Obesity and Other Nutrition Related Abnormalities in Pre-Dialysis Chronic Kidney Disease (CKD) Participants
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Socio-Demographics
3.2. Clinical
3.3. Anthropometry
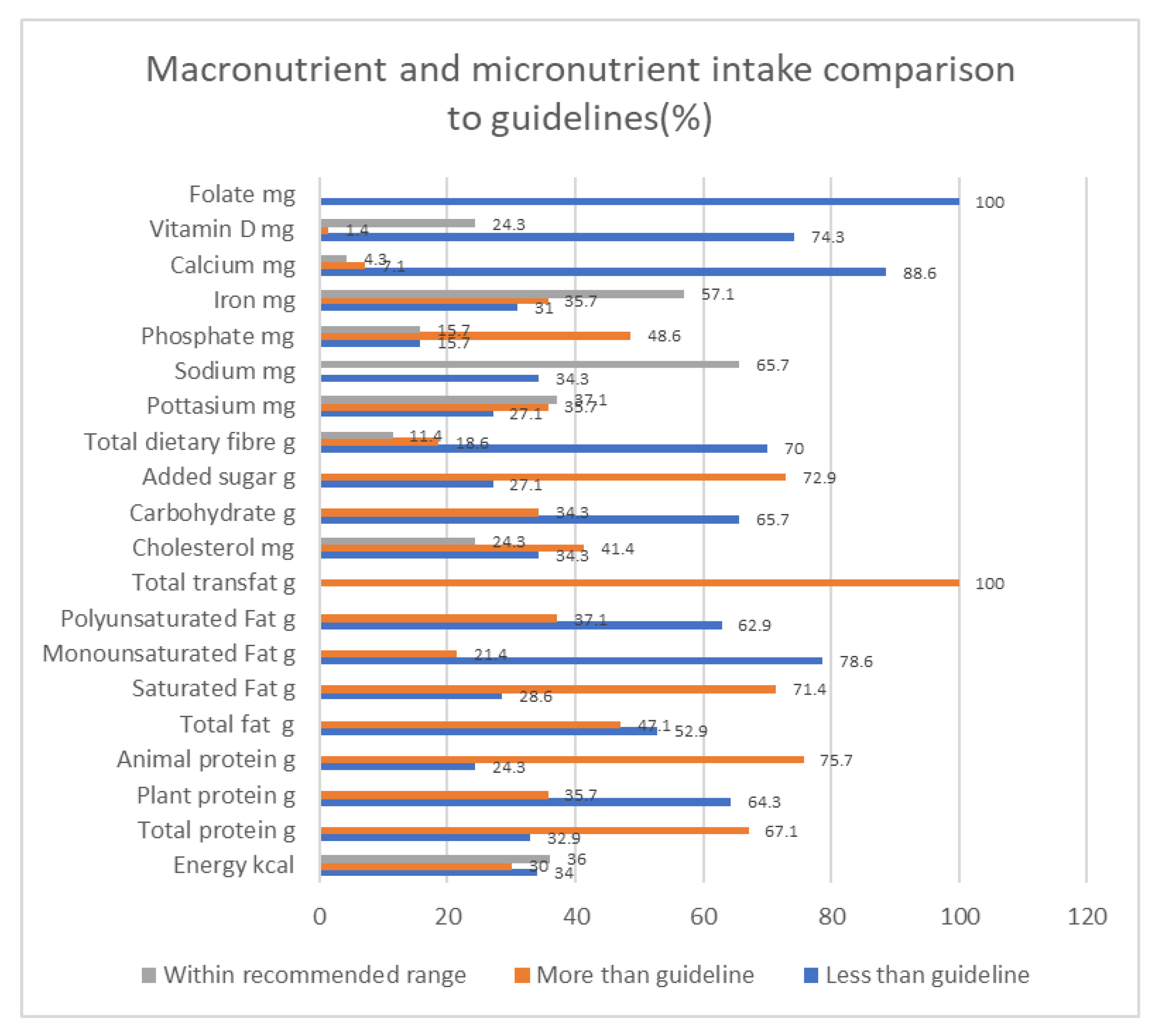
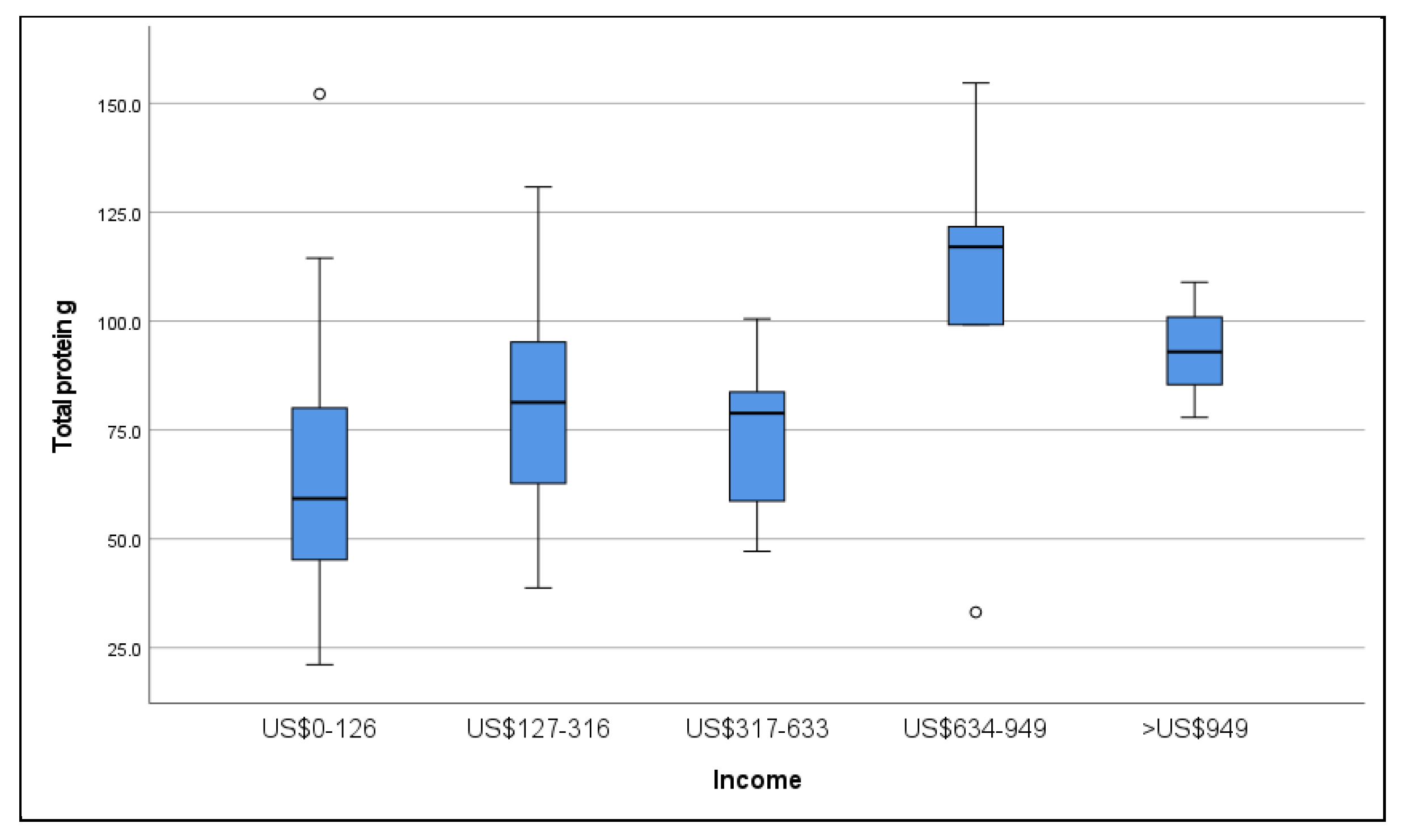
3.4. Dietary Intake
3.5. Biochemistry
4. Discussion
Limitations of Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Measurement | Formula | Cut-Off Values | Interpretation |
|---|---|---|---|
| BMI [17] | Weight/height2 | <18.49 kg/m2 | underweight |
| 18.5–24.99 kg/m2 | normal weight | ||
| 25–29.99 kg/m2 | overweight | ||
| >30 kg/m2 | obese | ||
| Adjusted body weight [44] | aBWef= BWef + [(SBW − BWef) × 0.25] ef: oedema free weight | ||
| MUAC [45] | <23 cm females | Malnourished | |
| <22 cm males | Malnourished | ||
| >28 females | Overweight | ||
| >29 males | Overweight | ||
| >30 females and males | Obese | ||
| WC [46] | <80 cm for females <94 cm for males | Normal | |
| between 80–88 cm for females between 94–102cm for males | Increased risk for disease | ||
| >88 cm for females and >102 cm for males | High risk for disease | ||
| AFA/AMA area [44] | AFA = [MAC(cm) × TSF(cm)/2 π × TSF(cm)2]/4 π | <5th percentile | Wasted |
| AMA = [MAC (cm) − (π × Triceps Skinfold Thickness (cm))]2/4 π | |||
| ≥5th and ≤15th percentile | Below average muscle/fat | ||
| ≥15th and ≤85th | Average muscle/fat | ||
| ≥85th and ≤95th percentile | Above average muscle/fat | ||
| >95th percentile | High muscle/fat |
References
- Chan, M.; Kelly, J.; Batterham, M.; Tapsell, L. A High Prevalence of Abnormal Nutrition Parameters Found in Predialysis End-Stage Kidney Disease: Is It a Result of Uremia or Poor Eating Habits? J. Ren. Nutr. 2014, 24, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, J.; Dahl, H.; Lervaag Welland, N.; Sandnes, K.; Sæle, K.; Sekse, I.; Marti, H.P. High rates of central obesity and sarcopenia in CKD irrespective of renal replacement therapy—An observational cross-sectional study. BMC Nephrol. 2018, 19, 259. [Google Scholar]
- Hall, M.E.; do Carmo, J.M.; da Silva, A.A.; Juncos, L.A.; Wang, Z.; Hall, J.E. Obesity, Hypertension and chronic kidney disease. Int. J. Nephrol. Renov. Dis. 2014, 7, 75–88. [Google Scholar] [CrossRef]
- Chandra, A.; Biersmith, M.; Tolouian, R. Obesity and kidney protection. J. Nephropathol. 2014, 3, 91–97. [Google Scholar] [PubMed]
- Herrington, W.G.; Smith, M.; Bankhead, C.; Matsushita, K.; Stevens, S.; Jolt, T.; Hobbs, F.R.; Coresh, J.; Woodward, M. Body-mass index and risk of advanced chronic kidney disease: Prospective analyses from a primary care cohort of 1.4 million adults in England. PLoS ONE 2017, 12, e0173515. [Google Scholar] [CrossRef]
- Fouque, D.; Pelletier, S.; Mafra, D.; Chauveau, P. Nutrition and chronic kidney disease. Kidney Int. 2011, 80, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Greene, T.; Chumlea, W.C.; Hollinger, D.; Maroni, B.J.; Merrill, D.; Scherch, L.K.; Schulman, G.; Wang, S.R.; Zimmer, G.S.; et al. Relationship between nutritional status and the glomerular filtration rate: Results from the MDRD study. Kidney Int. 2000, 57, 1688–1703. [Google Scholar] [CrossRef]
- Biruete, A.; Jeong, J.H.; Barnes, J.L.; Wilund, K.R. Modified Nutritional Recommendations to Improve Dietary Patterns and Outcomes in Hemodialysis Patients. J. Ren. Nutr. 2017, 27, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Mukai, H.; Lindholm, B.; Heimbürger, O.; Barany, P.; Stenvinkel, P.; Qureshi, A.R. Clinical global assessment of nutritional status as predictor of mortality in chronic kidney disease patients. PLoS ONE 2017, 12, e0186659. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.A.; Lazarus, R.; Kelly, J.J. Prevalence and prognostic significance of malnutrition in chronic renal insufficiency. J. Ren. Nutr. 2001, 11, 16–22. [Google Scholar] [CrossRef]
- Silva, M.I.B.; Vale, B.S.; Lemos, C.C.; Torres, M.R.; Bregman, R. Body Adiposity Index Assess Body Fat with High Accuracy in Nondialyzed Chronic Kidney Disease Patients. Obesity 2012, 21, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.Y.; Lee, K.-B.; Han, S.H.; Kim, Y.H.; Kim, Y.-S.; Lee, S.W.; Oh, Y.K.; Chae, D.W.; Ahn, C. Nutritional status in adults with predialysis chronic kidney disease: KNOW-CKD study. J. Korean Med. Sci. 2017, 32, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, O.A.; Okaka, E.I. Malnutrition in pre-dialysis chronic kidney disease patients in a teaching hospital in Southern Nigeria. Afr. Health Sci. 2016, 16, 234–241. [Google Scholar]
- Prakash, J.; Raja, R.; Mishra, R.; Vohra, R.; Sharma, N.; Wani, I.; Parekh, A. High prevalence of malnutrition and inflammation in undialyzed patients with chronic renal failure in developing countries: A single center experience from Eastern India. Ren. Fail. 2007, 29, 811–816. [Google Scholar] [CrossRef]
- Lu, J.L.; Kalantar-Zadeh, K.; Ma, J.Z.; Quarles, L.D.; Kovesdy, C.P. Association of body mass index with outcomes in patients with CKD. J. Am. Soc. Nephrol. 2014, 25, 2088–2096. [Google Scholar] [CrossRef]
- WHO. Global Database on Body Mass Index. Available online: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed on 6 December 2017).
- NHANES. Anthropometry Procedures Manual. National Health and Nutrition Examination Survey: Atlanta, US. 2007; pp. 1–102. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed on 16 April 2020).
- Sanzul, R.; Senekal, M.; Harbron, J.; Hoosen, F. The Dietary Intake and Practices of South African Marathon Runners. Honors Thesis, University of Cape Town, Cape Town, South Africa, 2014. Unpublished work. [Google Scholar]
- SAFOODS. SAMRC Food Composition Tables for South Africa, 5th ed.; South African Medical Research Council: Cape Town, South Africa; Available online: http://safoods.mrc.ac.za (accessed on 24 January 2017).
- Eknoyan, G.; Levin, N. K/DOQI Nutrition in Chronic Renal Failure. Am. J. Kidney Dis 2000, 35 (Suppl. S2), S1–S3. [Google Scholar] [CrossRef]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Tamura, M.K.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Department of Health, Medical Research Council. South Africa Demographic and Health Survey 2016; Statistics SA: Pretoria, South Africa, 2017. [Google Scholar] [CrossRef]
- Kyle, U.G.; Schutz, Y.; Dupertuis, Y.M.; Pichard, C. Body composition interpretation: Contributions of the fat-free mass index and the body fat mass index. Nutrition 2003, 19, 597–604. [Google Scholar] [CrossRef]
- Johansen, K.L.; Lee, C. Body composition in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Morelli, E.; Rizza, G.M.; Galetta, F.; Franzoni, F.; Barsotti, G. Nutritional status and dietary manipulation in predialysis chronic renal failure patients. J. Ren. Nutr. 2004, 14, 127–133. [Google Scholar] [CrossRef]
- Włodarek, D.; Głąbska, D.; Rojek-Trębicka, J. Assessment of diet in chronic kidney disease female predialysis patients. Ann. Agric. Environ. Med. 2014, 21, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Steiber, A.L. Clinical indicators associated with poor oral intake of patients with chronic renal failure. J. Ren. Nutr. 1999, 9, 84–88. [Google Scholar] [CrossRef]
- Heitmann, B.L.; Lissner, L. Dietary underreporting by obese individuals-Is it specific or non-Specific? BMJ 1995, 311, 986–989. [Google Scholar] [CrossRef]
- Fouque, D.; Mitch, W.E. Low-protein diets in chronic kidney disease: Are we finally reaching a consensus? Nephrol Dial. Transplant. 2015, 30, 6–8. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Comparison of high vs. normal/low protein diets on renal function in subjects without chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2014, 9, e97656. [Google Scholar] [CrossRef]
- Chironda, G.; Bhengu, B.R. Contributing Factors to Non-Adherence among Chronic Kidney Disease (CKD) Patients: A Systematic Review of Literature. Med. Clin. Rev. 2016, 2, 29. [Google Scholar] [CrossRef]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D.S. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.V.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Chonchol, M. Does Inflammation Affect Outcomes in Dialysis patients. Semin. Dial. 2018, 31, 388–397. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. Added sugars drive chronic kidney disease and its consequences: A comprehensive review. J. Insul. Resist. 2016, 1, 6. [Google Scholar] [CrossRef]
- Williams, S.; Malatesta, K.; Norris, K.C. Vitamin D and chronic kidney disease. Ethn. Dis. 2009, 19, S5–S8. [Google Scholar]
- Kendrick, J.; Kestenbaum, B.; Chonchol, M. Phosphate and Cardiovascular disease. Adv. Chronic Kidney Dis. 2011, 18, 113–119. [Google Scholar] [CrossRef]
- Piazzolla, G.; Candigliota, M.; Fanelli, M.; Castrovilli, A.; Berardi, E.; Antonica, G.; Battaglia, S.; Solfrizzi, V.; Sabbà, C.; Tortorella, C. Hyperhomocysteinemia is an independent risk factor of atherosclerosis in patients with metabolic syndrome. Diabetol. Metab. Syndr. 2019, 11, 87–89. [Google Scholar] [CrossRef]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Am. J. Cardiol. 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Lee, R.D.; Nieman, D.C. Nutritional Assessment, 6th ed.; McGraw-Hill Publishers: Columbus, OH, USA, 2012. [Google Scholar]
- Van Tonder, E.; Mace, L.; Steenkamp, L.; Tydeman-Edwards, R.; Gerber, K.; Friskin, D. Mid-upper arm circumference (MUAC) as a feasible tool in detecting adult malnutrition. S. Afr. J. Clin. Nutr. 2019, 32, 93–98. [Google Scholar] [CrossRef]
- WHO. Waist Circumference and Waist-Hip Ratio Report of a WHO Expert Consultation. Available online: http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf (accessed on 5 December 2017).
| n | Mean ± SD | ||
|---|---|---|---|
| Age (years) | 70 | 41.7 ± 11.8 | |
| n | Percent % | ||
| Gender | Male | 33 | 47.1 |
| Female | 37 | 52.9 | |
| Employment status | Full time | 29 | 41.4 |
| Part time | 5 | 7.1 | |
| Unemployed | 22 | 31.4 | |
| Pensioner/Grant holder | 4 | 5.7 | |
| Other | 10 | 14.2 | |
| Monthly Income | US $0–126 | 29 | 41.4 |
| US $127–316 | 18 | 25.7 | |
| US $317–633 | 15 | 21.4 | |
| US $634–949 | 5 | 7.1 | |
| >US $949 | 3 | 4.3 | |
| Education level | Primary school | 10 | 14.3 |
| Grade 8–11 | 32 | 45.7 | |
| Grade 12 | 20 | 28.6 | |
| University | 1 | 1.4 | |
| Technicon | 7 | 10.0 | |
| n | Mean ± SD | ||
|---|---|---|---|
| Blood pressure (systolic) mmHg | 64 | 146.0 ± 25.5 | |
| Blood pressure (diastolic) mmHg | 64 | 81.0 ± 15.3 | |
| n | % | ||
| Oedema | None | 44 | 62.9 |
| Mild | 15 | 21.4 | |
| Moderate | 8 | 11.4 | |
| Severe | 3 | 4.3 | |
| GFR stages | Stage 3 | 21 | 30.0 |
| Stage 4 | 18 | 25.7 | |
| Stage 5 | 31 | 44.2 | |
| Cause Renal Failure | Polycystic kidney disease | 6 | 8.6 |
| Hypertension | 35 | 50.0 | |
| Glomerular disease | 13 | 18.6 | |
| Other and unknown | 16 | 22.9 | |
| Total Group n = 70 | Male n = 33 | Female n = 37 | * p Value | |
|---|---|---|---|---|
| Mean ± SD | ||||
| Weight (kg) | 76.8 ± 25.4 | 82.9 ± 23 | 71.4 ± 19.7 | * 0.03 |
| BMI (unit) | 28.4 ± 7.0 | 28.4 ± 7.8 | 28.6 ± 6.4 | * 0.90 |
| Waist circumference (cm) | 92.1 ± 16.8 | 94.9 ±19.5 | 91.6 ± 13.7 | * 0.18 |
| MUAC (cm) | 31.0 ± 5.4 | 31.2 ± 5.1 | 30.5 ± 5.8 | * 0.84 |
| Triceps (mm) | 21.0 ± 9.1 | 17.0 ± 9.0 | 24.0 ± 8.0 | * 0.001 |
| BMI Categories | n (%) | |||
| Underweight | 3 (4.3) | 0 | 3 (8.1) | Chi2 = 8.9, p = ** 0.03 |
| Normal weight | 21 (30.0) | 13 (39.4) | 8 (21.7) | |
| Overweight | 21 (30.0) | 12 (36.4) | 9 (24.3) | |
| Obese | 25 (35.7) | 8 (24.2) | 17 (45.9) | |
| Waist circumference Categories | ||||
| Normal | 28 (40) | 18 (54.5) | 10 (27.0) | Chi2 = 8.0, p = ** 0.005 |
| Increased risk | 13 (18.6) | 7 (21.2) | 6 (16.2) | |
| High risk | 29 (41.4) | 8 (24.2) | 21 (56.8) | |
| MUAC Categories | n (%) | |||
| Undernourished | 5 (7.1) | 0 | 5 (13.5) | Chi2 = 3.0, p = ** 0.22 |
| Normal | 17 (24.3) | 9 (27.2) | 8 (21.6) | |
| Overweight | 9 (13.0) | 5 (15.1) | 4 (10.8) | |
| Obese | 39 (55.7) | 19 (57.5) | 20 (54.0) | |
| AMA Categories | n (%) | |||
| Wasted | 1 (1.4) | 1 (3.0) | 0 | Chi2 = 8.9, p = ** 0.06 |
| Below average muscle | 7 (10.0) | 6 (18.2) | 1 (2.7) | |
| Average muscle | 36 (51.4) | 17 (51.5) | 19 (51.4) | |
| Above average muscle | 13 (18.6) | 5 (15.2) | 8 (21.6) | |
| High muscle | 12 (17.1) | 3 (9.1) | 9 (24.3) | |
| AFA Categories | n (%) | |||
| Wasted | 5 (7.1) | 3 (9.1) | 2 (5.4) | Chi2 = 12.2, p = ** 0.02 |
| Below average fat | 5 (7.1) | 1 (3.0) | 4 (10.8) | |
| Average fat | 40 (57.1) | 18 (54.5) | 22 (59.5) | |
| Above average fat | 10 (14.3) | 2 (6.1) | 8 (21.6) | |
| Excess fat | 9 (12.9) | 8 (24.2) | 1 (2.7) | |
| Recommended Daily Allowances [21] | Actual Intake | |
|---|---|---|
| Mean ± SD | ||
| n = 70 | ||
| Energy kcal/kg | 25–35 [23] | 27 |
| 2041.7 ± 732 kcal/kg | ||
| Total protein g/kg | 0.6–0.8 | 1 |
| 0.55–0.6 g/kg [23] | 74.2 ± 28.4 g | |
| Plant protein | 50% of protein intake | 34.2% |
| 25.4 ± 10.7 g | ||
| Animal protein | 50% of protein intake | 64.8% |
| 48.1 ± 21.2 g | ||
| Total fat | 34% Energy | 35.2% |
| 80.0 ± 34.9 g | ||
| Saturated Fat | <7% of Energy | 10.7% |
| 24.3 ± 11.7 g | ||
| Monounsaturated Fat | <20% Energy | 12.2% |
| 27.7 ± 14.4 g | ||
| Polyunsaturated Fat | <10% Energy | 9.0% 20.6 ± 8.6 g |
| Total trans fat g | 0 | 0.7 ± 0.5 |
| Cholesterol mg | 200–300 | 278.2 ± 133.7 |
| Carbohydrate | 55% Energy | 49.3% E 251.9 ± 93.7 g |
| Added sugar g | 25 | 39.1 (23.0, 59.1) * |
| Total sugars g | NA | 69.9 ± 29.2 |
| Total dietary fiber g | 253–0 | 21.8 ± 9.7 |
| Calcium mg | 1000–1200 | 484.7 (349.0, 743.1) * |
| 800–1000 [23] | ||
| Iron mg | 101–8 | 13.0 ± 4.6 |
| Phosphate mg | 800–1000 | 1038.7 ± 420.6 |
| Sodium mg | 2400 | 2049 ± 965.1 |
| 2300 [23] | ||
| Potassium mg | 2000–3000 | 2691.2 ± 932.7 |
| Vitamin B6 mg | 5 | 3.2 ± 1.3 |
| Folate mg | 1000 | 291.8 ± 118.0 |
| Vitamin D mg | 5–10 | 2.7 (1.8, 5.2) * |
| Normal Ranges * | Actual Median and Interquartile Range | |
|---|---|---|
| Urea mmol/L | 2.1–7.1 | 16.3 (10.9, 25.3) |
| Creatinine umol/L | 64–104 | 287.0 (183, 477.5) |
| GFR mL/min·1.73 m2 | >60 | 19.0 (10.8, 31.2) |
| Potassium mmol/L | 3.5–5.1 | 4.8 (4.3, 5.2) |
| Sodium mmol/L | 136–141 | 142.0 (139, 144.0) |
| Phosphate mmol/L | 0.78–1.42 | 1.4 (1.1, 1.5) |
| Total Chol mmol/L (high risk) | <4.5 | 4.9 (3.9, 5.7) |
| LDL (high risk) mmol/L | <2.6 ** | 2.7 (2.1, 3.3) |
| HDL mmol/L | >1.2 | 1.1 (1.0, 1.4) |
| TG mmol/L | <1.7 | 1.7 (1.2, 2.5) |
| CRP mg/L | <3 ** | 5.0 (1, 9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahim, Z.; Moosa, M.R.; Blaauw, R. Obesity and Other Nutrition Related Abnormalities in Pre-Dialysis Chronic Kidney Disease (CKD) Participants. Nutrients 2020, 12, 3608. https://doi.org/10.3390/nu12123608
Ebrahim Z, Moosa MR, Blaauw R. Obesity and Other Nutrition Related Abnormalities in Pre-Dialysis Chronic Kidney Disease (CKD) Participants. Nutrients. 2020; 12(12):3608. https://doi.org/10.3390/nu12123608
Chicago/Turabian StyleEbrahim, Zarina, M. Rafique Moosa, and Renée Blaauw. 2020. "Obesity and Other Nutrition Related Abnormalities in Pre-Dialysis Chronic Kidney Disease (CKD) Participants" Nutrients 12, no. 12: 3608. https://doi.org/10.3390/nu12123608
APA StyleEbrahim, Z., Moosa, M. R., & Blaauw, R. (2020). Obesity and Other Nutrition Related Abnormalities in Pre-Dialysis Chronic Kidney Disease (CKD) Participants. Nutrients, 12(12), 3608. https://doi.org/10.3390/nu12123608





