Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agents and Chemicals
2.2. Animals
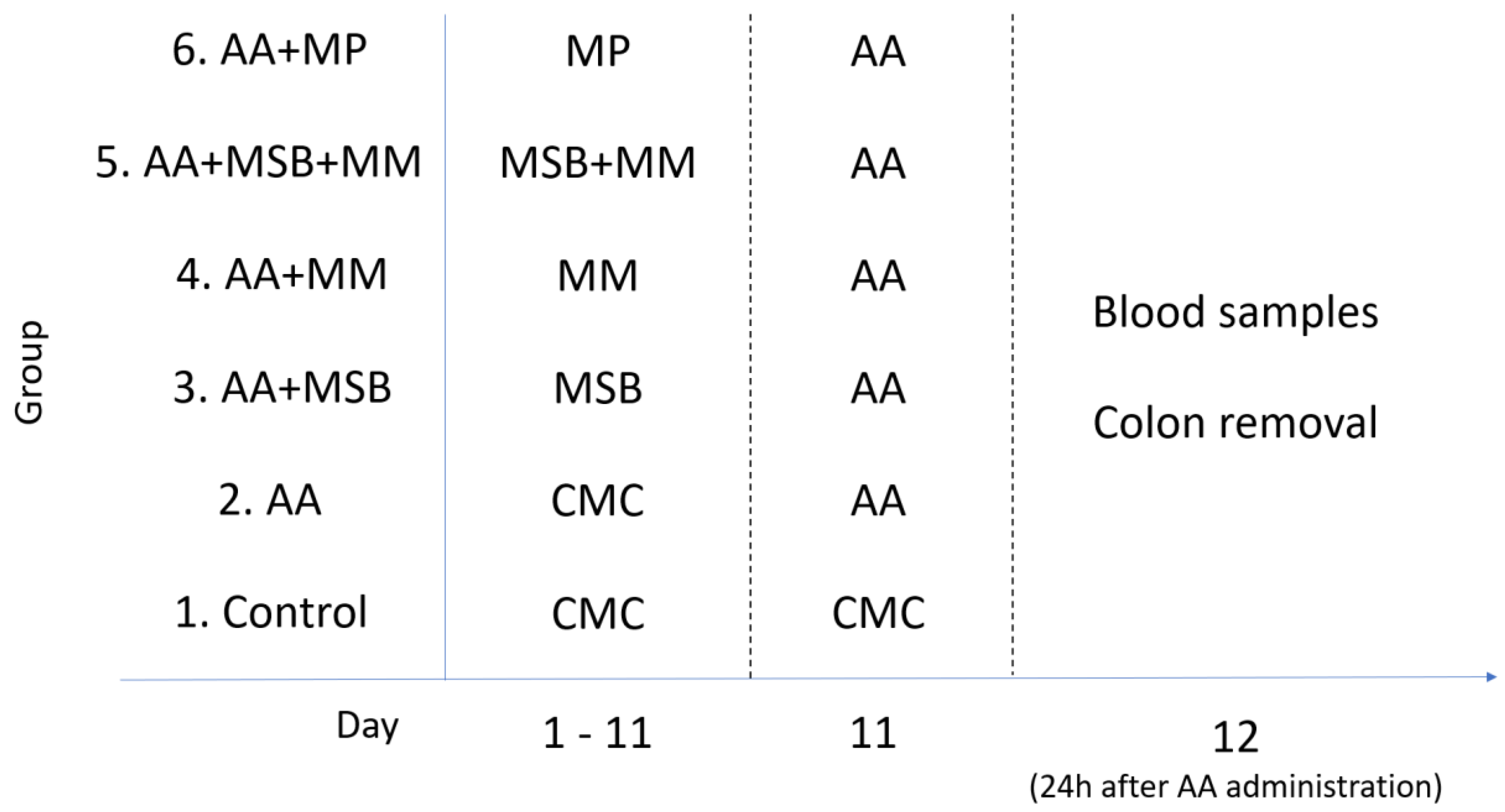
2.3. Experimental Design
2.4. Induction of Colitis
2.5. Evaluation of Inflammatory and Cellular Markers
2.6. Assessment of Oxidative Stress
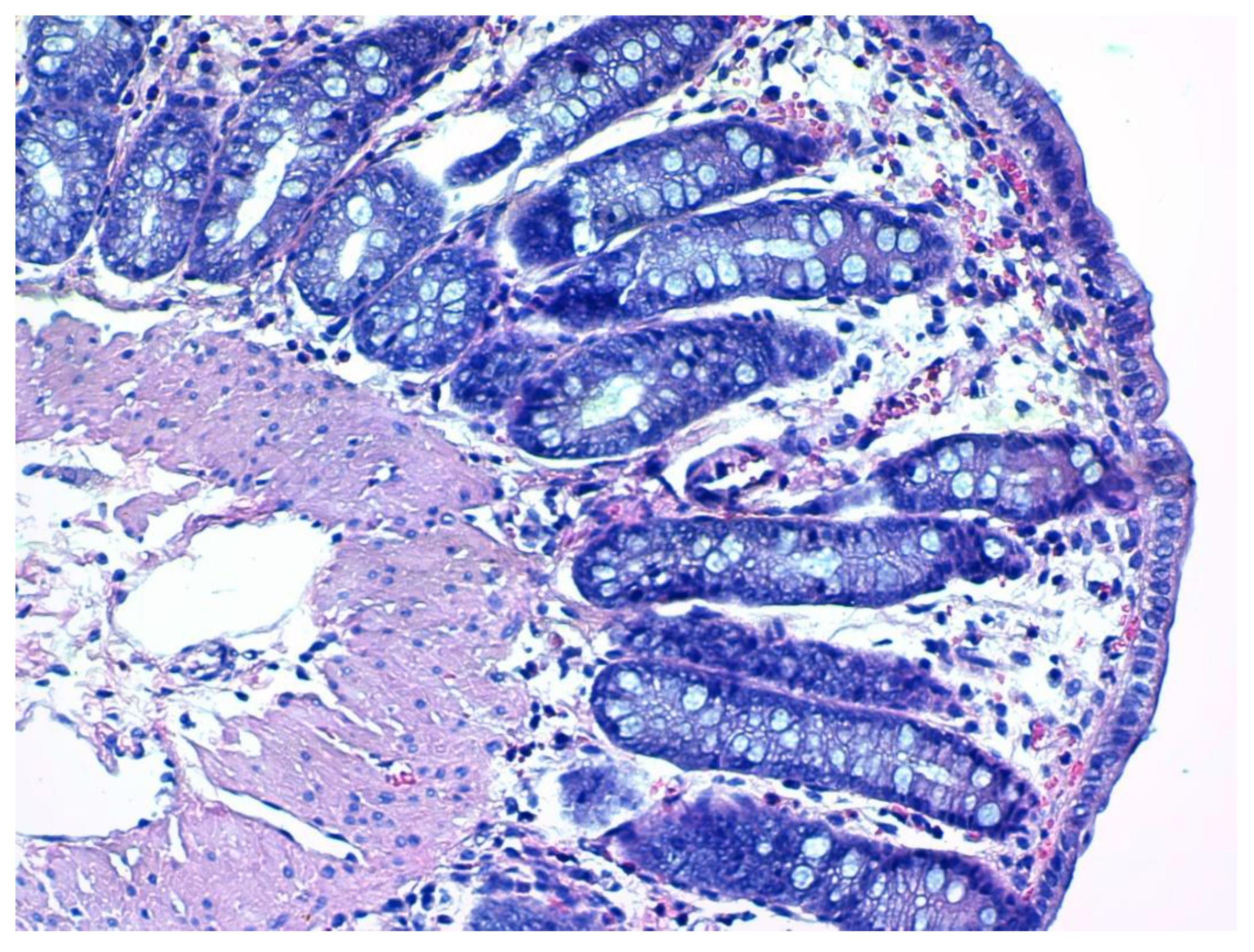
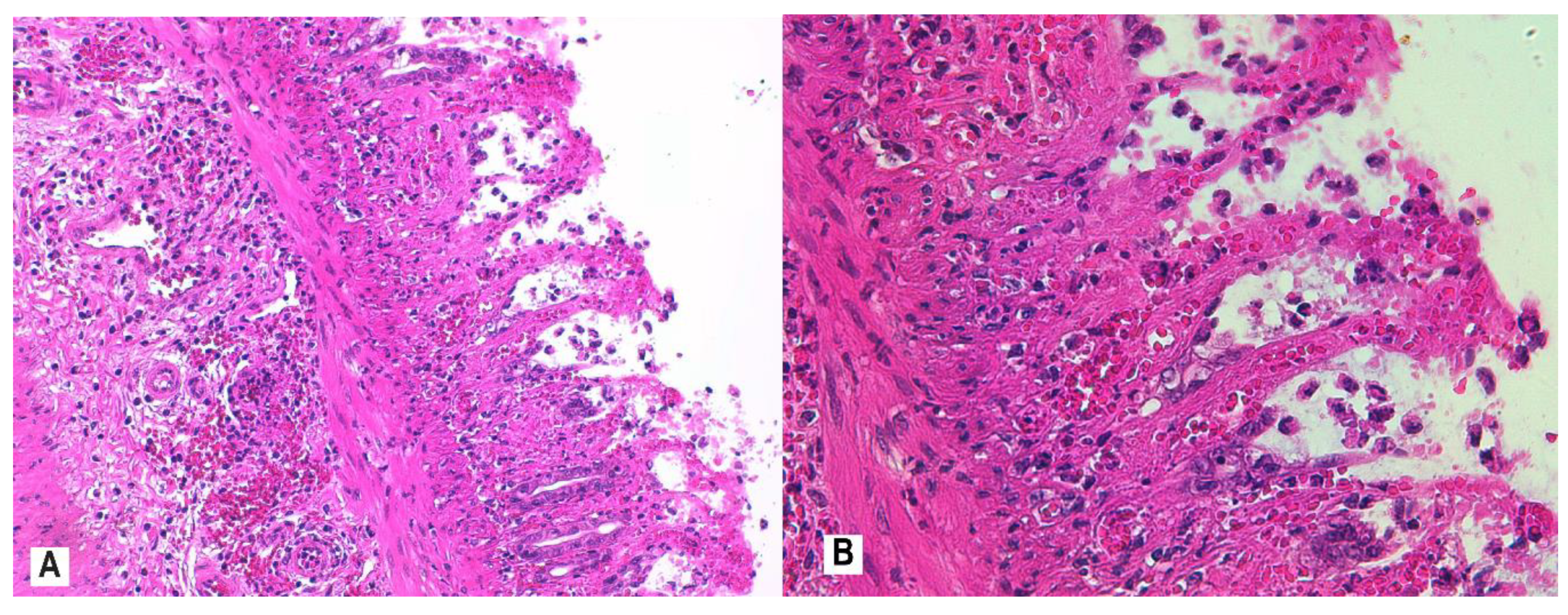
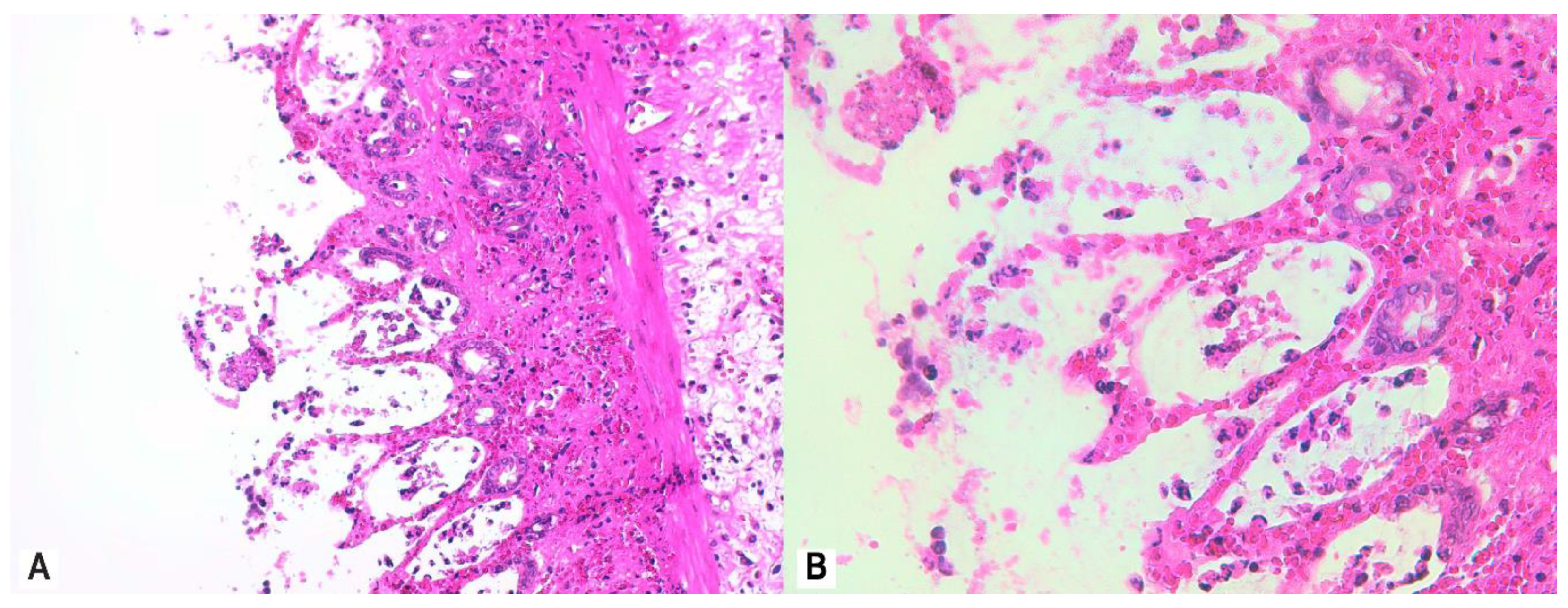
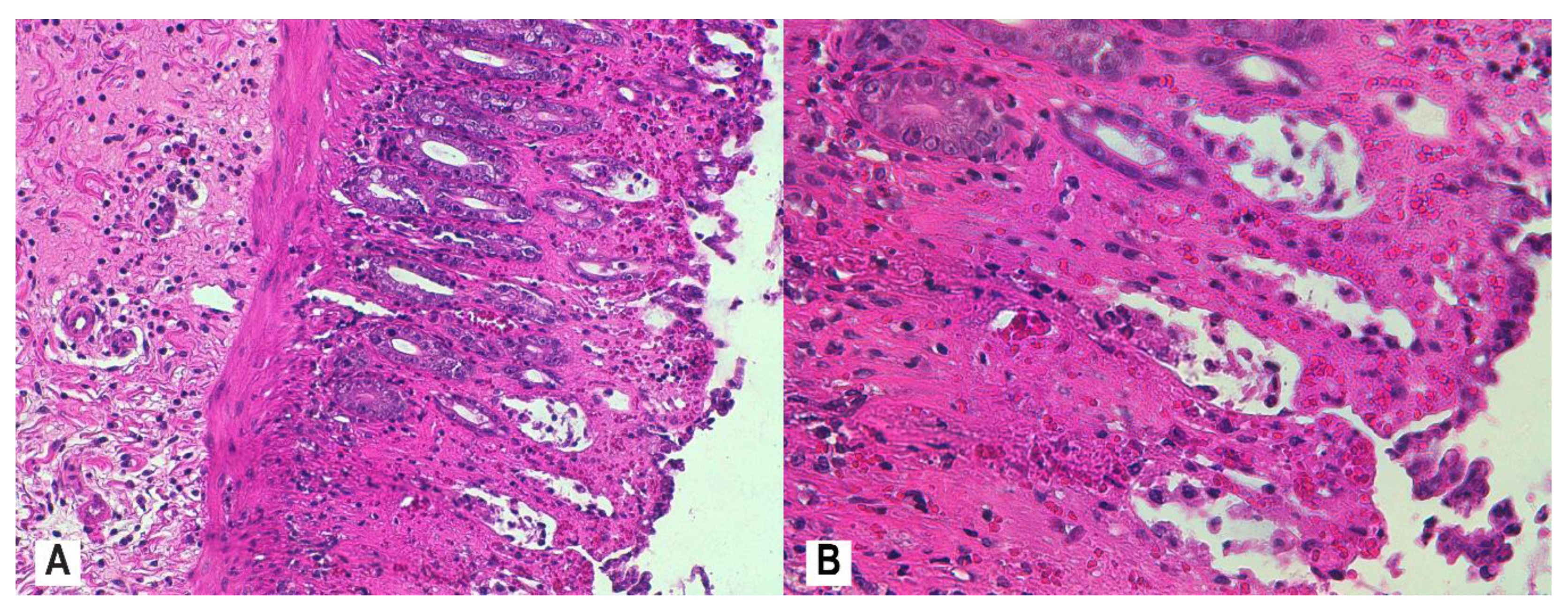
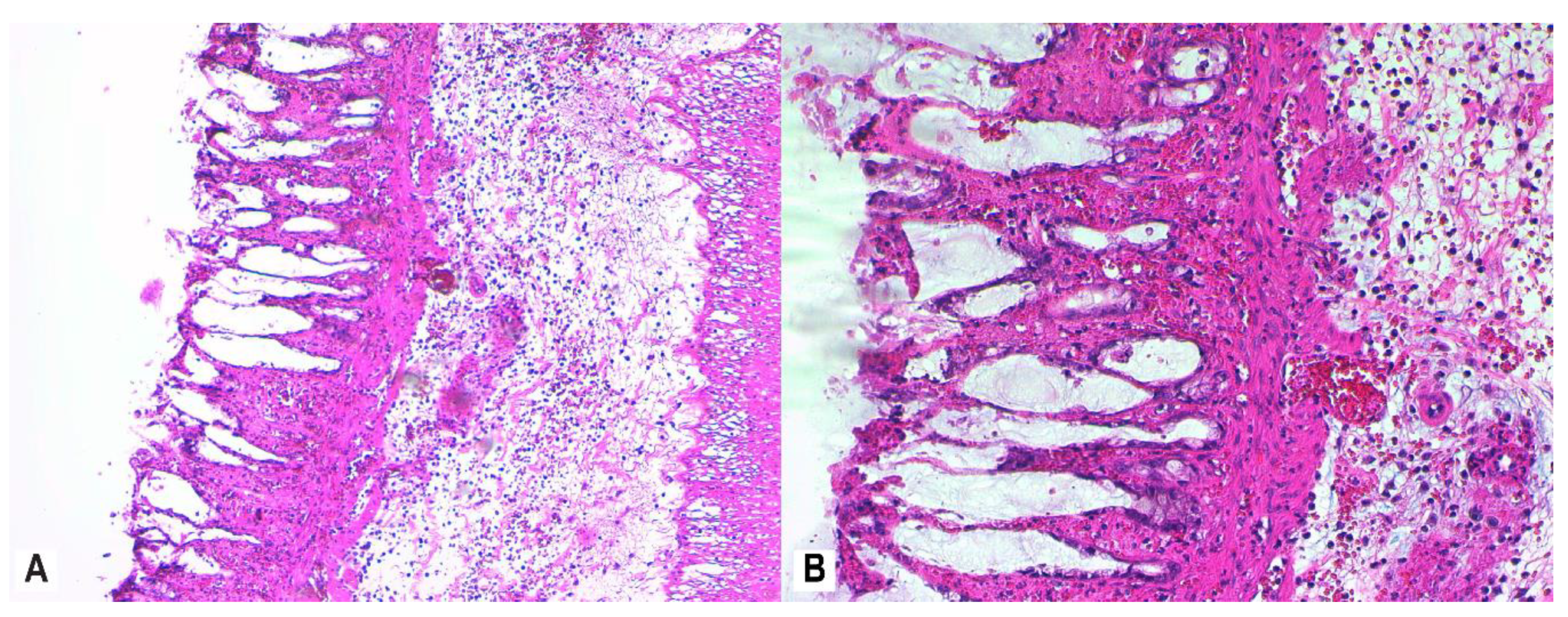
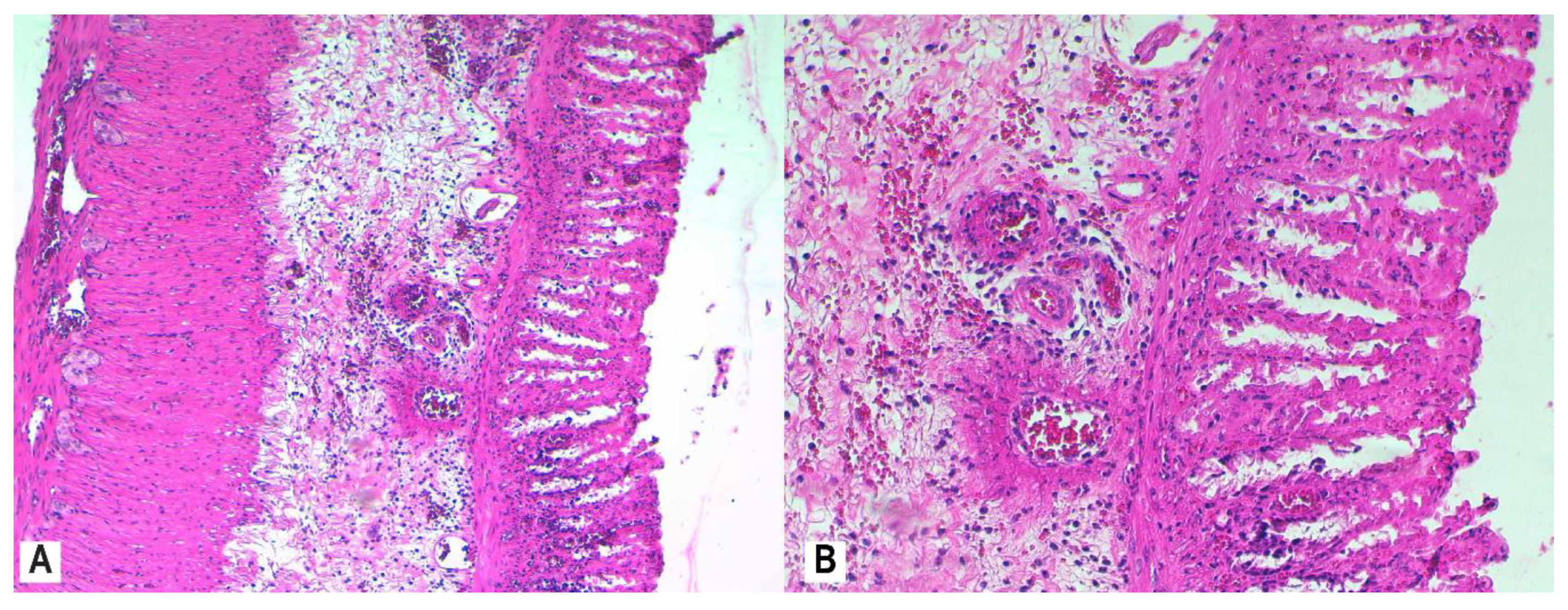
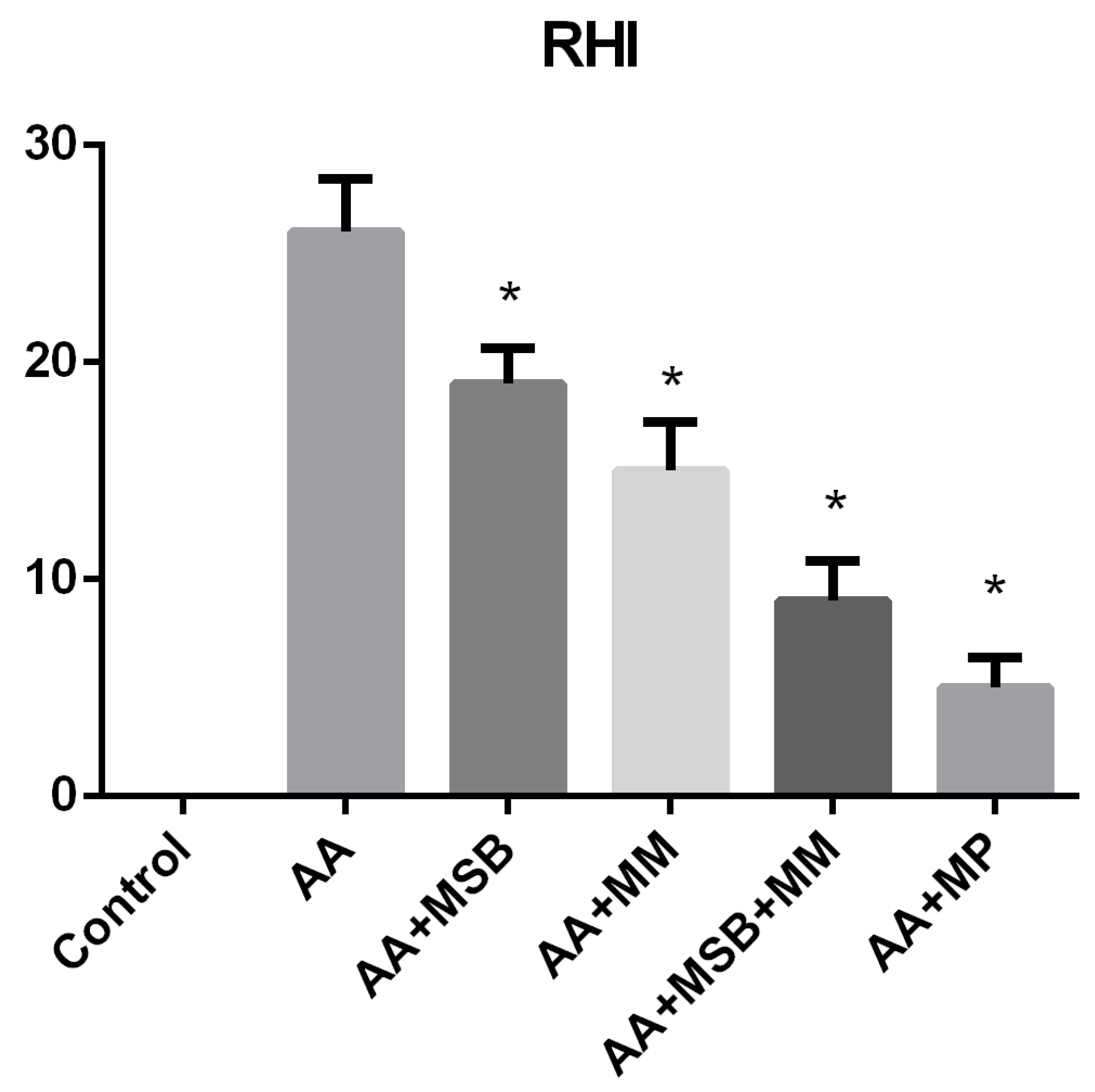
2.7. Histological Assessment and Score
2.8. Statistical Analyses
3. Results
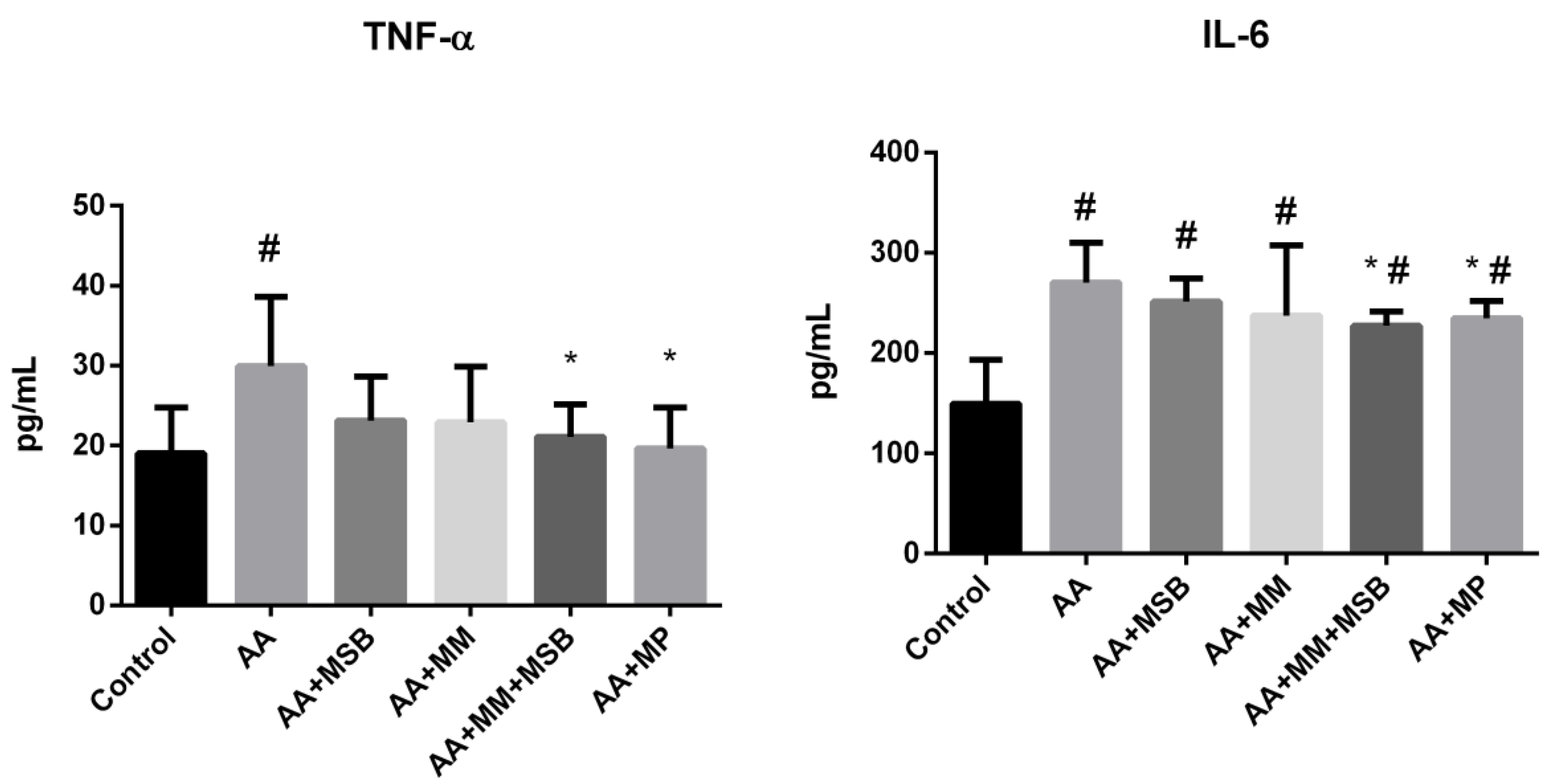
3.1. TNF-α and IL-6 Levels
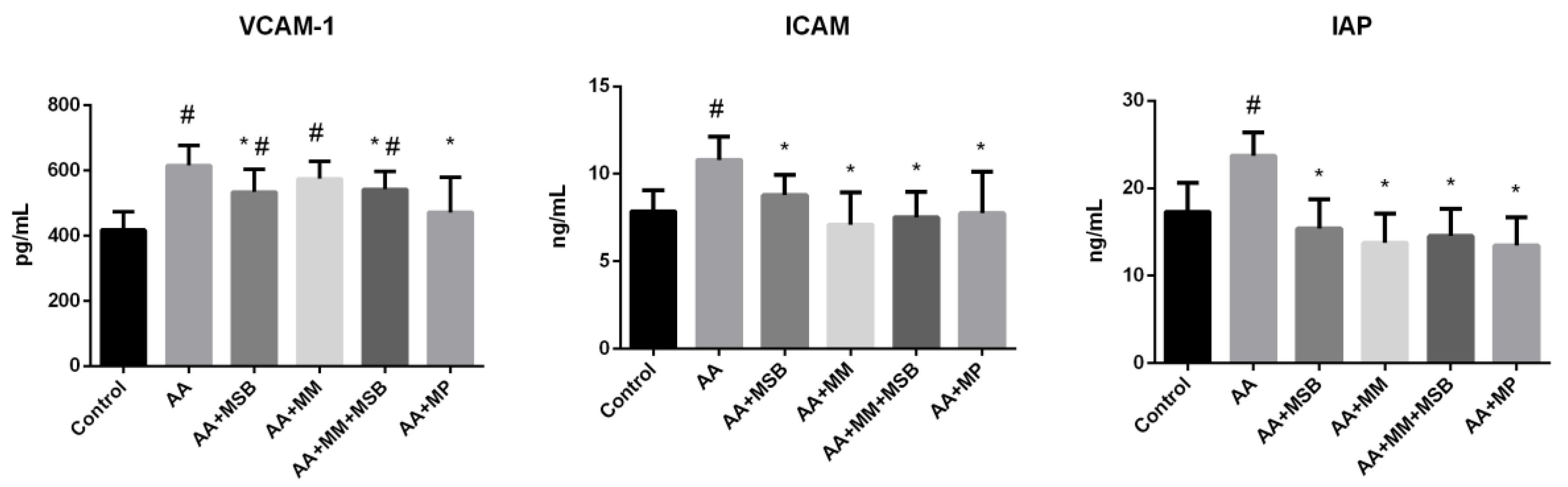
3.2. ICAM-1, VCAM-1, and IAP Levels
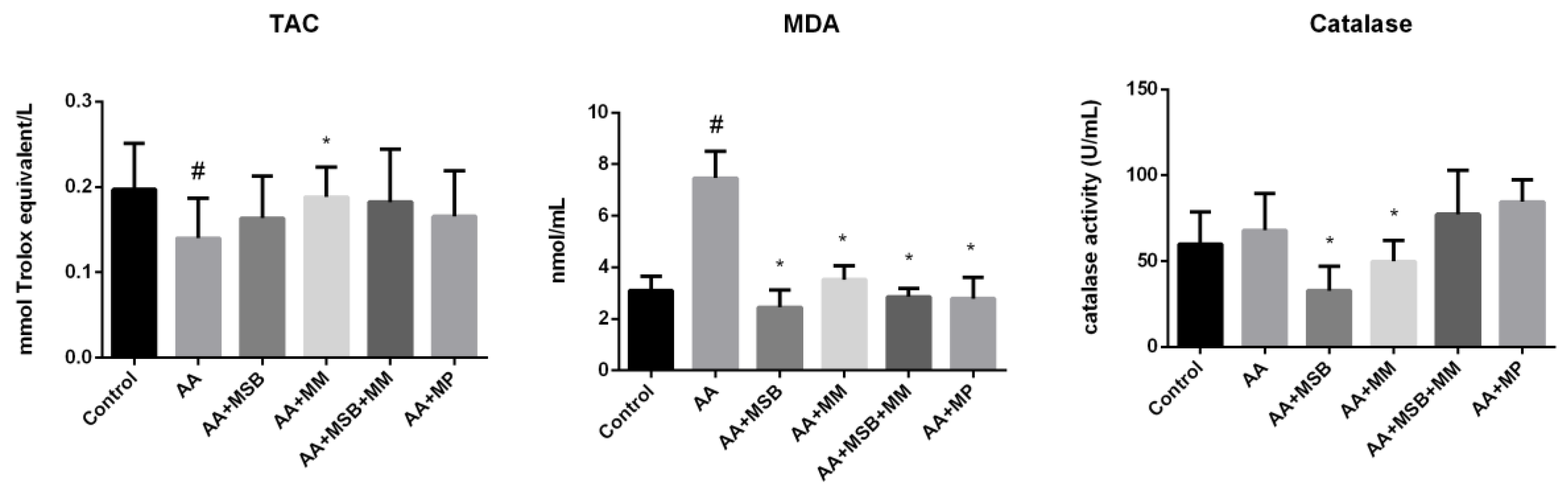
3.3. TAC, MDA, and Catalase
3.4. Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care Clin. Off. Pract. 2017, 44, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetinkaya, A.; Bulbuloglu, E.; Kantarceken, B.; Ciralik, H.; Kurutas, E.B.; Buyukbese, M.A.; Gumusalan, Y. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig. Dis. Sci. 2006, 51, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, A.; Bulbuloglu, E.; Kurutas, E.B.; Ciralik, H.; Kantarceken, B.; Buyukbese, M.A. Beneficial effects of N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J. Exp. Med. 2005, 206, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Reiff, C.; Kelly, D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int. J. Med. Microbiol. 2010, 300, 25–33. [Google Scholar] [CrossRef]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef]
- Grisham, M.B. Oxidants and free radicals in inflammatory bowel disease. Lancet 1994, 344, 859–861. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Kim, S.; Son, M.J.; Son, C.Y.; Kim, J.Y.; Kronbichler, A.; Lee, K.H.; Shin, J. Il Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun. Rev. 2019, 18, 439–454. [Google Scholar] [CrossRef]
- Schäcke, H.; Döcke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The human gut microbiome—A potential controller of wellness and disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkusa, T.; Koido, S. Intestinal microbiota and ulcerative colitis. J. Infect. Chemother. 2015, 21, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Catinean, A.; Neag, M.A.; Mitre, A.O.; Bocsan, C.I.; Buzoianu, A.D. Microbiota and Immune-Mediated Skin Diseases—An Overview. Microorganisms 2019, 7, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catinean, A.; Neag, A.M.; Nita, A.; Buzea, M.; Buzoianu, A.D. Bacillus spp. spores-a promising treatment option for patients with irritable bowel syndrome. Nutrients 2019, 11, 1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchaert, C.; Strubbe, B.; Peeters, H. Fecal microbiota transplantation in ulcerative colitis. Acta Gastroenterol. Belg. 2019, 82, 519–528. [Google Scholar] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.; Albin, A.; Stahl, U. Spore-forming bacteria and their utilisation as probiotics. Benef. Microbes 2012, 3, 67–75. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Henning, A.L.; Bowman, E.M.; Gary, M.A.; Carbajal, K.M. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 117. [Google Scholar] [CrossRef]
- Campbell, J.M.; Polo, J.; Russell, L.E.; Crenshaw, J.D. Review of spray-dried plasma’s impact on intestinal barrier function. Livest. Sci. 2010, 133, 239–241. [Google Scholar] [CrossRef]
- Petschow, B.W.; Burnett, B.; Shaw, A.L.; Weaver, E.M.; Klein, G.L. Serum-derived bovine immunoglobulin/protein isolate: Postulated mechanism of action for management of enteropathy. Clin. Exp. Gastroenterol. 2014, 7, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bosque, A.; Amat, C.; Polo, J.; Campbell, J.M.; Crenshaw, J.; Russell, L.; Moretó, M. Spray-dried animal plasma prevents the effects of Staphylococcus aureus enterotoxin B on intestinal barrier function in weaned rats. J. Nutr. 2006, 136, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liao, S.F. Physiological effects of dietary amino acids on gut health and functions of swine. Front. Vet. Sci. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Mettraux, C.; Moennoz, D.; Godin, J.P.; Vuichoud, J.; Rochat, F.; Breuillé, D.; Obled, C.; Corthésy-Theulaz, I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J. Nutr. 2006, 136, 1558–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peddireddy, V.; Siva Prasad, B.; Gundimeda, S.D.; Penagaluru, P.R.; Mundluru, H.P. Assessment of 8-oxo-7, 8-dihydro-2′-deoxyguanosine and malondialdehyde levels as oxidative stress markers and antioxidant status in non-small cell lung cancer. Biomarkers 2012, 17, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Arif, M.; Arif, F.; Arora, N.; Straub, K.D. Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biol. 2017, 12, 325–339. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Mosli, M.H.; Feagan, B.G.; Zou, G.; Sandborn, W.J.; D’Haens, G.; Khanna, R.; Shackelton, L.M.; Walker, C.W.; Nelson, S.; Vandervoort, M.K.; et al. Development and validation of a histological index for UC. Gut 2017, 66, 50–58. [Google Scholar] [CrossRef]
- Geboes, K.; Riddell, R.; Öst, A.; Jensfelt, B.; Persson, T.; Löfberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Tam, N.K.M.; Uyen, N.Q.; Hong, H.A.; Duc, L.H.; Hoa, T.T.; Serra, C.R.; Henriques, A.O.; Cutting, S.M. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 2006, 188, 2692–2700. [Google Scholar] [CrossRef] [Green Version]
- Owusu, G.; Obiri, D.D.; Ainooson, G.K.; Osafo, N.; Antwi, A.O.; Duduyemi, B.M.; Ansah, C. Acetic Acid-Induced Ulcerative Colitis in Sprague Dawley Rats Is Suppressed by Hydroethanolic Extract of Cordia vignei Leaves through Reduced Serum Levels of TNF-α and IL-6. Int. J. Chronic Dis. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Xu, B.; Shi, F.; Du, M.; Li, Y.; Yu, T.; Chen, L. Protective effect of Methane-Rich saline on acetic Acid-Induced ulcerative colitis via blocking the TLR4/NF-κB/MAPK pathway and promoting IL-10/JAK1/STAT3-Mediated anti-inflammatory response. Oxid. Med. Cell. Longev. 2019, 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharon, P.; Stenson, W.F. Metabolism of Arachidonic Acid in Acetic Acid Colitis in Rats: Similarity to Human Inflammatory Bowel Disease. Gastroenterology 1985, 88, 55–63. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y.L.; Xu, M.; Yu, C.C.; Lian, M.Q.; Tang, Z.Y.; Li, C.X.; Lin, Y. Geniposide ameliorates TNBS-induced experimental colitis in rats via reducing inflammatory cytokine release and restoring impaired intestinal barrier function. Acta Pharmacol. Sin. 2017, 38, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bosque, A.; Miró, L.; Polo, J.; Russell, L.; Campbell, J.; Weaver, E.; Crenshaw, J.; Moretó, M. Dietary Plasma Protein Supplements Prevent the Release of Mucosal Proinflammatory Mediators in Intestinal Inflammation in Rats. J. Nutr. 2010, 140, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship with Mucosal Immunity in Inflammatory Bowel Disease. Cmgh 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neag, M.A.; Catinean, A.; Muntean, D.M.; Pop, M.R.; Bocsan, C.I.; Botan, E.C.; Buzoianu, A.D. Probiotic bacillus spores protect against acetaminophen induced acute liver injury in rats. Nutrients 2020, 12, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafa, H.; Benkhelifa, S.; Aityounes, S.; Saoula, H.; Belhadef, S.; Belkhelfa, M.; Boukercha, A.; Toumi, R.; Soufli, I.; Moralès, O.; et al. All-Trans Retinoic Acid Modulates TLR4/NF- B Signaling Pathway Targeting TNF- α and Nitric Oxide Synthase 2 Expression in Colonic Mucosa during Ulcerative Colitis and Colitis Associated Cancer. Mediat. Inflamm. 2017, 2017, 16. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal relationship between diet-induced gut microbiota changes and diabetes: A novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bosque, A.; Miró, L.; Maijó, M.; Polo, J.; Campbell, J.; Russell, L.; Crenshaw, J.; Weaver, E.; Moretó, M. Dietary intervention with serum-derived bovine immunoglobulins protects barrier function in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, 1012–1018. [Google Scholar] [CrossRef]
- Ma, N.; Ma, X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr. Rev. Food Sci. Food Saf. 2019, 18, 221–242. [Google Scholar] [CrossRef] [Green Version]
- Danese, S.; Fiocchi, C. Endothelial Cell-Immune Cell Interaction in IBD. Dig. Dis. 2016, 34, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.F.S.; Guerra, G.C.B.; Pintado, M.M.E.; Sousa, Y.R.F.; Algieri, F.; Rodriguez-Nogales, A.; Araújo, R.F.; Gálvez, J.; Queiroga, R.D.C.R.E.; Rodriguez-Cabezas, M.E. Intestinal anti-inflammatory effects of goat whey on DNBS-induced colitis in mice. PLoS ONE 2017, 12, e0185382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, S.; Llopis, M.; Antolín, M.; Gironella, M.; Sans, M.; Malagelada, J.R.; Piqué, J.M.; Guarner, F.; Panés, J. Lactobacillus casei prevents the upregulation of ICAM-1 expression and leukocyte recruitment in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Duysburgh, C.; Van den Abbeele, P.; Krishnan, K.; Bayne, T.F.; Marzorati, M. A synbiotic concept containing spore-forming Bacillus strains and a prebiotic fiber blend consistently enhanced metabolic activity by modulation of the gut microbiome in vitro. Int. J. Pharm. X 2019, 1, 100021. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kołodziej, B.; Naruszewicz, M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: The role of NF-κB and PPARα. J. Nutr. Biochem. 2004, 15, 220–228. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef]
- Estaki, M.; DeCoffe, D.; Gibson, D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 2014, 20, 15650–15656. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2020. [Google Scholar] [CrossRef]
- Kováč, J.; Vítězová, M.; Kushkevych, I. Metabolic activity of sulfate-reducing bacteria from rodents with colitis. Open Med. 2018, 13, 344–349. [Google Scholar] [CrossRef]
- Liu, B.; Piao, X.; Guo, L.; Liu, S.; Chai, F.; Gao, L. Ursolic acid protects against ulcerative colitis via anti-inflammatory and antioxidant effects in mice. Mol. Med. Rep. 2016, 13, 4779–4785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Akabawy, G.; El-Sherif, N.M. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2019, 111, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.R.; Yuan, L.X.; Stryker, S.J.; Ratliff, P.; Telford, G.L.; Opara, E.C. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig. Dis. Sci. 2000, 45, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, Z.; Liu, T.; Wan, H.; Qi, D. Protective effect of L-cysteine on biomarkers and peripheral nervous system in streptozotocin-induced diabetic rats. J. Funct. Foods 2019, 61, 103263. [Google Scholar] [CrossRef]
- Li, J.M.; Zhang, X.Y.; Yuan, C.; Miao, L.P.; Yan, H.X.; Dong, X.Y.; Lu, J.J.; Zou, X.T. Effects of dietary L-threonine levels on antioxidant capacity, digestive enzyme activities, and antibody production of Xinyang green-shell laying hens. J. Appl. Poult. Res. 2016, 25, 422–427. [Google Scholar] [CrossRef]
- Zhou, X.; He, L.; Wu, C.; Zhang, Y.; Wu, X.; Yin, Y. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol. Nutr. Food Res. 2017, 61, 1–13. [Google Scholar] [CrossRef]
- Pérez-Bosque, A.; Miró, L.; Maijó, M.; Polo, J.; Campbell, J.M.; Russell, L.; Crenshaw, J.D.; Weaver, E.; Moretó, M. Oral serum-derived bovine immunoglobulin/protein isolate has immunomodulatory effects on the colon of mice that spontaneously develop colitis. PLoS ONE 2016, 11, e0154823. [Google Scholar] [CrossRef]
- Kotowicz, N.; Bhardwaj, R.K.; Ferreira, W.T.; Hong, H.A.; Olender, A.; Ramirez, J.; Cutting, S.M. Safety and probiotic evaluation of two Bacillus strains producing antioxidant compounds. Benef. Microbes 2019, 10, 759–771. [Google Scholar] [CrossRef]
- Dost, T.; Ozkayran, H.; Gokalp, F.; Yenisey, C.; Birincioglu, M. The effect of hypericum perforatum (St. John’s Wort) on experimental colitis in rat. Dig. Dis. Sci. 2009, 54, 1214–1221. [Google Scholar] [CrossRef]
- Zerin, M.; Karakilçik, A.Z.; BItiren, M.; Musa, D.; Özgönül, A.; Selek, Ş.; Nazligül, Y.; Uzunköy, A. Vitamin C modulates oxidative stress-induced colitis in rats. Turk. J. Med. Sci. 2010, 40, 871–879. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; van Duijn, W.; Mieremet-Ooms, M.A.C.; van Hogezand, R.A.; Lamers, C.B.H.W.; Verspaget, H.W. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J. Pathol. 2003, 201, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Vochyánová, Z.; Pokorná, M.; Rotrekl, D.; Smékal, V.; Fictum, P.; Suchý, P.; Gajdziok, J.; Šmejkal, K.; Hošek, J. Prenylated flavonoid morusin protects against TNBS-induced colitis in rats. PLoS ONE 2017, 12, e0182464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasion, V.S.; Burnett, B.P. Survival and digestibility of orally-administered immunoglobulin preparations containing IgG through the gastrointestinal tract in humans. Nutr. J. 2015, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catinean, A.; Neag, M.A.; Krishnan, K.; Muntean, D.M.; Bocsan, C.I.; Pop, R.M.; Mitre, A.O.; Melincovici, C.S.; Buzoianu, A.D. Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis. Nutrients 2020, 12, 3607. https://doi.org/10.3390/nu12123607
Catinean A, Neag MA, Krishnan K, Muntean DM, Bocsan CI, Pop RM, Mitre AO, Melincovici CS, Buzoianu AD. Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis. Nutrients. 2020; 12(12):3607. https://doi.org/10.3390/nu12123607
Chicago/Turabian StyleCatinean, Adrian, Maria Adriana Neag, Kiran Krishnan, Dana Maria Muntean, Corina Ioana Bocsan, Raluca Maria Pop, Andrei Otto Mitre, Carmen Stanca Melincovici, and Anca Dana Buzoianu. 2020. "Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis" Nutrients 12, no. 12: 3607. https://doi.org/10.3390/nu12123607
APA StyleCatinean, A., Neag, M. A., Krishnan, K., Muntean, D. M., Bocsan, C. I., Pop, R. M., Mitre, A. O., Melincovici, C. S., & Buzoianu, A. D. (2020). Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis. Nutrients, 12(12), 3607. https://doi.org/10.3390/nu12123607







