.; Hanif, W.; Khunti, K. Ethnicity and COVID-19: An urgent public health research priority. Lancet 2020, 395, 1421–1422. [Google Scholar] [CrossRef]
- Khunti, K.; Singh, A.K.; Pareek, M.; Hanif, W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ 2020, 369, m1548. [Google Scholar] [CrossRef] [Green Version]
- Eggemoen, Å.R.; Knutsen, K.V.; Dalen, I.; Jenum, A.K. Vitamin D status in recently arrived immigrants from Africa and Asia: A cross-sectional study from Norway of children, adolescents and adults. BMJ Open 2013, 3, e003293. [Google Scholar] [CrossRef] [Green Version]
- Lawson, M.; Thomas, M. Vitamin D concentrations in Asian children aged 2 years living in England: Population survey. BMJ 1999, 318, 28. [Google Scholar] [CrossRef] [Green Version]
- Finch, P.J.; Ang, L.; Colston, K.W.; Nisbet, J.; Maxwell, J.D. Blunted seasonal variation in serum 25-hydroxy vitamin D and increased risk of osteomalacia in vegetarian London Asians. Eur. J. Clin. Nutr. 1992, 46, 509–515. [Google Scholar]
- Van der Meer, I.M.; Karamali, N.S.; Boeke, A.J.; Lips, P.; Middelkoop, B.J.; Verhoeven, I.; Wuister, J.D. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am. J. Clin. Nutr. 2006, 84, 350–353. [Google Scholar] [CrossRef]
- Madar, A.A.; Stene, L.C.; Meyer, H.E. Vitamin D status among immigrant mothers from Pakistan, Turkey and Somalia and their infants attending child health clinics in Norway. Br. J. Nutr. 2009, 101, 1052–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck-Nielsen, S.S.; Jensen, T.K.; Gram, J.; Brixen, K.; Brock-Jacobsen, B. Nutritional rickets in Denmark: A retrospective review of children’s medical records from 1985 to 2005. Eur. J. Pediatr. 2009, 168, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982, 319, 74–76. [Google Scholar] [CrossRef]
- O’Sullivan, F.; Laird, E.; Kelly, D.; van Geffen, J.; van Weele, M.; McNulty, H.; Hoey, L.; Healy, M.; McCarroll, K.; Cunningham, C.; et al. Ambient UVB Dose and Sun Enjoyment Are Important Predictors of Vitamin D Status in an Older Population. J. Nutr. 2017, 147, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Man, R.; Li, L.J.; Cheng, C.Y.; Wong, T.; Lamoureux, E.; Sabanayagam, C. Prevalence and determinants of suboptimal vitamin D levels in a multiethnic Asian population. Nutrients 2017, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Kift, R.; Berry, J.L.; Vail, A.; Durkin, M.T.; Rhodes, L.E.; Webb, A.R. Lifestyle factors including less cutaneous sun exposure contribute to starkly lower vitamin D levels in UK South Asians compared with the white population. Br. J. Dermatol. 2013, 169, 1272–1278. [Google Scholar] [CrossRef]
- Ogunkolade, W.B.; Boucher, B.J.; Bustin, S.A.; Burrin, J.M.; Noonan, K.; Mannan, N.; Hitman, G.A. Vitamin D metabolism in peripheral blood mononuclear cells is influenced by chewing “betel nut” (Areca catechu) and vitamin D status. J. Clin. Endocrinol. Metab. 2006, 91, 2612–2617. [Google Scholar] [CrossRef] [Green Version]
- Food Safety Authority of Ireland Updated Healthy Eating Guidelines Guide to Improve Nation’s Diet. Available online: https://www.fsai.ie/news_centre/press_releases/healthy_eating_guidelines_28012019.html (accessed on 28 May 2020).
- Mygind, A.; Traulsen, J.M.; Nørgaard, L.S.; Bissell, P. The ambiguity of ethnicity as risk factor of vitamin D deficiency—A case study of Danish vitamin D policy documents. Health Policy 2011, 102, 56–63. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition (SACN) Report 2016. Vitamin D and Health. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 25 May 2020).
- National Institute for Health and Care Excellence. Vitamin D: Implementation of Existing Guidance to Prevent Deficiency; Public Health Draft Guidance, May 2014; Available online: http://guidance.nice.org.uk/PHG/71 (accessed on 28 May 2020).



| Male | Female | p-Value | |
|---|---|---|---|
| n 95 | n 91 | ||
| Age (years) | 31.0 (27.0, 36.0) | 33.0 (26.0, 38.0) | 0.178 |
| <18 n (%) | 2 (2.1) | 4 (4.4) | 0.437 |
| 18 to 50 n (%) | 90 (94.7) | 78 (85.7) | 0.047 |
| >50 n (%) | 3 (3.2) | 9 (9.9) | 0.077 |
| Season sampled n (%) | |||
| Winter | 42 (44.2) | 18 (19.8) | 0.001 |
| Spring | 17 (17.9) | 16 (17.6) | 0.956 |
| Summer | 16 (16.8) | 23 (25.3) | 0.207 |
| Autumn | 20 (21.1) | 34 (37.3) | 0.016 |
| Bone biochemistry | |||
| 25(OH)D (nmol/L) | 18.0 (27.0, 36.0) | 25.0 (17.0, 30.0) | 0.004 |
| PTH (pg/mL) | 41.6 (31.7, 53.8) | 43.8 (35.1, 71.0) | 0.044 |
| Calcium (mmol/L) | 2.35 (2.29, 2.41) | 2.26 (2.22, 2.35) | <0.001 |
| <30 nmol/L (Deficient) | 30–50 nmol/L (Insufficient) | >50 nmol/L (Sufficient) | |
|---|---|---|---|
| Total | |||
| PTH (pg/mL) | 45.5 (36.5, 65.1) a | 41.8 (28.1, 50.0) a,b | 36.1 (25.4, 43.8) b |
| Calcium (mmol/L) | 2.33 (2.25, 2.39) | 2.32 (2.25, 2.38) | 2.26 (2.24, 2.35) |
| Men | |||
| PTH (pg/mL) | 45.5 (33.1, 58.1) a | 37.1 (28.9, 46.1) a | 20.4 (16.6, 39.2) b |
| Calcium (mmol/L) | 2.35 (2.31, 2.41) | 2.35 (2.30, 2.43) | 2.26 (2.21, 2.37) |
| Women | |||
| PTH (pg/mL) | 45.8 (38.9, 89.0) | 46.3 (25.6, 55.7) | 39.3 (33.2, 48.1) |
| Calcium (mmol/L) | 2.25 (2.20, 2.35) | 2.26 (2.19, 2.37) | 2.27 (2.24, 2.35) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laird, E.; Walsh, J.B.; Lanham-New, S.; O’Sullivan, M.; Kenny, R.A.; Scully, H.; Crowley, V.; Healy, M. A High Prevalence of Vitamin D Deficiency Observed in an Irish South East Asian Population: A Cross-Sectional Observation Study. Nutrients 2020, 12, 3674. https://doi.org/10.3390/nu12123674
Laird E, Walsh JB, Lanham-New S, O’Sullivan M, Kenny RA, Scully H, Crowley V, Healy M. A High Prevalence of Vitamin D Deficiency Observed in an Irish South East Asian Population: A Cross-Sectional Observation Study. Nutrients. 2020; 12(12):3674. https://doi.org/10.3390/nu12123674
Chicago/Turabian StyleLaird, Eamon, James Bernard Walsh, Susan Lanham-New, Maria O’Sullivan, Rose Anne Kenny, Helena Scully, Vivion Crowley, and Martin Healy. 2020. "A High Prevalence of Vitamin D Deficiency Observed in an Irish South East Asian Population: A Cross-Sectional Observation Study" Nutrients 12, no. 12: 3674. https://doi.org/10.3390/nu12123674
APA StyleLaird, E., Walsh, J. B., Lanham-New, S., O’Sullivan, M., Kenny, R. A., Scully, H., Crowley, V., & Healy, M. (2020). A High Prevalence of Vitamin D Deficiency Observed in an Irish South East Asian Population: A Cross-Sectional Observation Study. Nutrients, 12(12), 3674. https://doi.org/10.3390/nu12123674






