Phenethyl Isothiocyanate Protects against High Fat/Cholesterol Diet-Induced Obesity and Atherosclerosis in C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Collection of Serum and Tissue Samples
2.3. Biochemical Analyses
2.4. Analyses of Blood Glucose and Insulin
2.5. Histopathological Examination of Aorta and Liver Tissues
2.6. Preparation of Whole Cell and Nuclear Lysates
2.7. Histone Extraction and Purification
2.8. Immunoblotting
2.9. Quantitative Polymerase Chain Reaction (qPCR)
2.10. Statistical Analysis
3. Results
3.1. PEITC Supplementation Significantly Attenuated Body Weight Gain and Food Efficiency in C57BL/6 Mice Fed HFCD
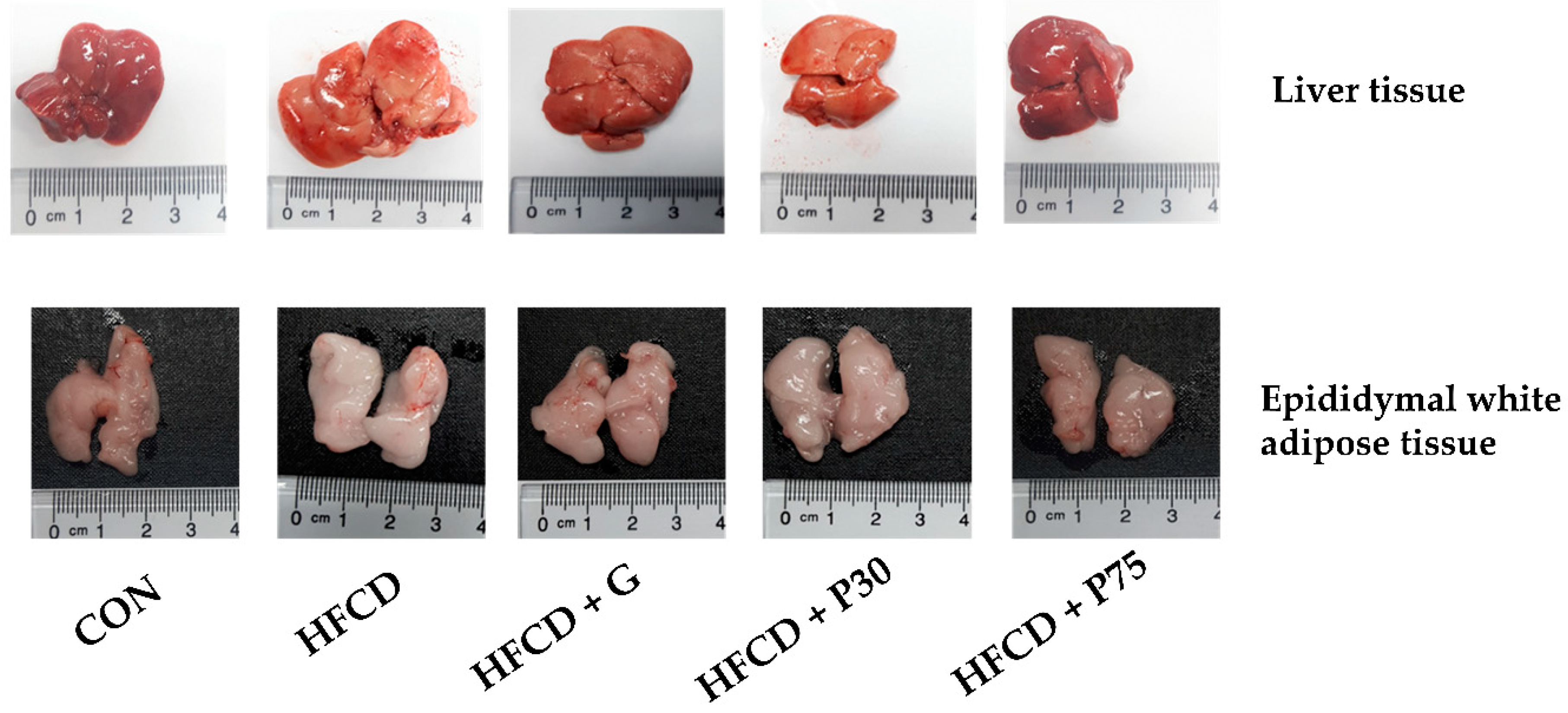
3.2. Tissue Weights in C57BL/6 Mice Fed HFCD
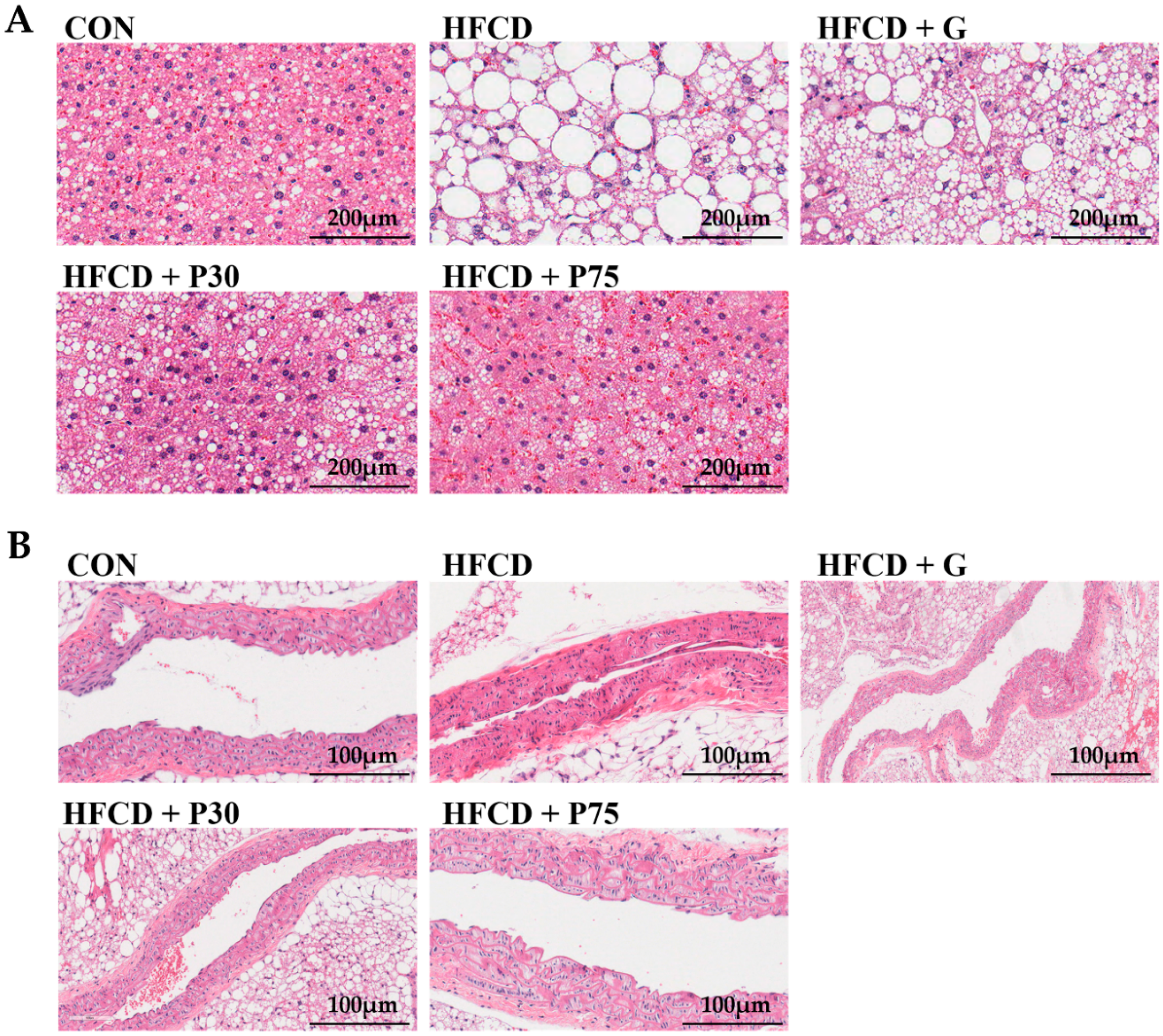
3.3. PEITC Supplementation Significantly Ameliorated HFCD-Induced Aorta and Hepatic Injury in C57BL/6 Mice
3.4. PEITC Supplementation Improved the Serum Lipid Profile in Mice
3.5. PEITC Supplementation Improved the Levels of Fasting Glucose and Insulin in Serum, and the HOMA Values
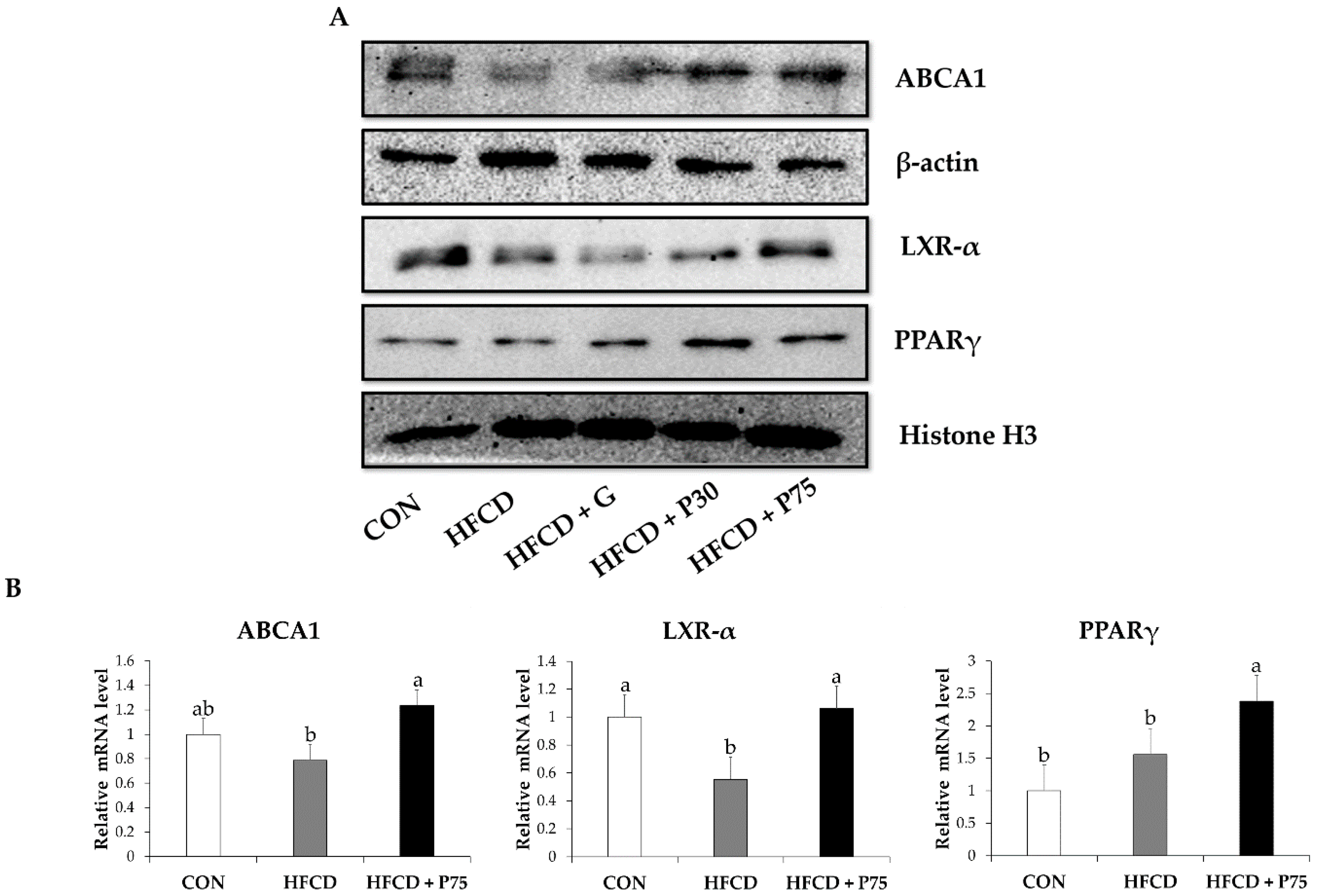
3.6. PEITC Modulated the Levels of Proteins of the Reverse Cholesterol Transport Pathway in the Liver
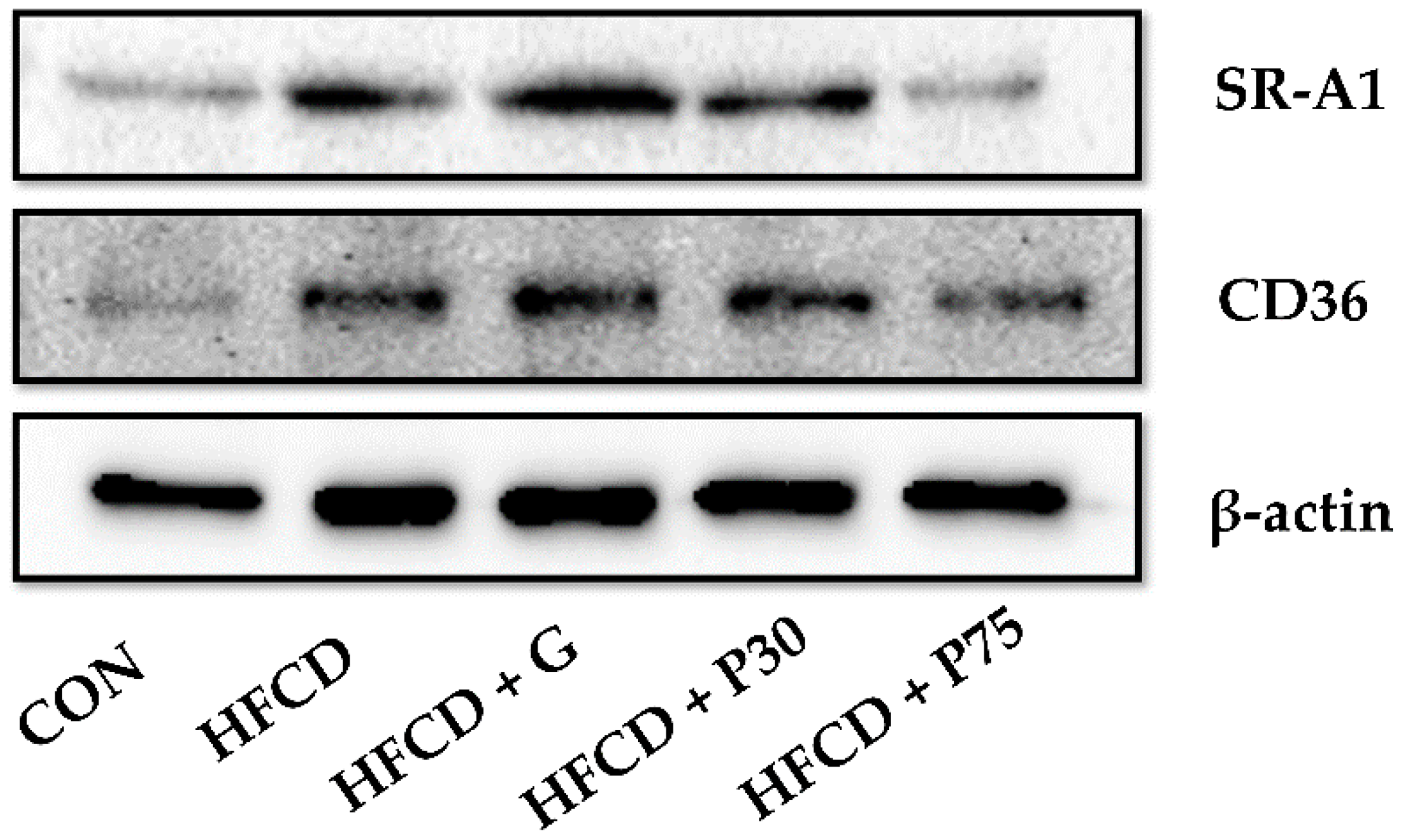
3.7. PEITC Modulated the Protein Levels of Lipid Accumulation Genes in the Liver
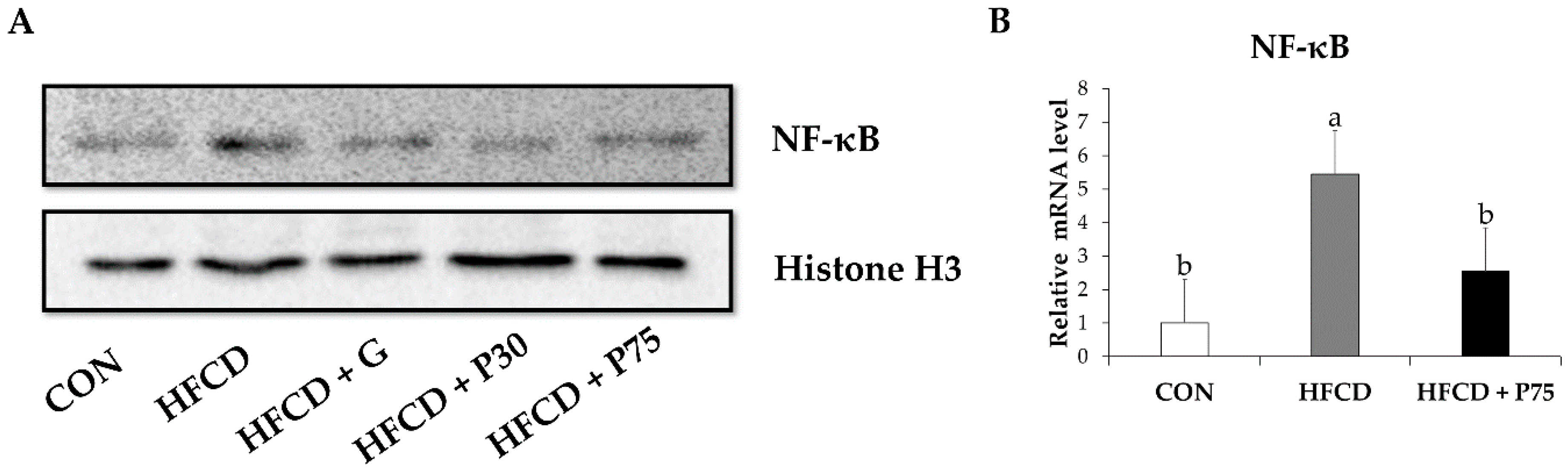
3.8. PEITC Modulated the Protein Levels of Inflammation Genes in the Liver
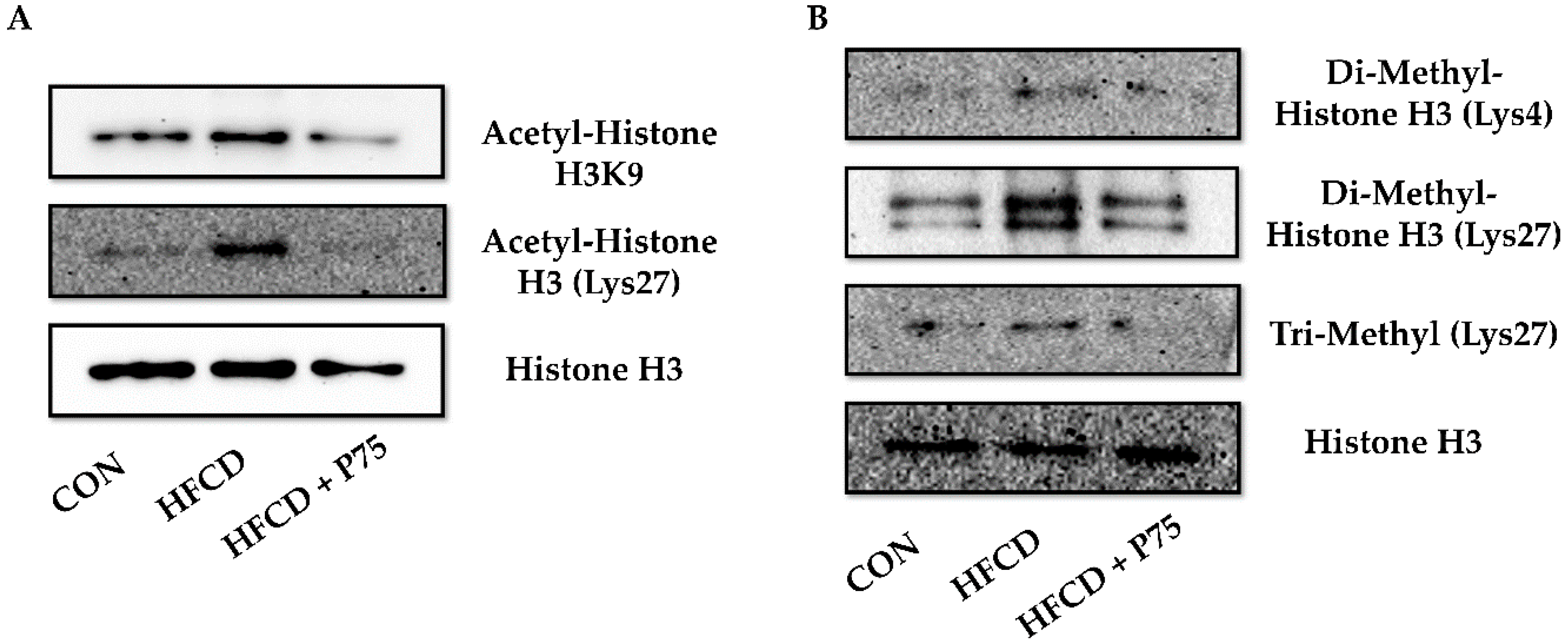
3.9. PEITC Supplementation Abrogated the HFCD-Induced Increase in Total H3K4 Dimethylation, H3K27 Di-/Trimethylation, and H3K9 and H3K27 Acetylation Expression
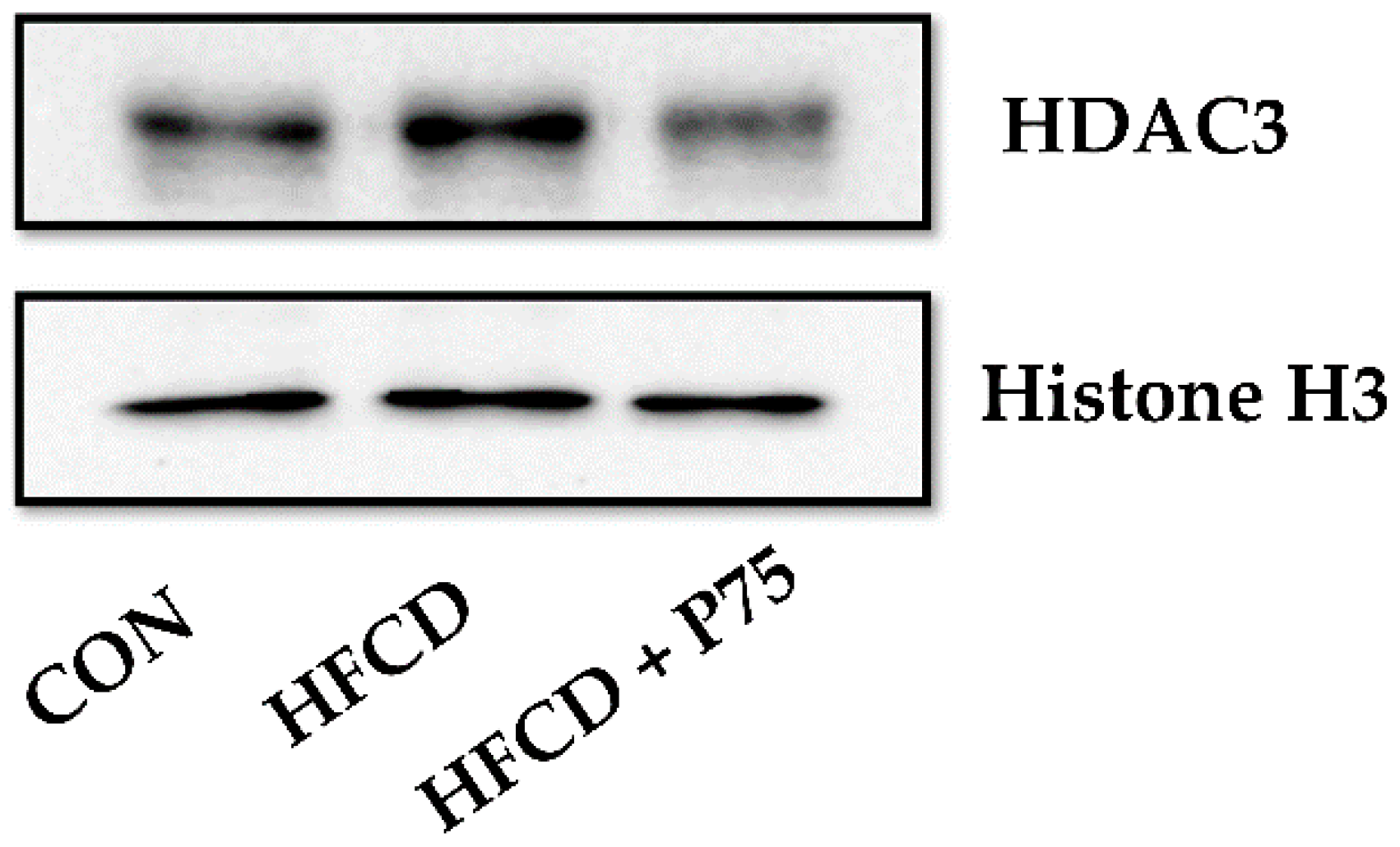
3.10. PEITC Supplementation Abrogated the HFCD-Induced Increase in HDAC3 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- King, V.L.; Hatch, N.W.; Chan, H.W.; de Beer, M.C.; de Beer, F.C.; Tannock, L.R. A murine model of obesity with accelerated atherosclerosis. Obesity 2010, 18, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, G.N. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol. Metab. 2016, 27, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Fargion, S.; Porzio, M.; Fracanzani, A.L. Nonalcoholic fatty liver disease and vascular disease: State-of-the-art. World J. Gastroenterol. 2014, 20, 13306–13324. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, G.G.; Chen, Z.; Gan, C.; McCommis, K.S.; Soufi, N.; Chrast, R.; Mitra, M.S.; Yang, K.; Gross, R.W.; Finck, B.N. Liver-specific loss of lipin-1-mediated phosphatidic acid phosphatase activity does not mitigate intrahepatic TG accumulation in mice. J. Lipid Res. 2015, 56, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Febbraio, M.; Podrez, E.A.; Smith, J.D.; Hajjar, D.P.; Hazen, S.L.; Hoff, H.F.; Sharma, K.; Silverstein, R.L. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Investig. 2000, 105, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.G.; Tran, J.L.; Erion, D.M.; Vera, N.B.; Febbraio, M.; Weiss, E.J. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed mice. Endocrinology 2016, 157, 570–585. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.K.; Lai, X.; Jia, S.J. Epigenetics in atherosclerosis: A clinical perspective. Discov. Med. 2015, 19, 73–80. [Google Scholar]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef]
- Soumian, S.; Albrecht, C.; Davies, A.H.; Gibbs, R.G. ABCA1 and atherosclerosis. Vasc. Med. 2005, 10, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Pamukcu, B.; Lip, G.Y.; Shantsila, E. The nuclear factor--kappa B pathway in atherosclerosis: A potential therapeutic target for atherothrombotic vascular disease. Thromb. Res. 2011, 128, 117–123. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero-Herrera, I.; Chen, X.; Ramos, S.; Devaraj, S. (−)-Epicatechin attenuates high-glucose-induced inflammation by epigenetic modulation in human monocytes. Eur. J. Nutr. 2017, 56, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Yun, J.M. Combination treatments with luteolin and fisetin enhance anti-inflammatory effects in high glucose-treated THP-1 cells through histone acetyltransferase/histone deacetylase regulation. J. Med. Food 2017, 20, 782–789. [Google Scholar] [CrossRef]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3′-diindolylmethane: Anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, F.U.; Rehman, M.S.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an alternative epigenetic modulator: Mechanism of action and potential effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Khyzha, N.; Alizada, A.; Wilson, M.D.; Fish, J.E. Epigenetics of atherosclerosis: Emerging mechanisms and methods. Trends Mol. Med. 2017, 23, 332–347. [Google Scholar] [CrossRef]
- Shao, D.; Lian, Z.; Di, Y.; Zhang, L.; Rajoka, M.S.R.; Zhang, Y.; Kong, J.; Jiang, C.; Shi, J. Dietary compounds have potential in controlling atherosclerosis by modulating macrophage cholesterol metabolism and inflammation via miRNA. NPJ Sci. Food 2018, 2, 13. [Google Scholar] [CrossRef]
- Nergiz-Ünal, R.; Kuijpers, M.J.; de Witt, S.M.; Heeneman, S.; Feijge, M.A.; Garcia Caraballo, S.C.; Biessen, E.A.; Haenen, G.R.; Cosemans, J.M.; Heemskerk, J.W. Atheroprotective effect of dietary walnut intake in ApoE-deficient mice: Involvement of lipids and coagulation factors. Thromb. Res. 2013, 131, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Eastham, L.L.; Howard, C.M.; Balachandran, P.; Pasco, D.S.; Claudio, P.P. Eating green: Shining light on the use of dietary phytochemicals as a modern approach in the prevention and treatment of head and neck cancers. Curr. Top Med. Chem. 2018, 18, 182–191. [Google Scholar] [CrossRef]
- Chou, Y.C.; Chang, M.Y.; Wang, M.J.; Harnod, T.; Hung, C.H.; Lee, H.T.; Shen, C.C.; Chung, J.G. PEITC induces apoptosis of human brain glioblastoma GBM8401 cells through the extrinsic- and intrinsic -signaling pathways. Neurochem. Int. 2015, 81, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Wright, S.E.; Kim, S.H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta (BBA) Rev. Cancer 2014, 1846, 405–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam-Ubol, A.; Fitzgerald, A.L.; Ritdej, A.; Phonyiam, T.; Zhang, H.; Myers, J.N.; Huang, P.; Trachootham, D. Sensory acceptable equivalent doses of β-phenylethyl isothiocyanate (PEITC) induce cell cycle arrest and retard the growth of p53 mutated oral cancer in vitro and in vivo. Food Funct. 2018, 9, 3640–3656. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br. J. Nutr. 2007, 98, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattanata, N.; Klaynongsruang, S.; Daduang, S.; Tavichakorntrakool, R.; Limpaiboon, T.; Lekphrom, R.; Boonsiri, P.; Daduang, J. Inhibitory effects of gallic acid isolated from caesalpinia mimosoides lamk on cholangiocarcinoma cell lines and foodborne pathogenic bacteria. Asian Pac. J. Cancer Prev. 2016, 17, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Daduang, J.; Palasap, A.; Daduang, S.; Boonsiri, P.; Suwannalert, P.; Limpaiboon, T. Gallic acid enhancement of gold nanoparticle anticancer activity in cervical cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 169–174. [Google Scholar] [CrossRef]
- Hsiang, C.Y.; Hseu, Y.C.; Chang, Y.C.; Kumar, K.J.; Ho, T.Y.; Yang, H.L. Toona sinensis and its major bioactive compound gallic acid inhibit LPS-induced inflammation in nuclear factor-κB transgenic mice as evaluated by in vivo bioluminescence imaging. Food Chem. 2013, 136, 426–434. [Google Scholar] [CrossRef]
- Jang, A.; Srinivasan, P.; Lee, N.Y.; Song, H.P.; Lee, J.W.; Lee, M.; Jo, C. Comparison of hypolipidemic activity of synthetic gallic acid-linoleic acid ester with mixture of gallic acid and linoleic acid, gallic acid, and linoleic acid on high-fat diet induced obesity in C57BL/6 Cr Slc mice. Chem. Biol. Interact. 2008, 174, 109–117. [Google Scholar] [CrossRef]
- Dobiásová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, X.; Chu, X.; Wang, J.; Xie, B.; Ge, J.; Guo, Y.; Li, X.; Yang, G. Allicin improves metabolism in high-fat diet-induced obese mice by modulating the gut microbiota. Nutrients 2019, 11, 2909. [Google Scholar] [CrossRef] [Green Version]
- Thon, M.P.; Hemmler, A.; Glinzer, A.; Mayr, M.; Wildgruber, M.; Zernecke-Madsen, A.; Gee, M.W. A multiphysics approach for modeling early atherosclerosis. Biomech. Model. Mechanobiol. 2018, 17, 617–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Barozzi, I.; Termanini, A.; Prosperini, E.; Recchiuti, A.; Dalli, J.; Mietton, F.; Matteoli, G.; Hiebert, S.; Natoli, G. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc. Natl. Acad. Sci. USA 2012, 109, E2865–E2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife 2015, 4, e08009. [Google Scholar] [CrossRef] [PubMed]
- Torrejón, C.; Uauy, R. Quality of fat intake, atherosclerosis and coronary disease: Effects of saturated and trans fatty acids. Rev. Med. Chil. 2011, 139, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kim, J.H.; Jang, Y.S.; Kim, C.H.; Lee, J.Y.; Lim, S.S. Anti-obesity effect of Solidago virgaurea var. gigantea extract through regulation of adipogenesis and lipogenesis pathways in high-fat diet-induced obese mice (C57BL/6N). Food Nutr. Res. 2017, 61, 1273479. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.; Wang, F.; Zeng, Y.; Chen, X.; Xu, X. Salusin-α attenuates hepatic steatosis and atherosclerosis in high fat diet-fed low density lipoprotein receptor deficient mice. Eur. J. Pharmacol. 2018, 830, 76–86. [Google Scholar] [CrossRef]
- Jiang, W.; Agrawal, D.K.; Boosani, C.S. Cell-specific histone modifications in atherosclerosis (Review). Mol. Med. Rep. 2018, 18, 1215–1224. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Srivastava, S.K. Inhibition of Integrin-HER2 signaling by Cucurbitacin B leads to in vitro and in vivo breast tumor growth suppression. Oncotarget 2014, 5, 1812–1828. [Google Scholar] [CrossRef] [Green Version]
- Chuang, W.T.; Liu, Y.T.; Huang, C.S.; Lo, C.W.; Yao, H.T.; Chen, H.W.; Lii, C.K. Benzyl isothiocyanate and phenethyl isothiocyanate inhibit adipogenesis and hepatosteatosis in mice with obesity induced by a high-fat diet. J. Agric. Food Chem. 2019, 67, 7136–7146. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Diet and murine atherosclerosis. Arter. Thromb. Vasc. Biol. 2006, 26, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, J.M.; Paraíso, A.F.; de Oliveira, M.V.; Martins, A.M.; Neto, J.F.; Guimarães, A.L.; de Paula, A.M.; Qureshi, M.; Santos, S.H. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 2014, 30, 915–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, M.M.; Murphy, R.T.; Ryan, A.W. Epigenetic modulation in the treatment of atherosclerotic disease. Front. Genet. 2014, 5, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Z.; Liu, H.; Su, X.; Fang, Z.; Dong, Z.; Yu, C.; Luo, K. Role of elevated liver transaminase levels in the diagnosis of liver injury after blunt abdominal trauma. Exp. Ther. Med. 2012, 4, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Ross, R. Atherosclerosis--An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Hopkins, P.N.; Wu, L.L.; Hunt, S.C.; Brinton, E.A. Plasma triglycerides and type III hyperlipidemia are independently associated with premature familial coronary artery disease. J. Am. Coll. Cardiol. 2005, 45, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Morishita, S.; Saito, T.; Mishima, Y.; Mizutani, A.; Hirai, Y.; Koyama, S.; Kawakami, M. Strains and species differences in experimental hyperlipidemia. Nihon Yakurigaku Zasshi 1986, 87, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Xiaoling, Y.; Li, Z.; ShuQiang, L.; Shengchao, M.; Anning, Y.; Ning, D.; Nan, L.; Yuexia, J.; Xiaoming, Y.; Guizhong, L.; et al. Hyperhomocysteinemia in ApoE-/- mice leads to overexpression of enhancer of zeste homolog 2 via miR-92a regulation. PLoS ONE 2016, 11, e0167744. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Graves, R.A.; Budavari, A.I.; Spiegelman, B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- Duval, C.; Thissen, U.; Keshtkar, S.; Accart, B.; Stienstra, R.; Boekschoten, M.V.; Roskams, T.; Kersten, S.; Müller, M. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57BL/6 mice. Diabetes 2010, 59, 3181–3191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpstra, V.; van Amersfoort, E.S.; van Velzen, A.G.; Kuiper, J.; van Berkel, T.J. Hepatic and extrahepatic scavenger receptors: Function in relation to disease. Arter. Thromb. Vasc. Biol. 2000, 20, 1860–1872. [Google Scholar] [CrossRef] [Green Version]
- Bieghs, V.; Wouters, K.; van Gorp, P.J.; Gijbels, M.J.; de Winther, M.P.; Binder, C.J.; Lütjohann, D.; Febbraio, M.; Moore, K.J.; van Bilsen, M.; et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology 2010, 138, 2477–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Luo, D.; Liu, L.; Liang, F.X.; Yu, Z.M.; Chen, R. Electroacupuncture: A feasible Sirt1 promoter which modulates metainflammation in diet-induced obesity rats. Evid. Based Complement. Altern. Med. 2018, 2018, 5302049. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Weng, Z. The correlation between histone modifications and gene expression. Epigenomics 2013, 5, 113–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeksema, M.A.; Gijbels, M.J.; Van den Bossche, J.; van der Velden, S.; Sijm, A.; Neele, A.E.; Seijkens, T.; Stöger, J.L.; Meiler, S.; Boshuizen, M.C.; et al. Targeting macrophage Histone deacetylase 3 stabilizes atherosclerotic lesions. EMBO Mol. Med. 2014, 6, 1124–1132. [Google Scholar] [CrossRef]
- Roy, N.; Elangovan, I.; Kopanja, D.; Bagchi, S.; Raychaudhuri, P. Tumor regression by phenethyl isothiocyanate involves DDB2. Cancer Biol. Ther. 2013, 14, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Khor, T.O.; Cheung, W.K.; Prawan, A.; Reddy, B.S.; Kong, A.N. Chemoprevention of familial adenomatous polyposis in Apc(Min/+) mice by phenethyl isothiocyanate (PEITC). Mol. Carcinog. 2008, 47, 321–325. [Google Scholar] [CrossRef]
- Gupta, R.; Bhatt, L.K.; Momin, M. Potent antitumor activity of laccaic acid and phenethyl isothiocyanate combination in colorectal cancer via dual inhibition of DNA methyltransferase-1 and Histone deacetylase-1. Toxicol. Appl. Pharmacol. 2019, 377, 114631. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Cao, L.; Zhang, Q.; Song, Q.; Meng, Z.; Wu, X.; Xu, K. Inhibition of autophagy potentiates the anti-metastasis effect of phenethyl isothiocyanate through JAK2/STAT3 pathway in lung cancer cells. Mol. Carcinog. 2018, 57, 522–535. [Google Scholar] [CrossRef] [PubMed]
| CON | HFCD | HFCD + G | HFCD + P30 | HFCD + P75 | |
|---|---|---|---|---|---|
| Baseline body weight (g) | 19.85 ± 0.33 a | 19.87 ± 0.31 a | 19.88 ± 0.3 a | 19.95 ± 0.33 a | 19.87 ± 0.31 a |
| Final body weight (g) | 38.03 ± 0.61 b | 47.72 ± 0.7 a | 44.25 ± 1.89 a | 44.68 ± 0.87 a | 37.07 ± 1.66 b |
| Body weight change (g) | 18.18 ± 0.47 b | 27.85 ± 0.55 a | 25.03 ± 1.78 a | 24.73 ± 0.78 b | 17.2 ± 1.55 b |
| Food efficiency ratio (%) 1) | 6.79 ± 0.17 b | 9.55 ± 0.19 a | 7.38 ± 0.53 b | 5.6 ± 0.18 c | 4.63 ± 0.42 c |
| Average daily food intake (g/day) | 2.91 ± 0 b | 3.17 ± 0.01 b | 3.35 ± 0.01 ab | 4.36 ± 0.01 a | 4.04 ± 0.01 ab |
| CON | HFCD | HFCD + G | HFCD + P30 | HFCD + P75 | |
|---|---|---|---|---|---|
| Liver (g) | 1.45 ± 0.08 b | 3.09 ± 0.21 a | 2.8 ± 0.16 a | 2.7 ± 0.15 a | 2.1 ± 0.26 b |
| Epididymal white adipose tissue (g) | 1.94 ± 0.07 b | 2.1 ± 0.11 b | 2.17 ± 0.1 b | 2.47 ± 0.09 a | 1.81 ± 0.15 b |
| Retroperitoneal adipose tissue (g) | 0.76 ± 0.08 b | 1.04 ± 0.1 a | 1.02 ± 0.08 a | 0.98 ± 0.09 a | 0.62 ± 0.07 b |
| Kidney (g) | 0.35 ± 0.03 b | 0.36 ± 0.01 a b | 0.4 ± 0.02 a | 0.33 ± 0.01 b | 0.35 ± 0.02 a b |
| CON | HFCD | HFCD + G | HFCD + P30 | HFCD + P75 | |
|---|---|---|---|---|---|
| AST | 36.48 ± 8.17 ab | 50.96 ± 8.87 a | 18.53 ± 4.81 b | 25.88 ± 6.02 b | 44.22 ± 10.32 a |
| ALT | 62.05 ± 14.13 a | 112.6 ± 19.98 a | 85.49 ± 17.57 a | 64.76 ± 14.77 a | 73.92 ± 16.97 a |
| TC (mg/dL) | 69.18 ± 1.96 bc | 113.13 ± 4.52 a | 76.33 ± 2.51 b | 71.05 ± 2.92 bc | 67.06 ± 5.75 c |
| TG (mg/dL) | 67.39 ± 6.94 b | 94.31 ± 7.05 a | 76.43 ± 6.59 a b | 68.03 ± 5.39 b | 68.5 ± 8.45 b |
| HDL-C (mg/dL) | 47.38 ± 5.17 a | 43.91 ± 4.04 a | 41.49 ± 6.18 a | 46.32 ± 5.62 a | 47.56 ± 2.64 a |
| LDL-C (mg/dL) | 23.53 ± 4.9 b | 46.97 ± 6.69 a | 34.41 ± 6.54 ab | 22.31 ± 6.37 b | 27.12 ± 6.19 ab |
| NEFA | 0.035 ± 0.00 a | 0.045 ± 0.01 a | 0.032 ± 0.00 a | 0.036 ± 0.00 a | 0.030 ± 0.00 a |
| AI 1) | 0.17 ± 0.03 b | 0.33 ± 0.03 a | 0.33 ± 0.07 a | 0.24 ± 0.07 ab | 0.14 ± 0.04 b |
| CON | HFCD | HFCD + G | HFCD + P30 | HFCD + P75 | |
|---|---|---|---|---|---|
| Serum insulin (ng/mL) | 0.47 ± 0.11 bc | 1.45 ± 0.15 a | 0.83 ± 0.13 b | 0.62 ± 0.04 bc | 0.31 ± 0.19 c |
| Fasting glucose (mg/dL) | 146.67 ± 19.22 b | 216 ± 15.04 a | 221.67 ± 3.48 a | 170 ± 13.65 a b | 147.67 ± 33.21 b |
| HOMA-IR 1) | 0.17 ± 0.06 c | 0.77 ± 0.06 a | 0.45 ± 0.08 b | 0.26 ± 0.02 bc | 0.14 ± 0.1 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, M.-H.; Im, Y.-S.; Seo, A.-R.; Kim, K.Y.; Moon, H.-R.; Yun, J.-M. Phenethyl Isothiocyanate Protects against High Fat/Cholesterol Diet-Induced Obesity and Atherosclerosis in C57BL/6 Mice. Nutrients 2020, 12, 3657. https://doi.org/10.3390/nu12123657
Gwon M-H, Im Y-S, Seo A-R, Kim KY, Moon H-R, Yun J-M. Phenethyl Isothiocyanate Protects against High Fat/Cholesterol Diet-Induced Obesity and Atherosclerosis in C57BL/6 Mice. Nutrients. 2020; 12(12):3657. https://doi.org/10.3390/nu12123657
Chicago/Turabian StyleGwon, Min-Hee, Young-Sun Im, A-Reum Seo, Kyoung Yun Kim, Ha-Rin Moon, and Jung-Mi Yun. 2020. "Phenethyl Isothiocyanate Protects against High Fat/Cholesterol Diet-Induced Obesity and Atherosclerosis in C57BL/6 Mice" Nutrients 12, no. 12: 3657. https://doi.org/10.3390/nu12123657
APA StyleGwon, M.-H., Im, Y.-S., Seo, A.-R., Kim, K. Y., Moon, H.-R., & Yun, J.-M. (2020). Phenethyl Isothiocyanate Protects against High Fat/Cholesterol Diet-Induced Obesity and Atherosclerosis in C57BL/6 Mice. Nutrients, 12(12), 3657. https://doi.org/10.3390/nu12123657





