Abstract
Vitamin K acts as a coenzyme of carboxylase, catalyzing the carboxylation of several vitamin K dependent proteins. Beyond its well-known effects on blood coagulation, it also exerts relevant effects on bone and the vascular system. In this review, we point out the relevance of an adequate vitamin K intake to obtain sufficient levels of carboxylated (active form) vitamin K dependent proteins (such as Osteocalcin and matrix Gla protein) to prevent bone health. Another bone-related action of Vitamin K is being a ligand of the nuclear steroid and xenobiotic receptor (SXR). We also discuss the recommended intake, deficiency, and assessment of vitamin K. Furthermore, we review the few available studies that have as pre-specified outcome bone fractures, indicating that we need more clinical studies to confirm that vitamin K is a potential therapeutic agent for bone fractures.
1. Introduction
Nutrition represents one of the most relevant modifiable for osteoporosis prevention and bone health. [1]. Although impairment of bone remodeling is the cornerstone of the pathogenesis of primary osteoporosis, other related mechanisms have to be investigated. Intrinsic changes within the osteoblast and osteoclast lineage that may occur with ageing leading to an increased cellular senescence that is considered a hallmark of ageing may play a role [2,3].
Several studies have shown that nutrients and vitamins, including vitamin D, vitamin C, and recently vitamin K play an important role in the maintenance of optimal bone health, especially among older adults [4]. Recently, a growing interest has been directed to vitamin K, mainly involved in the blood coagulation pathway, since it maintains the activity of coagulation factors in the liver [5]. Epidemiological studies suggested that a vitamin K deficiency is associated with several diseases, including osteoporosis and vascular calcification [6].
2. Vitamin K Structure, Source, Cycle, Metabolism
Vitamin K is the term used to name a family of fat-soluble compounds, which although differ in origin and/or function, share a common 2-methyl-1,4-naphthoquinone ring, but differ in the (lipophilic) side chains linked at the 3-position. The three main forms are vitamin PK or phylloquinone (PK), vitamin K2 or menaquinones (MKn) and vitamin K3 or menadione. These three forms can be differentiated by the 3-position: PK has an isoprenoid side chain, whereas the MK form possesses a phytyl side chain and is characterized by a variable number of connected isoprenoid units (MKn), and menadione has no side chain and is a synthetic analogue [4].
The sources of vitamin K are different depending on the vitamers. PK can be found mainly in green leafy vegetables (e.g., kale), vegetables in the Brassica genus (e.g., Brussels sprouts, broccoli), fruits (e.g., avocado, kiwi, and green grapes), herbs (e.g., cilantro, parsley), and green and herbal teas. Other dietary sources are plant oils such as soybean, canola, and olive oils [7].
Fermented foods such as fermented butter or cheese, curdled cheese, egg yolk, and beef liver are sources of MKn. Natto, a traditional Japanese soybean-based food, produced by fermentation using Bacillus subtilis, is a source of Menaquinone-7 (MK-7) [7]. Regarding MKn produced by intestinal bacterial flora, MK-6, MK-7, and MK-8 are sinthetyzed respectively by Eubacterium lentum, Veillonella and Enterobacteria while MK-10 and MK-11 by Bacteroides. Differently MK-4, is converted from PK through a side chain removal/addition mechanism in specific tissues (pancreas, testes, and vessel wall) having menadione as intermediate molecule, but its synthetic form is added in animal food and is then metabolized by the liver into MK-4 [4,8].
Vitamin PK, MK-4, and MK-7 are the currently commercially available formulations. The synthetic form of vitamin PK, also known as phytonadione, is used to treat and/or prevent vitamin K deficiency, bleeding and reverse the consequences of an overdose of anticoagulant drugs [9]. Dietary vitamin K is absorbed in the small intestine, being fat-soluble, both forms of vitamin K need a normal pancreatic function and the presence of bile salts for their absorption and are transported in plasma by lipoproteins [10]. Plasma concentrations of PK in healthy fasting people are around of 0.5 nM, an order of magnitude remarkably lower than blood concentrations of other fat soluble vitamins (A, D, and E). Concentrations of MKs in plasma, such as MK-4, are very low or undetectable [11]. The low plasma concentrations of vitamin K are physiologically associated with tissue reserves. As vitamin K is stored in the body in a limited amount without a regular dietary intake, it would run out quickly. For this reason, the human body recycles vitamin K, compensating for its limited storage capacity [4].
Vitamin K acts as a cofactor for a single microsomal enzyme, namely carboxyglutamyl carboxylase (GGCX) which catalyzes the carboxylation (and thereby activation) of vitamin-K-dependent proteins (VKDPs) [12]. Vitamin-K-dependent carboxylase catalyzes the posttranslational γ-carboxylation of glutamic acid (Glu) residues, placed in vitamin K-dependent proteins (also known as Gla proteins), to γ-carboxylated glutamic acid (Gla) residues. Among the Gla protein family, 17 different members have been recongnized: S prothrombin, factor VII, factor IX, factor X, protein C, protein S and protein Z belonging to the coagulative cascade; (matrix Gla protein,(MGP), Osteocalcin (OC), growth arrest-specific protein 6 (Gas6), Gla-rich protein (GRP) playing a role in modulating bone and vascular mineralization; periostin and periostin-like factor, two proline-rich Gla proteins, and two transmembrane Gla proteins. Vitamin K was previously considered only an essential factor for blood coagulation [5].
Considering that some Gla-proteins are involved in bone metabolism and vascular health, it is possible that their reduced carboxylation may lead to bone metabolism impairment and/or an increase in vascular calcification [13]. Recently, it was found that vitamin k may also have regulatory functions in energy metabolism and a protective role against vascular calcification and age-related bone loss. The coenzymatic (active) form of vitamin K is hydroquinone (KH2), which is produced by a quinone reductase at the expense of NADPH. This reaction leads to the oxidation of KH2, to epoxide (KO). KH2 is restituted by KO reduction through two reductase activities: vitamin K epoxide reductase (VKOR) which first transforms KO into quinine and then vitamin K reductase reduces K quinone to the K hydroquinone (KH2). The activity of vitamin K reductase is needed to synthesize KH2, the active cofactor for the GGCX in the endoplasmic reticulum, as well as to reduce ‘new’ molecules of vitamin K that are introduced into the cycle. The vitamin K recycling reduces its dietary requirements ensuring its availability for the important coenzymatic function of carboxylation [14].
The involvement of vitamin K in bone metabolism does not occur exclusively by the γ-carboxylation reaction. MK4, binds to the nuclear receptor, steroid, and xenobiotic receptor (SXR) and its murine ortholog, pregnane X receptor (PXR), a nuclear receptor whose primary function is to regulate the expression of genes encoding enzymes involved in steroid metabolism and detoxification of xenobiotics and various drugs [15,16,17]. Vitamin PK is not able to activate SXR directly, suggesting vitamin PK potentially contribute to this mechanism of action after being converted into MK-4 [18] (Figure 1).
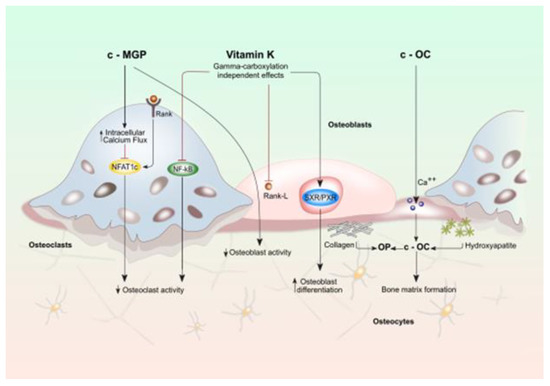
Figure 1.
Effects of vitamin K on activity of osteoblasts and osteoclasts. Bone cell function is modulated by both vitamin k dependent carboxylated proteins (c-MGP and c-OC) and vitamin K directly (gamma-carboxylation independent effects). Vitamin K (VK) plays a considerable role in maintaining bone strength since it regulates bone remodeling by promoting the osteoblast-to-osteocyte transition and by limiting osteoclastogenesis. This effect would take place mainly through the VK-dependent activation of bone Gla-proteins: Osteocalcin (OC) and matrix Gla protein (MGP protein). OC secreted by osteoblasts, plays an essential role in the synthesis and regulation of bone matrix. The undercarboxylated OC, shows a reduced calcium and hydroxyapatite binding activity, while, the active carboxylated (cOC) form is mainly involved in bone mineralization as it allows the interaction between its calcium-binding Gla residues with the calcium ions of hydroxyapatite: the main mechanism that enables OC to contribute to the formation of hydroxyapatite crystals. Oc acts also as an inhibitor of bone mineralization thus regulating the rate of mineral maturation (see also the text for the details).
The presence of SXR has been demonstrated in the liver, intestine, and human osteoblastic cells. After binding with a ligand, SXR forms a complex with retinoid X receptor which in turn binds to an SXR responsive element on the target gene promoter that rules the transcription. MK-4, in an SXR-dependent way, plays a pivotal role in bone health by inducing expression of genes coding proteins such as matrilin-2 (Matn2), tsukushi (Tsk), and CD14 which are involved in bone remodeling [4,6,18] (Figure 1). Specifically, Tsk encodes a protein that has a collagen-accumulating effect, while Matn2 is a widely distributed extracellular matrix protein like collagen and CD14 regulates osteoblastogenesis and osteoclastogenesis by inducing differentiation of B cells [4,14,19]. The effect of vitamin K through SXR on bone collagen content may be important for bone quality. The material properties of bone, degree of mineralization, and microdamage accumulation are all influenced by collagen cross-link formation. In particular the type-I collagen fibers wired with crosslinks fibers form the framework that binds matrix proteins and mineral crystals. If the arrangement of collagen fibers is altered and the mineral crystal remains immature, these changes in material properties can cause an impairment of bone elasticity [20,21]. Moreover, SXR/PXR promotes bone formation and blunts bone resorption, suggesting that SXR/PXR may play a pivotal role in maintaining bone homeostasis [19].
The effect of VK on bone health and remodeling also involves MGP that promotes bone formation by upregulating Wnt/β-catenin signaling but also exerts an inhibitory effect on bone mineralization. In the late stage of osteoclast differentiation, MGP is highly expressed thus outlining a negative-feedback loop to make osteoclast formation under tight control (see also the text for the details).
Recent evidence suggests that VK regulates osteoblastogenesis and osteoclastogenesis through the nuclear factor κB (NF-κB) signal transduction pathway. NF-κB signaling exerts two functions: one the one hand it stimulates osteoclasts development and resorption while on the other it inhibits osteoblasts differentiation and activity. VK2 prevents NF-κB activation, in a γ-carboxylation-independent manner, leading to bone formation and reducing bone resorption. [22,23] (Figure 1).
3. Vitamin K and VKDPs
VK plays a considerable role in maintaining bone strength since it regulates bone remodeling by promoting the osteoblast-to-osteocyte transition and by limiting osteoclastogenesis. This effect would take place through the VK-dependent activation of the following bone proteins: Osteocalcin (OC), matrix Gla protein (MGP protein), Gla-rich protein, protein S, and growth arrest specific 6 protein (Gas6). While the role of OC and MGP in bone health and in cardiovascular health seems sufficiently detailed, the role of the other bone VKDPs appears less well-defined [9,24].
OC is the most plentiful non-collagenous protein in bone, mainly secreted by osteoblasts, with a smaller amount produced by chondrocytes. It plays an essential role in the synthesis and regulation of bone matrix. The carboxylation pathway, requiring vitamin K as a cofactor, is crucial for the transformation of OC from the undercarboxylated form into the fully functional carboxylated form. The plasma levels of OC are considered measures of bone formation [25] and there is a general consensus in considering it a marker of bone formation.
The undercarboxylated OC (ucOC), the inactive form, shows a reduced calcium and hydroxyapatite binding activity, while the active carboxylated (cOC) form is mainly involved in bone mineralization as it allows the interaction between its calcium-binding Gla residues with the calcium ions of hydroxyapatite. This property has been proposed as the main mechanism that enables OC to contribute to the formation of hydroxyapatite crystals [26]. The level of ucOC represents a more sensitive marker of vitamin K status in humans and therefore can detect subclinical vitamin K deficiency [27]. Indeed, a high ucOC level, considered a measure of low vitamin K levels and intake can be released during osteoclastic resorption [28]. Furthermore, the OC also acts as an inhibitor of bone mineralization thus regulating the rate of mineral maturation. This finding is reflected in the ability of OC to inhibit the precipitation of calcium salts from saturated solutions [29] and to prevent over-mineralization as observed in rodents treated chronically with warfarin [30]. Moreover, Ducy et al., in OC null mouse, showed an age-dependent increase in bone formation rate and bone mass [31].
Finally it has been hypothesized that the OC can also perform a mechanical function within the bone matrix since it binds hydroxyapatite and forms a complex with collagen through the matrix protein osteopontin, acting as a bridge between the matrix and mineral component of bone tissue [32,33,34].
The effect of VK on bone health and remodeling is not limited to OC but also involves MGP, another member of the Gla family. MGP is a 12-KDa gamma-carboxyglutamic acid-containing protein, synthesized by vascular smooth muscle cells, endothelial cells, osteoblasts, chondrocytes, and osteoclasts. MGP is one of the most potent endogenous inhibitors of vascular calcification in vivo. It directly prevents calcium phosphate precipitation through binding calcification crystals in blood vessels to form vesicles and apoptotic bodies, and inhibits trans-differentiation of vascular smooth muscles cells into an osteogenic phenotype by binding with BMP2 [35,36,37,38]. MGP plays a multifaceted effect in bone health since it not only promotes bone formation by upregulating Wnt/β-catenin signaling, but it also exerts an inhibitory effect on bone mineralization [39]. In addition, MGP inhibits osteoblast mineralization and affects bone mass by regulating the deposition of the bone matrix [40,41].
Notwithstanding the low MGP expression in mature osteoclast, its role in osteoclastogenesis is relevant. It is likely that extracellular MGP produced by other cells, such as osteoblasts and chondrocytes, may regulate the cross talk between osteoclasts and these cells.
Osteoclasts differentiate from cells of monocyte/macrophage lineage upon the sequential stimulation by two pivotal factors: the monocyte/macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-kappa-B ligand (RANKL). RANKL selectively induces the nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), the key transcriptional factor in osteoclastogenesis [41,42] whose nuclear translocation and activation [43,44] is regulated by the activation of calcium-calcineurin pathway [45]. Osteoclast differentiation and bone resorption are accelerated by MGP depletion and are suppressed by MGP overexpression. MGP downgrades several mechanisms involved in osteoclast differentiation and function; in particular, it attenuates the integrin-induced activation of Src/Rac1 canonical pathway and interferes with the Ca2 flux induction of NFATc1 activation [45,46]. Despite MGP expression is induced significantly by RANKL, it reduces osteoclast differentiation and formation. It has been hypothesized that in the late stage of osteoclast differentiation, MGP is highly expressed thus outlining a negative-feedback loop to make osteoclast formation under tight control [45]. Recently, these experimental data were confirmed by Evenoepel et al. who showed an association between poor vitamin K status, defined by dephosphorylated uncarboxylated MGP (dp-ucMGP) > 500 nmol/L, and an increased risk of incident fractures in 468 end-stage renal disease patients [47].
Posttranslational modification of MGP (i.e., phosphorylation of up to three serine residues and gammacarboxylation of up to five glutamate residues) may explain its different biological effects. While the role of carboxylation, which depends on Vitamin K, is better understood and determines MGP’s bioactivity as a calcification inhibitor, the function of phosphorylation is not yet clarified. Recent data suggest a role of phosphorylation in regulating MGP secretion into the extracellular milieu [48,49]. Such reactions do not proceed in parallel, so at least four different molecules can be found in circulation: (i) (dp-ucMGP; (ii) dephosphorylated carboxylated MGP (dp-cMGP); (iii) phosphorylated uncarboxylated MGP (p-ucMGP); (iv) phosphorylated carboxylated MGP (p-cMGP) according to the state of carboxylation and/or phosphorylation. The active form is both phosphorylated and carboxylated (p-cMGP) and its synthesis is stimulated by vitamin D [50].
4. Vitamin K Recommended Intake, Deficiency, and Assessment
The recommended daily intake (RDI) or adequate intake (AI) of vitamin K is aimed at ensuring normal blood coagulation [16]. There is some variability of these recommended target values across various organizations. The National Academy of Medicine in the US stated the AI of vitamin PK at 120 μg/day for adult men and 90 μg/day for adult women [51]. The World Health Organization and the Food and Agriculture Organization recommended dosages for vitamin PK at 65 μg/day for men and 55 μg/day for women, based on a calculated requirement of 1 μg/day/kg body weight [52]. Finally, the European Commission has established a recommended daily allowance (RDA) for vitamin K at 75 μg/day [53]. In 2012, the Italian LARN (Reference Assumption Levels for Nutrients and Energy), proposed by the Human Nutrition Italian Society (SINU) suggested an intake of vitamin K stratified for age (140 or 170 μg/day for 18–59 and >60 years old, respectively) [54].
However, the studies carried out so far have suggested that a relatively higher vitamin K intake is required for bone and vascular health. Since vitamin K is stored mainly in the liver where it is used for the maintenance of the normal coagulation balance, a greater amount is required for extrahepatic tissues [55,56]. A Tsugawa et al., analyzed 1183 healthy adolescents, elaborating a new method for estimating vitamin K intake by a logarithmic regression equation. Authors showed that bone metabolism requires more vitamin K than blood coagulation: 155–188 and 62–54 μg/day, respectively [57].
Binkley et al. showed that a vitamin K intake > 250 μg is required for γ-carboxylation of OC. These studies suggest that the effect of vitamin K deficiency is more prominent on bone rather than on blood clotting [58].
Moreover, since no toxicity data are available, a safe upper limit for vitamin PK has not been established. Recent studies have suggested that vitamin K2 may be more biologically active than vitamin PK; however, lacking sufficient data, there are no recommendations for intake values for vitamin K2 [9]. The assessment of VK plasma levels is difficult as well as conditioned by numerous factors such as its low circulating concentration, the non-polar nature of the molecule and the interference of lipids. Additional variables that may influence VK plasma levels are affected by diet, inflammation, and the coexistence of chronic disease [59].
Undercarboxylated Gla-proteins, OC and MGP, have proven to be more sensitive than prothrombin time in detecting subclinical vitamin K deficiency representing functional tests useful to estimate vitamin K blood levels indirectly. Vitamin K deficiency status prevents VKDPs from acquiring their carboxylated form. The gamma-carboxyglutamate (Gla) domain is responsible for the high affinity binding of calcium ions, thus allowing coagulation factors and OC and MGP to interact with negatively charged membrane phospholipids.
Evaluation of the clinical impact of vitamin K requires the measurement of serum vitamin K homologue levels or dietary vitamin K intake. Vitamin K homologues include phylloquinone (vitamin PK) and menaquinones such as MK-4 and MK-7. Since any uncarboxylated VKDP is released by the cells into the bloodstream as a function of specific tissue activity, serum levels of uncarboxylated VKDPs can be a useful alternative marker of tissue-specific vitamin K deficiency or insufficiency.
Unfortunately, although several studies demonstrated a negative association between serum undercarboxylated VKDP levels and vitamin K status or dietary vitamin K intake, the criteria for detecting vitamin K deficiency in tissues are not defined.
While mild vitamin K deficiency does not induce considerable changes in coagulation pathway, extra-hepatic VKDP carboxylation is most affected by subclinical deficient states [60].
5. Vitamin K as Potential Therapeutic Target for Bone Fractures
Low plasma concentrations of VK are associated with a high risk of bone fractures in both northern Europeans and Asians populations of both sexes [61,62,63].
Several experimental studies have adressed the issue of the role of Vitamin K in bone metabolism. Wu et al. showed that both PK and MK (MK-4 and MK-7) inhibitor osteoclast-mediated effects on bone resorption in a dose dependent manner [64]. Furthermore, Rangel et al. demonstrated increased compact bone mass, increased bone formation markers and decreased bone resorption markers in ovariectomized (OVX) mice supplemented with Vitamin K [65]. Also, the effect of coadministration of vitamin K2 and other antiosteoporotic drugs, such as Teriparatide [66] and bisphosphonates, has been investigated [21].
In their meta-analysis, Hao et al. showed a statistically significant inverse association between dietary vitamin PK intake and risk of fractures (highest vs. the lowest intake, Relative Risks = 0.78, 95% CI: 0.56–0.99). The authors did not find any significant association between low vitamin PK and BMD [67]. Recently, 374 postmenopausal women with osteoporosis were studied showing a lower serum vitamin PK in the group with fractures (prevalent fractures: 0.53 (0.41), no fractures: 0.65 (0.66) μg/L, p = 0.04) and independently associated with fracture risk [68]. Dp-uc MGP was detectable in 97 (75%) participants with serum vitamin PK of 0.26 (0.15) μg/L, whilst PIVKA-II was above the clinical threshold in only 3.8% [68].
To date, a limited number of RCTs evaluated the effects of PK and K2 supplementation on fracture risk showing a potential positive effect and few trials are ongoing [69,70,71,72,73,74,75,76] (Table 1). In a double blind, randomized, controlled study, 244 postmenopausal women received MK-7 (180 μg MK-7/day) capsules or placebo for 3 years to investigate its effect on vertebral fractures. MK-7 significantly decreased the loss in vertebral height of the lower thoracic region at the mid-site of the vertebrae after 2 and 3 years [72] (Table 1). An interventional study 241 osteoporotic patients were enrolled in a 24-month randomized open-label study: in the control group (without treatment; n = 121) and the vitamin K2–treated group (n = 120), which received 45 mg/day orally MK-4 (45 mg/day orally). They found a reduction in the vitamin K2-treated group of the incidence of bone fractures (p = 0.0273) lower than the control group [76].

Table 1.
Published and ongoing randomized controlled trials on the relationship between vitamin K and bone fractures having fractures as pre-specified outcome. Abbreviations: PK and K2, vitamin PK and K2; MK4, menaquinone 4, MK7, menaquinone 7; RCT, randomized clinical trial; DB, double blind; ESRD, end-stage renal disease; HD, hemodialysis; PD, peritoneal dialysis; d, day; m, month; w, week; y, year; CAC, coronary artery calcification; AUs, Agatson units.
Recently, Mott et al. [77] published an update of their previous meta-analysis [78], mainly in light of the results of a review and statistical analysis, made by Bolland and colleagues [79] relative to 33 RCTs which raised serious doubts regarding their integrity and validity but also into consideration that more RCTs have been published in the meantime. Most of trials included in the systematic review by Mott et al. were carried out in postmenopausal or osteoporotic patients. Among studies reporting clinical fracture, six trials used vitamin K2 (five with 45 mg MK-4, one with 360 μg MK-7) and three used vitamin PK (ranging from 200 μg to 10 mg). Clinical fractures were lower in those who underwent vitamin K treatment (2.24% vs. 3.06%), however when the analysis was limited to low risk of bias studies, the effect was smaller (2.34% vs. 3.01%). The review by Mott et al. pointed out a considerable problem with differing methods for reporting and the diagnosis of fractures. Some trials did not provide useful data to be processed in meta-analysis; furthermore, most of the studies were conducted in Japanese populations and postmenopausal women; thus, further research is required to draw conclusions for the efficacy of vitamin K in other populations.
Participant selection based on baseline characteristics is an important issue and concerns both the BMD level of the participant and the presence of vitamin K deficiency. The enrollment in a trial of patients with normal BMD at baseline may make it hard to show its effect on fractures even if vitamin K does protect those with low BMD at baseline from fractures. Unfortunately, vitamin K baseline values were not available in many trials; a protective effect of vitamin K is likely to be achieved only in patients who are vitamin K deficient.
Mott et al., in their meta-analysis, classified the ECKO Trial as low risk of bias. In that trial, 440 postmenopausal women with osteopenia were randomized to either 5 mg of oral vitamin PK or placebo daily for 2 years. After 2 years, the investigators found daily vitamin PK supplementation increased serum vitamin PK levels by 10-fold, and decreased the percentage of undercarboxylated OC and total OC levels. However, vitamin PK supplementation had no effect on BMD or bone resorption, but determined a lower incidence of clinical fractures (HR = 0.45, p = 0.04) [74]. These data were corroborated by Fusaro et al. who carried out an observational study evaluating the association between PK and MKn levels and vertebral fractures assessed by quantitative vertebral morphometry in 387 hemodialysis patients. Authors found that 55.3% of patients had vertebral fractures and PK deficiency was the strongest predictor of vertebral fractures. Moreover, OC levels were lower in patients with high prevalence of vertebral fractures [80]. These findings suggest that the effect of vitamin PK on bone may not be related to an effect on BMD or bone turnover, but perhaps to an improvement of bone quality (e.g., material properties such as collagen crosslinks).
In Japan, MK4 is a well-established drug for osteoporosis treatment since 1960 (with a dosage of 45 mg/day orally) given the results of interventional studies that have demonstrated reduction in bone fractures incidence and improvement BMD [81].
6. Conclusions
Several points of experimental evidence seem to outline that vitamin K play an important role for bone health. Low vitamin K intake, low circulating levels of vitamin K, high serum levels of ucOC, or low serum levels of total OC have been associated with an increased risk of bone fractures in observational and RCT studies. However, the results of clinical trials are not resolutive and still remains matter of discussion whether supplementation with vitamin PK or vitamin K2 (or both) reduces the risk of vertebral or nonvertebral fractures given limitations inherent in the design of the trials that assessed these outcomes. Vitamin D status should be taken into account in future studies on vitamin K effects on bone, as there might be an interaction between the two molecules. Treating osteoporotic patients with vitamin K might have the additional advantage of protecting arteries from vascular calcification through its action on MGP.
The efficacy of vitamin K on fractures and bone quality needs to be ascertained in future large trials drawn to overcome problems still unsolved after previous studies and with sufficient statistical power to detect true and clinically meaningful effects. More evidence is needed about the effects of vitamin K supplementation at physiological and pharmacological doses and what the required dose of vitamin K is to ensure bone and vascular health.
Author Contributions
All authors have contributed, read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmadieh, H.; Arabi, A. Vitamins and bone health: Beyond calcium and vitamin D. Nutr. Rev. 2011, 69, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Curtis, E.M.; Cooper, C.; Harvey, N.C. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Investig. 2019, 42, 1149–1164. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Bone cell senescence: Mechanisms and perspectives. J. Bone Miner. Res. 2014, 29, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Mereu, M.C.; Aghi, A.; Iervasi, G.; Gallieni, M. Vitamin K and bone. Clin. Cases Miner. Bone Metab. 2017, 14, 200–206. [Google Scholar] [CrossRef]
- Fusaro, M.; Gallieni, M.; Porta, C.; Nickolas, T.L.; Khairallah, P. Correction to: Vitamin K effects in human health: New insights beyond bone and cardiovascular health. J. Nephrol. 2020, 33, 389. [Google Scholar] [CrossRef]
- Tsugawa, N.; Shiraki, M. Vitamin K Nutrition and Bone Health. Nutrients 2020, 12, 1909. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a diet supplement with impact in human health: Current evidence in age-related diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Teunissen, K.J.; Hamulyák, K.; Knapen, M.H.; Vik, H.; Vermeer, C. Vitamin K-containing dietary supplements: Comparison of synthetic vitamin PK and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef]
- Hamidi, M.S.; Gajic-Veljanoski, O.; Cheung, A.M. Vitamin K and bone health. J. Clin. Densitom. 2013, 16, 409–413. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 2008, 100, 530–547. [Google Scholar]
- Tsugawa, N.; Shiraki, M.; Suhara, Y.; Kamao, M.; Tanaka, K.; Okano, T. Vitamin K status of healthy Japanese women: Age-related vitamin K requirement for gamma-carboxylation of Osteocalcin. Am. J. Clin. Nutr. 2006, 83, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Gallieni, M.; Fusaro, M. Vitamin K and cardiovascular calcification in CKD: Is patient supplementation on the horizon? Kidney Int. 2014, 86, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Gallieni, M.; Rizzo, M.A.; Stucchi, A.; Delanaye, P.; Cavalier, E.; Moysés, R.M.A.; Jorgetti, V.; Iervasi, G.; Giannini, S.; et al. Vitamin K plasma levels determination in human health. Clin. Chem. Lab. Med. 2017, 55, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ouchi, Y.; Inoue, S. Vitamin K: Novel molecular mechanisms of action and its roles in osteoporosis. Geriatr. Gerontol. Int. 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef]
- Sato, T.; Inaba, N.; Yamashita, T. MK-7 and Its Effects on Bone Quality and Strength. Nutrients 2020, 12, 965. [Google Scholar] [CrossRef]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grün, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef]
- Hirota, Y.; Suhara, Y. New aspects of vitamin k research with synthetic ligands: Transcriptional activity via sxr and neural differentiation activity. Int. J. Mol. Sci. 2019, 20, 3006. [Google Scholar] [CrossRef]
- Azuma, K.; Inoue, S. Multiple modes of vitamin K actions in aging-related musculoskeletal disorders. Int. J. Mol. Sci. 2019, 20, 2844. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2010, 21, 195–214. [Google Scholar] [CrossRef]
- Kazama, J.J.; Iwasaki, Y.; Fukagawa, M. Uremic osteoporosis. Kidney Int. Suppl. 2013, 3, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Cosso, R.; Falchetti, A. Vitamin K and bone metabolism: The mith and the truth. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 301–317. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int. J. Mol. Med. 2011, 27, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Tuccinardi, D.; D’Onofrio, L.; Watanabe, M.; Maggi, D.; Maurizi, A.R.; Greto, V.; Buzzetti, R.; Napoli, N.; Pozzilli, P.; et al. Vitamin K and osteoporosis: Myth or reality? Metabolism 2017, 70, 57–71. [Google Scholar] [CrossRef]
- Wei, F.F.; Trenson, S.; Verhamme, P.; Vermeer, C.; Staessen, J.A. Vitamin K-Dependent matrix gla protein as multifaceted protector of vascular and tissue integrity. Hypertension 2019, 73, 1160–1169. [Google Scholar] [CrossRef]
- Price, P.A.; Otsuka, A.A.; Poser, J.W.; Kristaponis, J.; Raman, N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc. Natl. Acad. Sci. USA 1976, 73, 1447–1451. [Google Scholar] [CrossRef]
- McKeown, N.M.; Jacques, P.F.; Gundberg, C.M.; Peterson, J.W.; Tucker, K.L.; Kiel, D.P.; Wilson, P.W.; Booth, S.L. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J. Nutr. 2002, 132, 1329–1334. [Google Scholar] [CrossRef]
- Caluwé, R.; Verbeke, F.; De Vriese, A.S. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol. Dial. Transplant. 2020, 35, 23–33. [Google Scholar] [CrossRef]
- Van de Loo, P.G.; Soute, B.A.; van Haarlem, L.J.; Vermeer, C. The effect of Gla-containing proteins on the precipitation of insoluble salts. Biochem. Biophys. Res. Commun. 1987, 142, 113–119. [Google Scholar] [CrossRef]
- Price, P.A.; Williamson, M.K.; Haba, T.; Dell, R.B.; Jee, W.S. Excessive mineralization with growth plate closure in rats on chronic warfarin treatment. Proc. Natl. Acad. Sci. USA 1982, 79, 7734–7738. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in Osteocalcin-Deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Ritter, N.M.; Farach-Carson, M.C.; Butler, W.T. Evidence for the formation of a complex between osteopontin and Osteocalcin. J. Bone Miner. Res. 1992, 7, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.Q.; Sicheri, F.; Howard, A.J.; Yang, D.S. Bone recognition mechanism of porcine Osteocalcin from crystal structure. Nature 2003, 425, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.V.; Carr, S.A. Calcium-dependent alpha-helical structure in Osteocalcin. Biochemistry 1982, 21, 2538–2547. [Google Scholar] [CrossRef]
- Price, P.A.; Urist, M.R.; Otawara, Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983, 117, 765–771. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Munroe, P.B.; Olgunturk, R.O.; Fryns, J.P.; Van Maldergem, L.; Ziereisen, F.; Yuksel, B.; Gardiner, R.M.; Chung, E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999, 21, 142–144. [Google Scholar] [CrossRef]
- Zebboudj, A.F.; Imura, M.; Boström, K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J. Biol. Chem. 2002, 277, 4388–4394. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z.; Yan, K.; Wang, Y.; Yang, Y.; Wu, X. Matrix Gla Protein Promotes the Bone Formation by Up-Regulating Wnt/β-Catenin Signaling Pathway. Front. Endocrinol. (Lausanne) 2019, 10, 891. [Google Scholar] [CrossRef]
- Newman, B.; Gigout, L.I.; Sudre, L.; Grant, M.E.; Wallis, G.A. Coordinated expression of matrix Gla protein is required during endochondral ossification for chondrocyte survival. J. Cell Biol. 2001, 154, 659–666. [Google Scholar] [CrossRef]
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-dependent regulation of MGP in osteoblasts: Role of ERPK/2 and Fra-1. J. Bone Miner. Res. 2009, 24, 1856–1868. [Google Scholar] [CrossRef]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Lee, S.H.; Fisher, D.E.; Kook, H.; Kim, K.K.; Choi, Y.; Kim, N. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 2005, 280, 35209–35216. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in osteoclast differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Wang, N.; Li, J.; He, F.; Li, X.; Wu, S. Unexpected role of matrix gla protein in osteoclasts: Inhibiting osteoclast differentiation and bone resorption. Mol. Cell. Biol. 2019, 39. [Google Scholar] [CrossRef]
- Zou, W.; Kitaura, H.; Reeve, J.; Long, F.; Tybulewicz, V.L.; Shattil, S.J.; Ginsberg, M.H.; Ross, F.P.; Teitelbaum, S.L. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol. 2007, 176, 877–888. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Poor Vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J. Bone Miner. Res. 2019, 34, 262–269. [Google Scholar] [CrossRef]
- Wajih, N.; Borras, T.; Xue, W.; Hutson, S.M.; Wallin, R. Processing and transport of matrix gamma-carboxyglutamic acid protein and bone morphogenetic protein-2 in cultured human vascular smooth muscle cells: Evidence for an uptake mechanism for serum fetuin. J. Biol. Chem. 2004, 279, 43052–43060. [Google Scholar] [CrossRef]
- Murshed, M.; Schinke, T.; McKee, M.D.; Karsenty, G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell Biol. 2004, 165, 625–630. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Spronk, H.M.; Skepper, J.N.; Hackeng, T.M.; Shanahan, C.M.; Vermeer, C.; Weissberg, P.L.; Proudfoot, D. Post-translational modifications regulate matrix Gla protein function: Importance for inhibition of vascular smooth muscle cell calcification. J. Thromb. Haemost. 2007, 5, 2503–2511. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D.; The National Academies Press: Washington, DC, USA, 2011. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21796828 (accessed on 31 October 2020).
- Food and Agriculture Organization of the United Nations. World Health Organization and Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; pp. 108–129. [Google Scholar]
- European Community. Commission Directive 2008/100/EC of 28 October 2008 amending council directive 90/496/eec on nutrition labelling for food stuffs as regards recommended daily allowances, energy conversion factors and definitions. Off. J. Eur. Union 2008, 285, 9. [Google Scholar]
- Human Nutrition Italian Society (SINU). Italian LARN (Reference Assumption Levels for Nutrients and Energy). Available online: http://www.sinu.it/html/pag/10-VITAMINE-2.asp (accessed on 31 October 2020).
- Cranenburg, E.C.; Schurgers, L.J.; Vermeer, C. Vitamin K: The coagulation vitamin that became omnipotent. Thromb. Haemost. 2007, 98, 120–125. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Vitamin K, an example of triage theory: Is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, N.; Uenishi, K.; Ishida, H.; Minekami, T.; Doi, A.; Koike, S.; Takase, T.; Kamao, M.; Mimura, Y.; Okano, T. A novel method based on curvature analysis for estimating the dietary vitamin K requirement in adolescents. Clin. Nutr. 2012, 31, 255–260. [Google Scholar] [CrossRef]
- Binkley, N.C.; Krueger, D.C.; Kawahara, T.N.; Engelke, J.A.; Chappell, R.J.; Suttie, J.W. A high phylloquinone intake is required to achieve maximal Osteocalcin gamma-carboxylation. Am. J. Clin. Nutr. 2002, 76, 1055–1060. [Google Scholar] [CrossRef]
- Cozzolino, M.; Fusaro, M.; Ciceri, P.; Gasperoni, L.; Cianciolo, G. The Role of Vitamin K in Vascular Calcification. Adv. Chronic Kidney Dis. 2019, 26, 437–444. [Google Scholar] [CrossRef]
- Card, D.J.; Gorska, R.; Harrington, D.J. Laboratory assessment of vitamin K status. J. Clin. Pathol. 2020, 73, 70–75. [Google Scholar] [CrossRef]
- Torbergsen, A.C.; Watne, L.O.; Wyller, T.B.; Frihagen, F.; Strømsøe, K.; Bøhmer, T.; Mowe, M. Vitamin PK and 25(OH)D are independently and synergistically associated with a risk for hip fracture in an elderly population: A case control study. Clin. Nutr. 2015, 34, 101–106. [Google Scholar] [CrossRef]
- Finnes, T.E.; Lofthus, C.M.; Meyer, H.E.; Søgaard, A.J.; Tell, G.S.; Apalset, E.M.; Gjesdal, C.; Grimnes, G.; Schei, B.; Blomhoff, R.; et al. A combination of low serum concentrations of vitamins PK and D is associated with increased risk of hip fractures in elderly Norwegians: A NOREPOS study. Osteoporos. Int. 2016, 27, 1645–1652. [Google Scholar] [CrossRef]
- Yaegashi, Y.; Onoda, T.; Tanno, K.; Kuribayashi, T.; Sakata, K.; Orimo, H. Association of hip fracture incidence and intake of calcium, magnesium, vitamin D, and vitamin K. Eur. J. Epidemiol. 2008, 23, 219–225. [Google Scholar] [CrossRef]
- Wu, W.J.; Kim, M.S.; Ahn, B.Y. The inhibitory effect of vitamin K on RANKL-induced osteoclast differentiation and bone resorption. Food Funct. 2015, 6, 3351–3358. [Google Scholar] [CrossRef]
- Rangel, L.B.; de Siqueira, D.; do Rosário Soares, O.; Santana, H.S.; de Castro Miguel, E.; da Cunha, M.; de Abreu Oliveira, A.L.; Pedrosa, D.F.; Resgala, L.C.; Neto, H.A.; et al. Vitamin K supplementation modulates bone metabolism and ultra-structure of ovariectomized mice. Cell. Physiol. Biochem. 2018, 51, 356–374. [Google Scholar] [CrossRef]
- Nagura, N.; Komatsu, J.; Iwase, H.; Hosoda, H.; Ohbayashi, O.; Nagaoka, I.; Kaneko, K. Effects of the combination of vitamin K and teriparatide on the bone metabolism in ovariectomized rats. Biomed. Rep. 2015, 3, 295–300. [Google Scholar] [CrossRef][Green Version]
- Hao, G.; Zhang, B.; Gu, M.; Chen, C.; Zhang, Q.; Zhang, G.; Cao, X. Vitamin K intake and the risk of fractures: A meta-analysis. Med. (Baltim.) 2017, 96, e6725. [Google Scholar] [CrossRef]
- Moore, A.E.; Kim, E.; Dulnoan, D.; Dolan, A.L.; Voong, K.; Ahmad, I.; Gorska, R.; Harrington, D.J.; Hampson, G. Serum vitamin K. Bone 2020, 115630. [Google Scholar] [CrossRef]
- Tanaka, S.; Miyazaki, T.; Uemura, Y.; Miyakawa, N.; Gorai, I.; Nakamura, T.; Fukunaga, M.; Ohashi, Y.; Ohta, H.; Mori, S.; et al. Comparison of concurrent treatment with vitamin K. J. Bone Miner. Metab. 2017, 35, 385–395. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.L.; Zhu, H.M.; Wu, Y.Y.; Cheng, Q.; Wu, F.L.; Xing, X.P.; Liu, J.L.; Yu, W.; Meng, X.W. Menatetrenone versus alfacalcidol in the treatment of Chinese postmenopausal women with osteoporosis: A multicenter, randomized, double-blinded, double-dummy, positive drug-controlled clinical trial. Clin. Interv. Aging 2014, 9, 121–127. [Google Scholar] [CrossRef][Green Version]
- Kasukawa, Y.; Miyakoshi, N.; Ebina, T.; Aizawa, T.; Hongo, M.; Nozaka, K.; Ishikawa, Y.; Saito, H.; Chida, S.; Shimada, Y. Effects of risedronate alone or combined with vitamin K2 on serum undercarboxylated Osteocalcin and Osteocalcin levels in postmenopausal osteoporosis. J. Bone Miner. Metab. 2014, 32, 290–297. [Google Scholar] [CrossRef]
- Knapen, M.H.; Drummen, N.E.; Smit, E.; Vermeer, C.; Theuwissen, E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos. Int. 2013, 24, 2499–2507. [Google Scholar] [CrossRef]
- Inoue, T.; Fujita, T.; Kishimoto, H.; Makino, T.; Nakamura, T.; Sato, T.; Yamazaki, K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): A phase IV clinical study of 15-mg menatetrenone capsules. J. Bone Miner. Metab. 2009, 27, 66–75. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tile, L.; Lee, Y.; Tomlinson, G.; Hawker, G.; Scher, J.; Hu, H.; Vieth, R.; Thompson, L.; Jamal, S.; et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): A randomized controlled trial. PLoS Med. 2008, 5, e196. [Google Scholar] [CrossRef]
- Ishida, Y.; Kawai, S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi Osteoporosis Prevention Study. Am. J. Med. 2004, 117, 549–555. [Google Scholar] [CrossRef]
- Shiraki, M.; Shiraki, Y.; Aoki, C.; Miura, M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J. Bone Miner. Res. 2000, 15, 515–521. [Google Scholar] [CrossRef]
- Mott, A.; Bradley, T.; Wright, K.; Cockayne, E.S.; Shearer, M.J.; Adamson, J.; Lanham-New, S.A.; Torgerson, D.J. Effect of vitamin K on bone mineral density and fractures in adults: An updated systematic review and meta-analysis of randomised controlled trials. Osteoporos. Int. 2019, 30, 1543–1559. [Google Scholar] [CrossRef]
- Cockayne, S.; Adamson, J.; Lanham-New, S.; Shearer, M.J.; Gilbody, S.; Torgerson, D.J. Vitamin K and the prevention of fractures: Systematic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 1256–1261. [Google Scholar] [CrossRef]
- Bolland, M.J.; Avenell, A.; Gamble, G.D.; Grey, A. Systematic review and statistical analysis of the integrity of 33 randomized controlled trials. Neurology 2016, 87, 2391–2402. [Google Scholar] [CrossRef]
- Fusaro, M.; Noale, M.; Viola, V.; Galli, F.; Tripepi, G.; Vajente, N.; Plebani, M.; Zaninotto, M.; Guglielmi, G.; Miotto, D.; et al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. J. Bone Miner. Res. 2012, 27, 2271–2278. [Google Scholar] [CrossRef]
- Iwamoto, J.; Sato, Y.; Takeda, T.; Matsumoto, H. High-dose vitamin K supplementation reduces fracture incidence in postmenopausal women: A review of the literature. Nutr. Res. 2009, 29, 221–228. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).