Synergistic Effect of Omega-3 Fatty Acids and Oral-Hypoglycemic Drug on Lipid Normalization through Modulation of Hepatic Gene Expression in High Fat Diet with Low Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
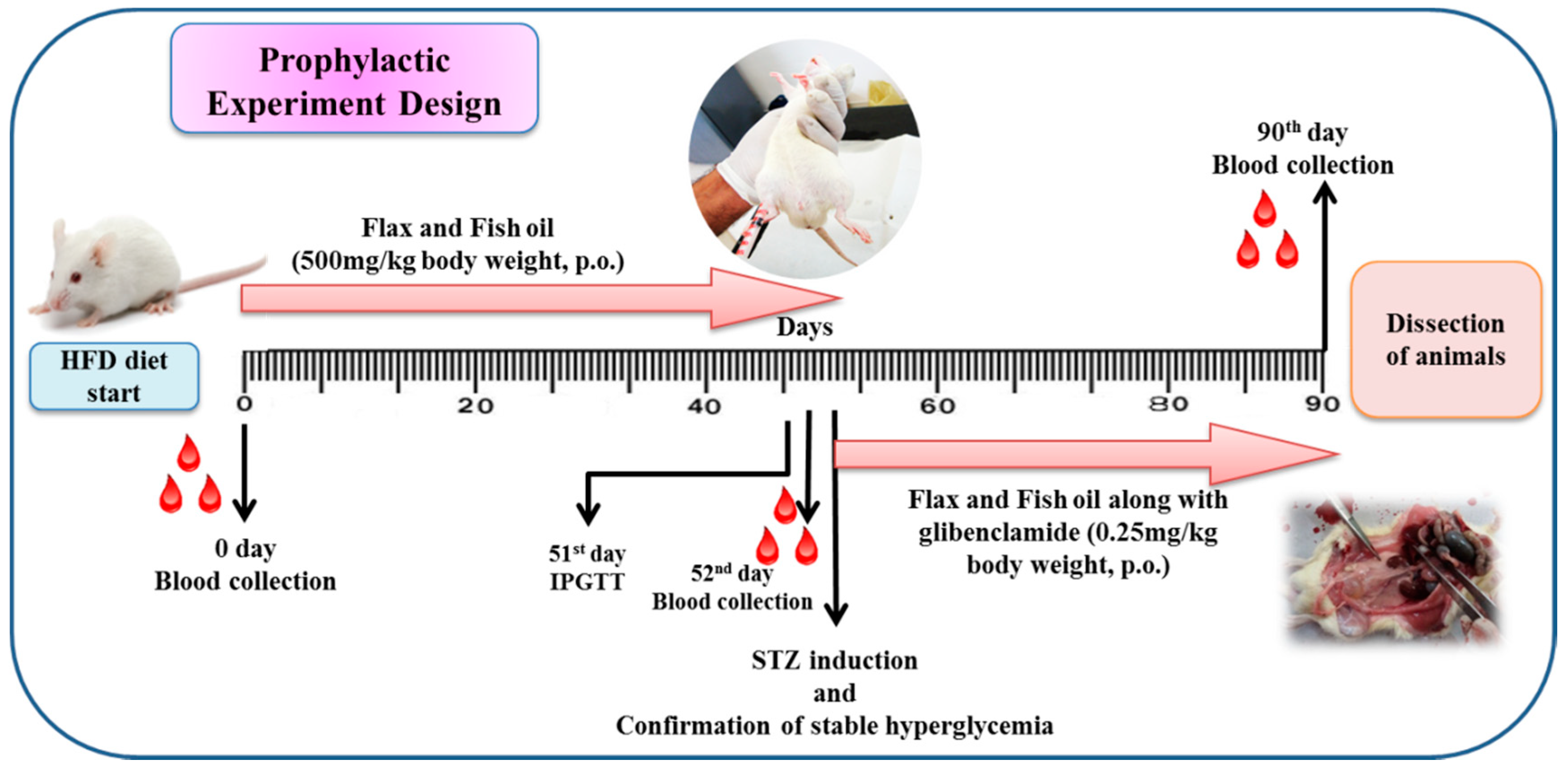
2.3. Experimental Design
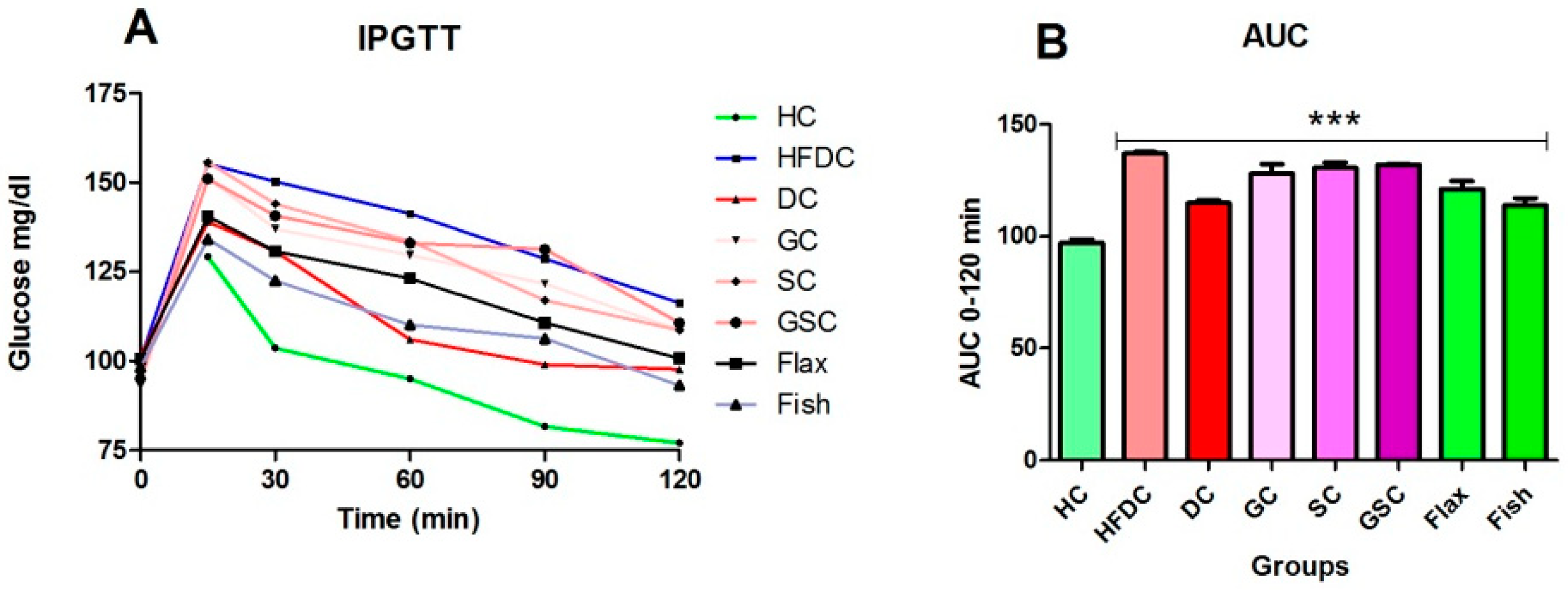
2.4. Intraperitoneal Glucose Tolerance Test (IPGTT)
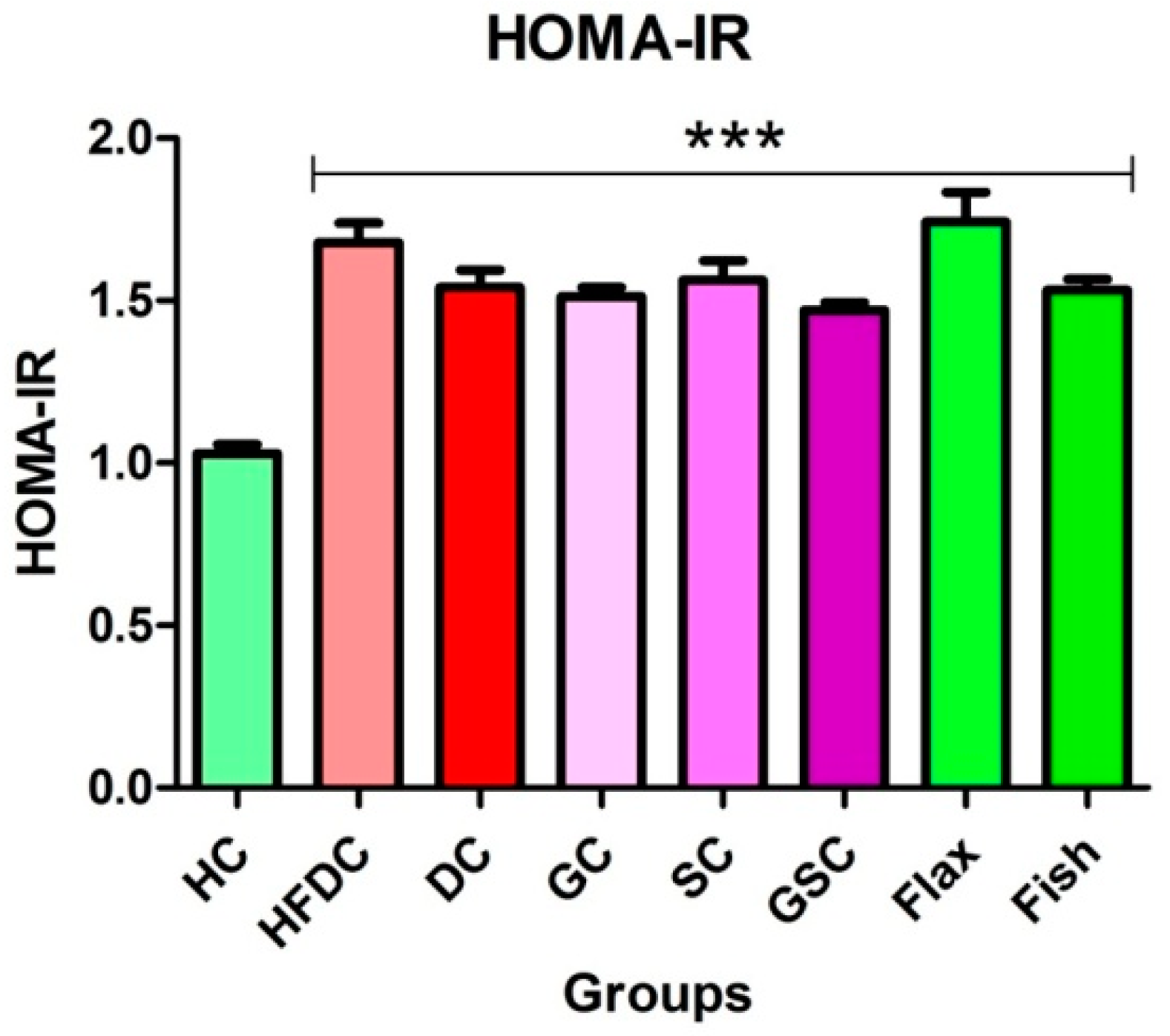
2.5. Assessment of Insulin Resistance
2.6. Biochemical Parameters
2.7. Selection of Gene for Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.8. Assessment of Hepatic Gene Expression by qRT-PCR
2.9. Histological Examination
2.10. Statistical Analysis
3. Results
3.1. Assessment of Average Body Weight, Feed and Water Consumption
3.2. Estimation of Organ Weight
3.3. Assessment of Biochemical Parameters at Zero Day
3.4. IPGTT and Area under the Curve (AUC) for the Experimental Groups
3.5. Insulin Resistance
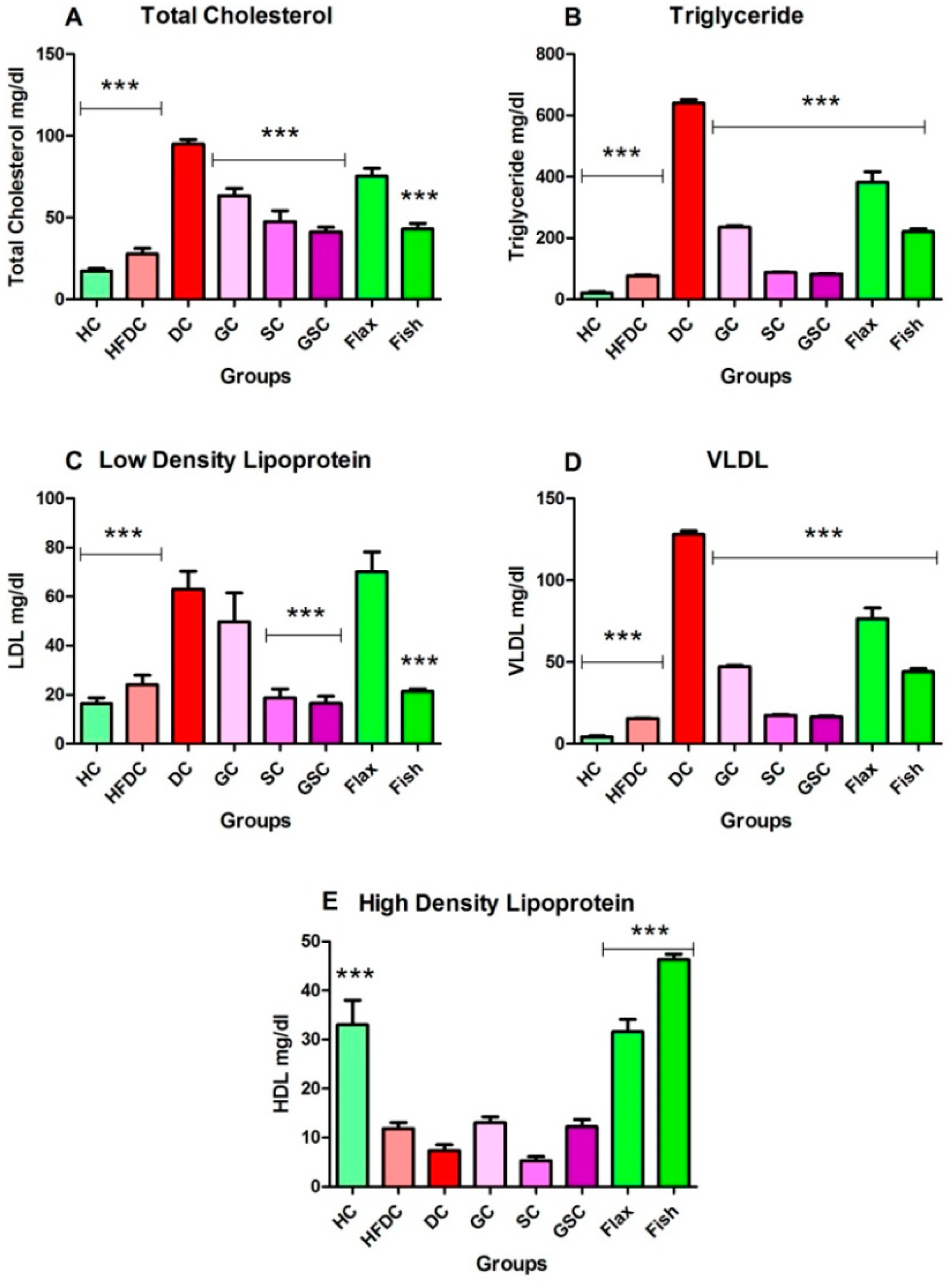
3.6. Estimation of Biochemical Parameters
3.6.1. Fish Oil Treatment Significantly Lowered Serum Glucose
3.6.2. Fish Oil Treatment Lowered Abnormal Lipid Profile
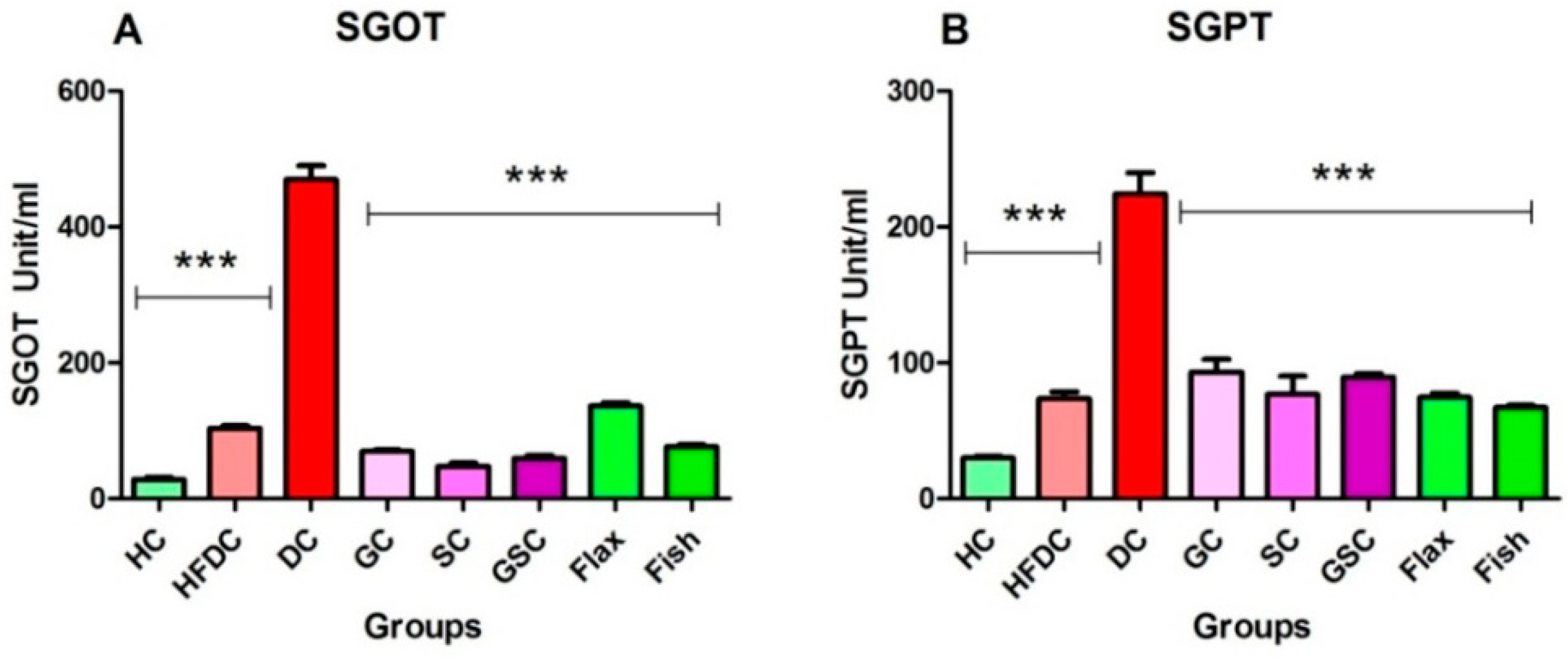
3.6.3. Flax and Fish Oil Interventions Decreases Level of Hepatic Enzymes
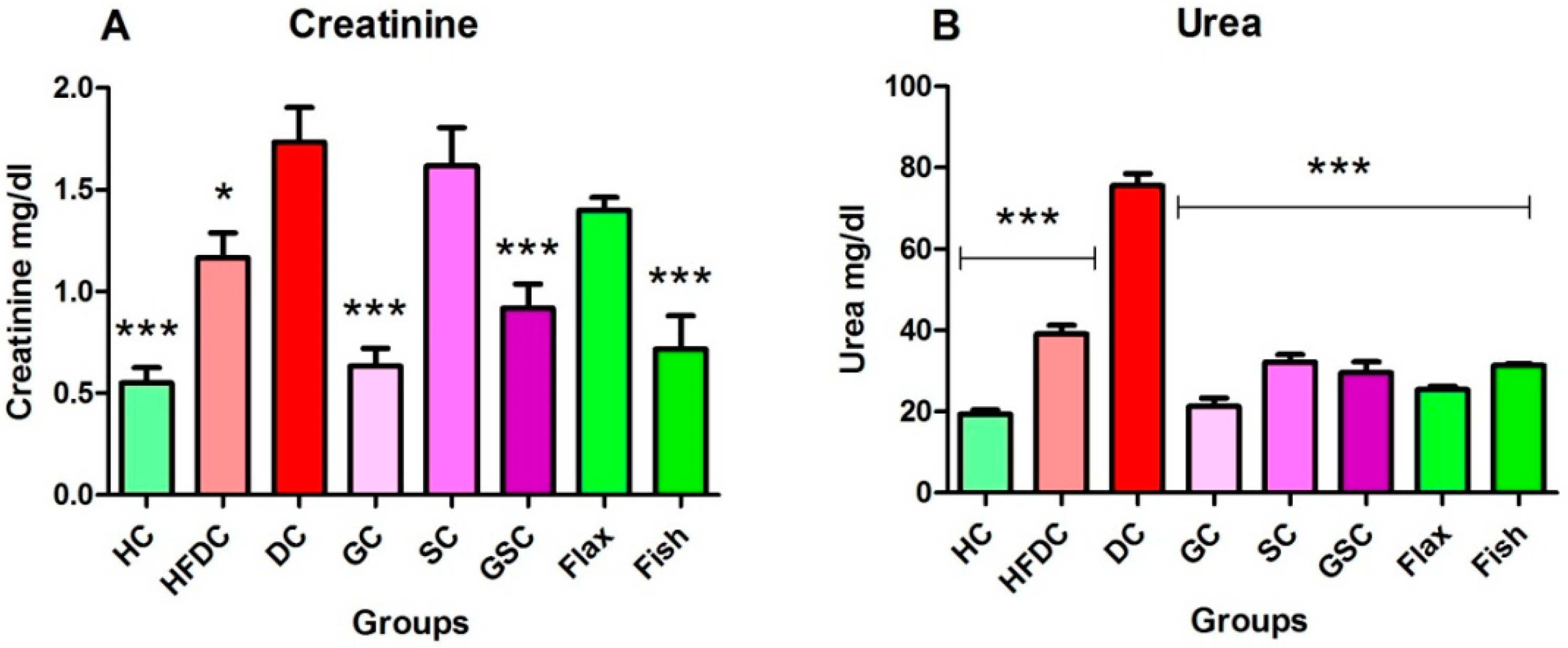
3.6.4. Flax and Fish Oil Intervention Improved Kidney Function
3.7. Expression of Transcription Factors and Their Regulatory Genes
3.7.1. Flax and Fish Oil Interventions Modulates the Expression of Transcription Factors Resulting in Lipid Normalization
3.7.2. Flax and Fish oil Intervention Modulates the Expression Fatty Acid Metabolism Genes which Results in Decreased Lipid Abnormality
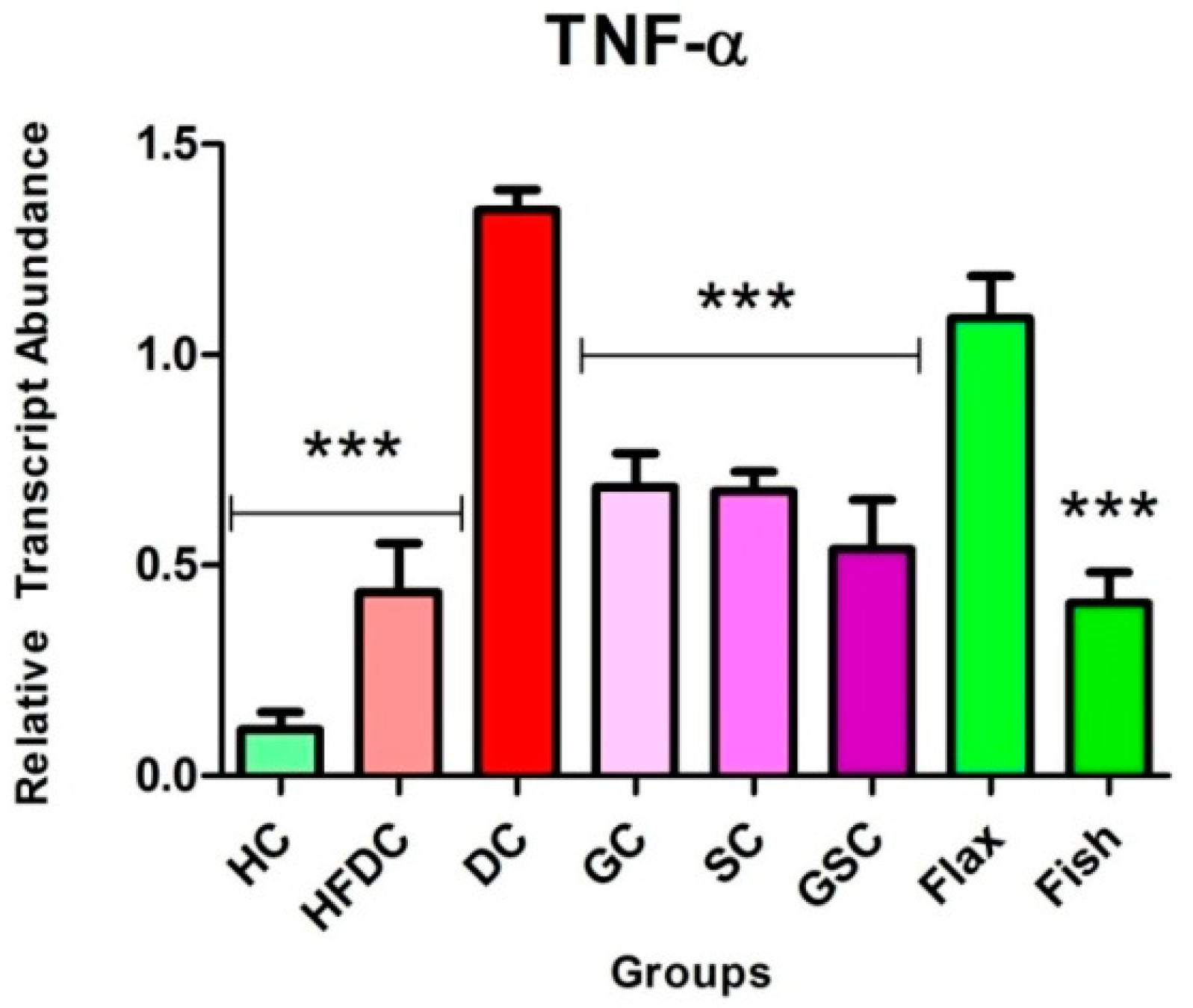
3.7.3. Fish Oil Intervention Downregulates the Expression of TNF-α
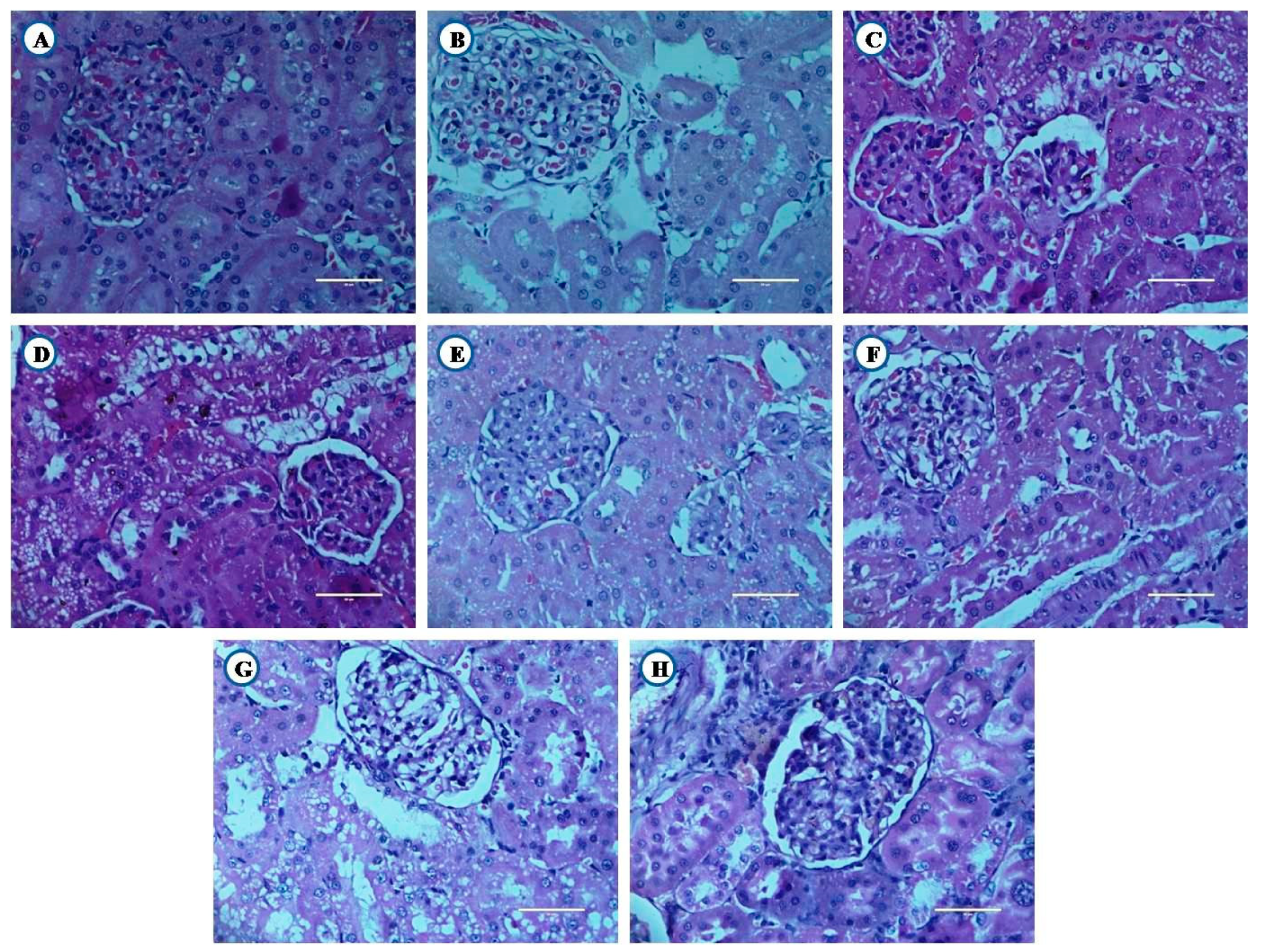
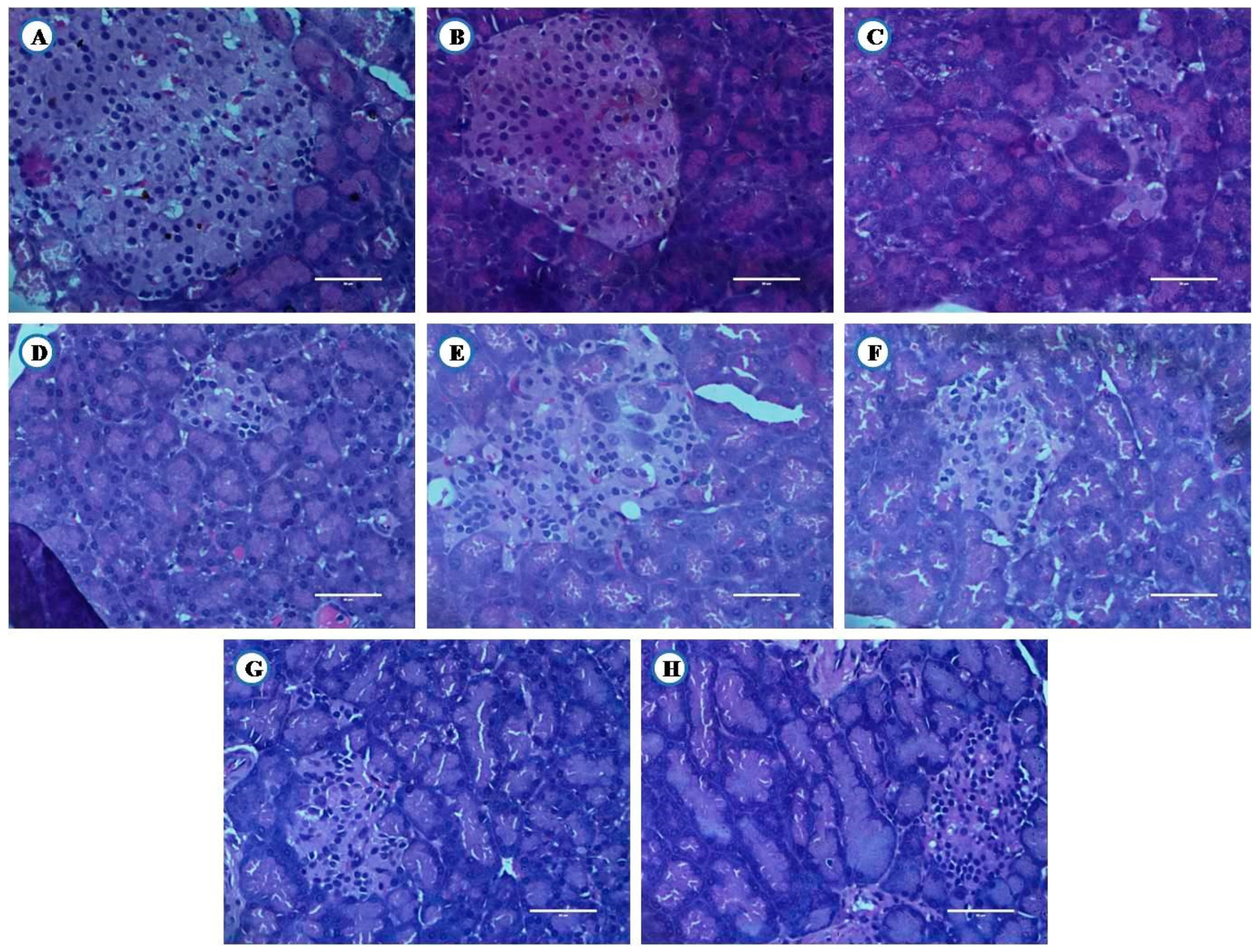
3.8. Histological Examination of Liver, Pancreas, and Kidney From Experimental Animals
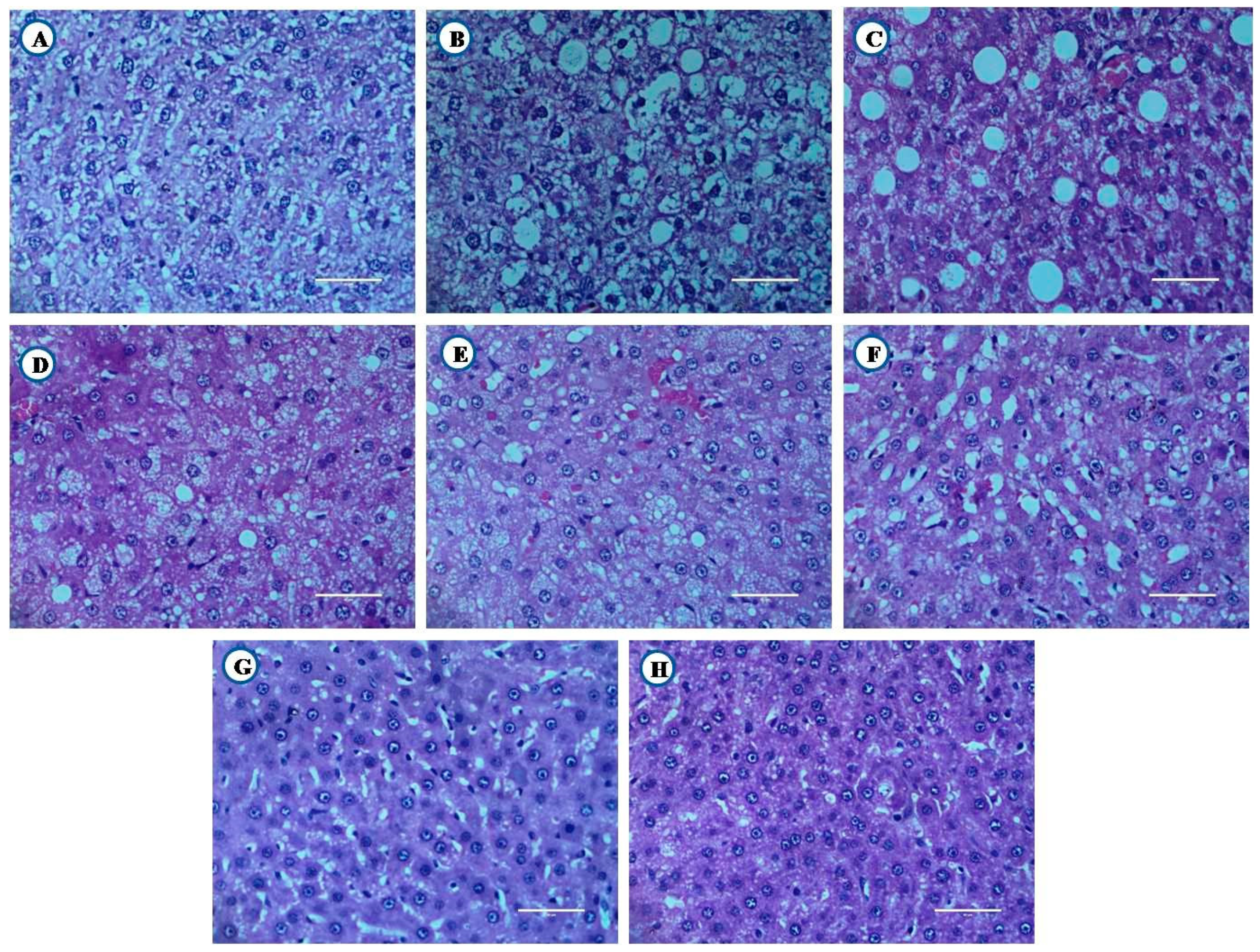
3.8.1. Histological Examination of Liver
3.8.2. Histological Examination of Kidney
3.8.3. Histological Examination of Pancreas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 366, l4697. [Google Scholar] [CrossRef] [Green Version]
- Adiels, M.; Olofsson, S.O.; Taskinen, M.R.; Boren, J. Diabetic dyslipidaemia. Curr. Opin. Lipidol. 2006, 17, 238–246. [Google Scholar] [CrossRef]
- Narindrarangkura, P.; Bosl, W.; Rangsin, R.; Hatthachote, P. Prevalence of dyslipidemia associated with complications in diabetic patients: A nationwide study in thailand. Lipids Health Dis. 2019, 18, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnaswami, V. Treatment of dyslipidemia in patients with type 2 diabetes. Lipids Health Dis. 2010, 9, 144. [Google Scholar]
- Mithal, A.; Majhi, D.; Shunmugavelu, M.; Talwarkar, P.G.; Vasnawala, H.; Raza, A.S. Prevalence of dyslipidemia in adult Indian diabetic patients: A cross sectional study (SOLID). Indian J. Endocrinol. Metab. 2014, 18, 642–647. [Google Scholar]
- Saydah, S.H.; Fradkin, J.; Cowie, C.C. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004, 291, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Parikh, R.M.; Joshi, S.R.; Menon, P.S.; Shah, N.S. Prevalence and pattern of diabetic dyslipidemia in Indian type 2 diabetic patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2010, 4, 10–12. [Google Scholar] [CrossRef]
- Goff, D.C.; Gerstein, H.C.; Ginsberg, H.N.; Cushman, W.C.; Margolis, K.L.; Byington, R.P.; Buse, J.B.; Genuth, S.; Probstfield, J.L.; Simons-Morton, D.G. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: Current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am. J. Cardiol. 2007, 99, 4i–20i. [Google Scholar] [CrossRef]
- Devarshi, P.P.; Jangale, N.M.; Ghule, A.E.; Bodhankar, S.L.; Harsulkar, A.M. Beneficial effects of flaxseed oil and fish oil diet are through modulation of different hepatic genes involved in lipid metabolism in streptozotocin-nicotinamide induced diabetic rats. Genes Nutr. 2013, 8, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ma, D.W. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients 2014, 18, 5184–5223. [Google Scholar] [CrossRef] [Green Version]
- Jangale, N.M.; Devarshi, P.P.; Dubal, A.A.; Ghule, A.E.; Koppikar, S.J.; Bodhankar, S.L.; Chougale, A.D.; Kulkarni, M.J.; Harsulkar, A.M. Dietary flaxseed oil and fish oil modulates expression of antioxidant and inflammatory genes with alleviation of protein glycation status and inflammation in liver of streptozotocin-nicotinamide induced diabetic rats. Food Chem. 2013, 141, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71 (Suppl. 1), 171S–175S. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Micha, R.; Imamura, F.; Pan, A.; Biggs, M.L.; Ajaz, O.; Djousse, L.; Hu, F.B.; Mozaffarian, D. Omega-3 fatty acids and incident type 2 diabetes: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 214–227. [Google Scholar] [CrossRef]
- Bang, H.O.; Dyerberg, J.; Nielsen, A.B. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet 1971, 1, 1143–1145. [Google Scholar] [CrossRef]
- Fialkow, J. Omega-3 fatty acid formulations in cardiovascular disease: Dietary supplements are not substitutes for prescription products. Am. J. Cardiovasc. Drugs 2016, 16, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Han, K.A.; Yu, J.; Lee, S.H.; Jeon, H.K.; Kim, S.H.; Kim, S.Y.; Han, K.H.; Won, K.; Kim, D.B.; et al. Efficacy and safety of adding omega-3 fatty acids in statin-treated patients with residual hypertriglyceridemia: ROMANTIC (Rosuvastatin-Omacor in residual hypertriglyceridemia), a randomized, double-blind, and placebo-controlled trial. Clin. Ther. 2018, 40, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar]
- Zhang, M.; Lv, X.Y.; Li, J.; Xu, Z.G.; Chen, L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 2008, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Binh, D.V.; Dung, N.T.K.; Thao, L.T.B.; Nhi, N.B.; Chi, P.V. Macro- and microvascular complications of diabetes induced by high-fat diet and low-dose streptozotocin injection in rats model. Int. J. Diabetes Res. 2013, 2, 50–55. [Google Scholar]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Khadke, S.P.; Kuvalekar, A.A.; Harsulkar, A.M.; Mantri, N. High energy intake induced overexpression of transcription factors and its regulatory genes involved in acceleration of hepatic lipogenesis: A rat model for type 2 diabetes. Biomedicines 2019, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Uma, B.; Hemantkumar, S.C.; Geetika, K.; Abul, K.N. Antidiabetic effects of Embelia ribes extract in high fat diet and low dose streptozotocin-induced type 2 diabetic rats. Front. Life Sci. 2013, 7, 186–196. [Google Scholar]
- Arshag, D.M. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 3150–3159. [Google Scholar]
- Rivellese, A.A.; Maffettone, A.; Iovine, C.; Di Marino, L.; Annuzzi, G.; Mancini, M.; Riccardi, G. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care 1996, 19, 1207–1213. [Google Scholar] [CrossRef]
- Montori, V.M.; Farmer, A.; Wollan, P.C.; Dinneen, S.F. Fish oil supplementation in type 2 diabetes: A quantitative systematic review. Diabetes Care 2000, 23, 1407–1415. [Google Scholar] [CrossRef] [Green Version]
- Ghadge, A.; Harsulkar, A.; Karandikar, M.; Pandit, V.; Kuvalekar, A. Comparative anti-inflammatory and lipid-normalizing effects of metformin and omega-3 fatty acids through modulation of transcription factors in diabetic rats. Genes Nutr. 2016, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Bassett, C.M.; Rodriguez-Leyva, D.; Pierce, G.N. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl. Physiol. Nutr. Metab. 2009, 34, 965–974. [Google Scholar] [CrossRef]
- Vijaimohan, K.; Jainu, M.; Sabitha, K.E.; Subramaniyam, S.; Anandhan, C.; Shyamala, D.C.S. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci. 2006, 79, 448–454. [Google Scholar] [CrossRef]
- Hendrich, S. (n-3) Fatty acids: Clinical trials in people with type 2 diabetes. Adv. Nutr. 2010, 1, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Kus, V.; Flachs, P.; Kuda, O.; Bardova, K.; Janovska, P.; Svobodova, M.; Jilkova, Z.M.; Rossmeisl, M.; Wang-Sattler, R.; Yu, Z.; et al. Unmasking differential effects of rosiglitazone and pioglitazone in the combination treatment with n-3 fatty acids in mice fed a high-fat diet. PLoS ONE 2011, 6, e27126. [Google Scholar] [CrossRef] [Green Version]
- Laila, A.E.; Noha, A.R.; Salma, M. Effects of omega-3 fatty acids and pioglitazone combination on insulin resistance through fibroblast growth factor 21 in type 2 diabetes mellitus. EJBAS 2015, 2, 75–86. [Google Scholar]
- Kaithwas, G.; Majumdar, D. In-vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. Eur. J. Lipid. Sci. Technol. 2012, 114, 1237–1245. [Google Scholar] [CrossRef]
- Mahmud, I.; Hossain, A.; Hossain, S.; Hannan, A.; Ali, L.; Hashimoto, M. Effects of Hilsa ilisa fish oil on the atherogenic lipid profile and glycaemic status of streptozotocin-treated type 1 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 76–81. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Shimano, H.; Horton, J.D.; Hammer, R.E.; Shimomura, I.; Brown, M.S.; Goldstein, J.L. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Investig. 1996, 98, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Shimano, H.; Horton, J.D.; Shimomura, I.; Hammer, R.E.; Brown, M.S.; Goldstein, J.L. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J. Clin. Investig. 1997, 99, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, N.; Kato, M.; Shundo, Y.; Tajiri, H.; Tanaka, M.; Yamashita, N.; Kohjima, M.; Kotoh, K.; Nakamuta, M.; Takayanagi, R.; et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 2008, 38, 1122–1129. [Google Scholar] [CrossRef]
- Moon, Y.A.; Liang, G.; Xie, X.; Frank-Kamenetsky, M.; Fitzgerald, K.; Koteliansky, V.; Brown, M.S.; Goldstein, J.L.; Horton, J.D. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012, 15, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Mandave, P.; Khadke, S.; Karandikar, M.; Pandit, V.; Ranjekar, P.; Kuvalekar, A.; Mantri, N. Antidiabetic, lipid normalizing, and nephroprotective actions of the strawberry: A potent supplementary fruit. Int. J. Mol. Sci. 2017, 18, 124. [Google Scholar] [CrossRef] [Green Version]
- Davidson, M.H. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am. J. Cardiol. 2006, 98, 27–33. [Google Scholar] [CrossRef]
- Sharma, S.; Black, S.M. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov. Today Dis. Mech. 2009, 6, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κβ. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef]
- Popa, C.; Netea, M.G.; van Riel, P.L.; van der Meer, J.W.; Stalenhoef, A.F. The role of TNF-α in A in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Jagannathan-Bogdan, M.; McDonnell, M.E.; Shin, H.; Rehman, Q.; Hasturk, H.; Apovian, C.M.; Nikolajczyk, B.S. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J. Immunol. 2011, 186, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Keller, H.; Wahli, W. Peroxisome proliferator-activated receptors—A link between endocrinology and nutrition? Trends Endocrinol. Metab. 1993, 4, 291–296. [Google Scholar] [CrossRef]
- Schoonjans, K.; Staels, B.; Auwerx, J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Biophys. Acta 1996, 1302, 93–109. [Google Scholar] [CrossRef]
- Boord, J.B.; Fazio, S.; Linton, M.F. Cytoplasmic fatty acid-binding proteins: Emerging roles in metabolism and atherosclerosis. Curr. Opin. Lipidol. 2002, 13, 141–147. [Google Scholar] [CrossRef]
- Veerkamp, J.H.; van Moerkerk, H.T. Fatty acid-binding protein and its relation to fatty acid oxidation. Mol. Cell. Biochem. 1993, 123, 101–106. [Google Scholar] [CrossRef]
- Newberry, E.P.; Xie, Y.; Kennedy, S.; Han, X.; Buhman, K.K.; Luo, J.; Gross, R.W.; Davidson, N.O. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 2003, 278, 51664–51672. [Google Scholar] [CrossRef] [Green Version]
- Wolfrum, C.; Borrmann, C.M.; Borchers, T.; Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha—and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 2323–2328. [Google Scholar] [CrossRef] [Green Version]
- Dutta-Roy, A.K. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am. J. Clin. Nutr. 2000, 71 (Suppl. 1), 315S–322S. [Google Scholar] [CrossRef] [Green Version]




| Sr. No. | Ingredients | Weight (gm/Kg) |
|---|---|---|
| 1 | Powdered normal pellet diet | 700 |
| 2 | Lard oil | 300 |
| Sr No | Target Genes | Primers | Sequences |
|---|---|---|---|
| Housekeeping Gene | |||
| 1 | Glyceraldehyde-3-phosphate dehydrogenase | Forward Reverse | AGTTCAACGGCACAGTCAAG TACTCAGCACCAGCATCACC |
| Transcription Factors | |||
| 1 | Sterol Regulatory Element-Binding Proteins-1c | Forward Reverse | AAACCTGAAGTGGTAGAAAC TTATCCTCAAAGGCTGGG |
| 2 | Peroxisome Proliferator-Activated Receptor Gamma | Forward Reverse | AAGACAACAGACAAATCACC CAGGGATATTTTTGGCATACTC |
| 3 | Nuclear Factor-κβ | Forward Reverse | AAAAACGAGCCTAGAGATTG ACATCCTCTTCCTTGTCTTC |
| Fatty Acid Metabolism Genes | |||
| 1 | Fatty Acid Synthase | Forward Reverse | AAAAGGAAAGTAGAGTGTGC GACACATTCTGTTCACTACAG |
| 2 | Acetyl-CoA Carboxylase Alpha | Forward Reverse | AGCAGTATTTGAACACATGG CAGTTCCAAGAAGTAGAAGC |
| 3 | Carnitine Palmitoyltransferase 1 | Forward Reverse | CACTGATGAAGGAAGAAGAC CCAGTCACTCACGTAATTTG |
| 4 | Fatty Acid Binding Protein | Forward Reverse | TGGAGGGTGACAATAAAATG TCATGGTATTGGTGATTGTG |
| Inflammatory Marker | |||
| 1 | Tumor Necrosis Factors-α | Forward Reverse | CTCACACTCAGATCATCTTC GAGAACCTGGGAGTAGATAAG |
| Groups | Weight (gm) | Feed Intake (gm) | Water Intake (mL) |
|---|---|---|---|
| HC | 199 ± 2.73 | 102 ± 0.09 *** | 209.1 ± 1.1 *** |
| HFDC | 279 ± 4.66 *** | 181.8 ± 0.79 *** | 136.4 ± 0.9 *** |
| DC | 199 ± 2.73 | 110.2 ± 0.36 | 347.4 ± 0.3 |
| GC | 286 ± 9.11 *** | 162.1 ± 1.53 *** | 141.6 ± 0.5 *** |
| SC | 275 ± 3.62 *** | 143.9 ± 0.59 *** | 139.8 ± 1.1 *** |
| GSC | 267 ± 3.35 *** | 169.9 ± 1.22 *** | 133 ± 0.4 *** |
| Flax | 253 ± 4.96 *** | 114.9 ± 0.28 *** | 144.5 ± 0.6 *** |
| Fish | 315 ± 12.15 *** | 110.6 ± 0.94 | 142.6 ± 0.3 *** |
| Groups | Liver | Kidney | Adipose Tissue | Muscle | Heart |
|---|---|---|---|---|---|
| HC | 8.16 ± 0.17 *** | 2.14 ± 0.05 *** | 3.99 ± 0.09 *** | 2.51 ± 0.07 *** | 1.65 ± 0.05 |
| HFDC | 12.43 ± 0.19 | 1.64 ± 0.08 | 15.17 ± 0.71 | 4.84 ± 0.33 | 1.90 ± 0.03 *** |
| DC | 11.31 ± 1.12 | 1.81 ± 0.04 | 14.43 ± 0.74 | 4.94 ± 0.72 | 1.52 ± 0.09 |
| GC | 10.85 ± 0.34 | 2.67 ± 0.05 *** | 4.39 ± 0.39 *** | 4.12 ± 0.15 | 1.75 ± 0.09 |
| SC | 9.75 ± 0.36 | 2.27 ± 0.07 ** | 3.47 ± 0.15 *** | 5.58 ± 0.25 | 1.50 ± 0.08 |
| GSC | 10.37 ± 0.28 | 2.28 ± 0.14 ** | 3.90 ± 0.46 *** | 5.34 ± 0.35 | 1.43 ± 0.03 |
| Flax | 8.55 ± 0.19 *** | 2.43 ± 0.03 *** | 1.75 ± 0.04 *** | 4.29 ± 0.08 | 1.39 ± 0.01 |
| Fish | 8.59 ± 0.05 ** | 2.44 ± 0.13 *** | 2.88 ± 0.18 *** | 2.85 ± 0.08 *** | 1.90 ± 0.01 *** |
| Parameters | HC | HFDC | DC | GC | SC | GSC | Flax | Fish |
|---|---|---|---|---|---|---|---|---|
| Glu (mg/dl) | 67.95 ± 6.05 | 68.33 ± 4.38 | 66.20 ± 9.09 | 61.18 ± 8.61 | 73.10 ± 4.34 | 53.97 ± 3.18 | 60.85 ± 2.03 | 56.08 ± 4.17 |
| Lipid profile (mg/dl) | ||||||||
| TC | 43.58 ± 4.37 | 57.63 ± 5.97 | 51.07 ± 3.15 | 49.70 ± 6.35 | 42.30 ± 2.63 | 44.48 ± 4.52 | 41.13 ± 4.76 | 47.97 ± 3.35 |
| TGs | 30.58 ± 5.71 | 47.40 ± 2.20 | 46.72 ± 2.63 | 36.62 ± 6.48 | 35.8 ± 6.63 | 26.47 ± 3.67 | 41.77 ± 6.32 | 33.90 ± 4.66 |
| HDL | 17.95 ± 0.20 | 22.19 ± 2.35 | 23.70 ± 1.82 | 23.95 ± 0.95 | 23.20 ± 2.50 | 26.83 ± 0.00 | 22.49 ± 0.76 | 21.78 ± 3.30 |
| LDL | 17.56 ± 0.10 | 13.37 ± 1.47 | 12.27 ± 2.17 | 16.50 ± 0.25 | 14.50 ± 0.66 | 13.80 ± 2.21 | 12.43 ± 0.89 | 12.43 ± 0.53 |
| VLDL | 6.12 ± 1.13 | 9.50 ± 0.44 | 9.31 ± 0.52 | 7.33 ± 1.28 | 7.17 ± 1.32 | 5.30 ± 0.74 | 8.36 ± 1.26 | 6.78 ± 0.92 |
| Liver Function Test (Unit/mL) | ||||||||
| SGOT | 41.60 ± 2.57 | 39.30 ± 5.09 | 55.87 ± 1.50 | 57.63 ± 4.87 | 45.52 ± 6.17 | 44.03 ± 2.05 | 44.68 ± 2.63 | 46.40 ± 5.20 |
| SGPT | 31.35 ± 3.90 | 34.45 ± 2.12 | 25.51 ± 2.53 | 26.60 ± 3.10 | 38.82 ± 2.52 | 34.03 ± 4.02 | 34.66 ± 2.89 | 33.75 ± 1.13 |
| Kidney Function Test (mg/dl) | ||||||||
| Creatinine | 0.60 ± 0.08 | 0.70 ± 0.07 | 0.57 ± 0.07 | 0.54 ± 0.07 | 0.55 ± 0.09 | 0.46 ± 0.12 | 0.55 ± 0.08 | 0.57 ± 0.06 |
| Urea | 26.49 ± 3.05 | 26.24 ± 0.59 | 25.05 ± 3.82 | 29.22 ± 1.04 | 27.55 ± 3.25 | 30.48 ± 0.45 | 28.19 ± 1.07 | 22.53 ± 1.53 |
| Experimental Groups | Glucose (mM) | Insulin (μU/mL) |
|---|---|---|
| HC | 3.682 ± 0.10 | 6.293 ± 0.04 |
| HFDC | 5.208 ± 0.14 *** | 7.248 ± 0.11 *** |
| DC | 4.81 ± 0.15 *** | 7.21 ± 0.10 *** |
| GC | 4.995 ± 0.08 *** | 6.822 ± 0.17 * |
| SC | 5.134 ± 0.15 *** | 6.852 ± 0.17 * |
| GSC | 4.875 ± 0.02 *** | 6.788 ± 0.14 * |
| Flax | 6.105 ± 0.30 *** | 6.418 ± 0.12 |
| Fish | 5.458 ± 0.12 *** | 6.315 ± 0.03 |
| No. | Target Genes | Symbol | Efficiency |
|---|---|---|---|
| Housekeeping Gene | |||
| 1 | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 97.27 |
| Transcription Factors | |||
| 1 | Sterol Regulatory Element-Binding Proteins-1c | SREBP-1c | 91.14 |
| 2 | Peroxisome Proliferator-Activated Receptor Gamma | PPAR-γ | 91.81 |
| 3 | Nuclear Factor-κβ | NF-κβ | 101.90 |
| Fatty Acid Metabolism Genes | |||
| 1 | Fatty Acid Synthase | FASN | 91.67 |
| 2 | Acetyl-CoA Carboxylase Alpha | ACACA | 84.87 |
| 3 | Carnitine Palmitoyltransferase 1 | CPT 1 | 101.65 |
| 4 | Fatty Acid Binding Protein | FABP | 92.96 |
| Inflammatory Marker | |||
| 1 | Tumor Necrosis Factors-α | TNF-α | 107.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadke, S.; Mandave, P.; Kuvalekar, A.; Pandit, V.; Karandikar, M.; Mantri, N. Synergistic Effect of Omega-3 Fatty Acids and Oral-Hypoglycemic Drug on Lipid Normalization through Modulation of Hepatic Gene Expression in High Fat Diet with Low Streptozotocin-Induced Diabetic Rats. Nutrients 2020, 12, 3652. https://doi.org/10.3390/nu12123652
Khadke S, Mandave P, Kuvalekar A, Pandit V, Karandikar M, Mantri N. Synergistic Effect of Omega-3 Fatty Acids and Oral-Hypoglycemic Drug on Lipid Normalization through Modulation of Hepatic Gene Expression in High Fat Diet with Low Streptozotocin-Induced Diabetic Rats. Nutrients. 2020; 12(12):3652. https://doi.org/10.3390/nu12123652
Chicago/Turabian StyleKhadke, Suresh, Pallavi Mandave, Aniket Kuvalekar, Vijaya Pandit, Manjiri Karandikar, and Nitin Mantri. 2020. "Synergistic Effect of Omega-3 Fatty Acids and Oral-Hypoglycemic Drug on Lipid Normalization through Modulation of Hepatic Gene Expression in High Fat Diet with Low Streptozotocin-Induced Diabetic Rats" Nutrients 12, no. 12: 3652. https://doi.org/10.3390/nu12123652
APA StyleKhadke, S., Mandave, P., Kuvalekar, A., Pandit, V., Karandikar, M., & Mantri, N. (2020). Synergistic Effect of Omega-3 Fatty Acids and Oral-Hypoglycemic Drug on Lipid Normalization through Modulation of Hepatic Gene Expression in High Fat Diet with Low Streptozotocin-Induced Diabetic Rats. Nutrients, 12(12), 3652. https://doi.org/10.3390/nu12123652





