Fighting Sarcopenia in Ageing European Adults: The Importance of the Amount and Source of Dietary Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Dietary Intake
2.3. Anthropometry and Body Composition
2.4. Sarcopenia Risk Score
2.5. Adherence to Physical Activity Guidelines
2.6. Assessment of Metabolic Risk
2.7. Statistical Analysis
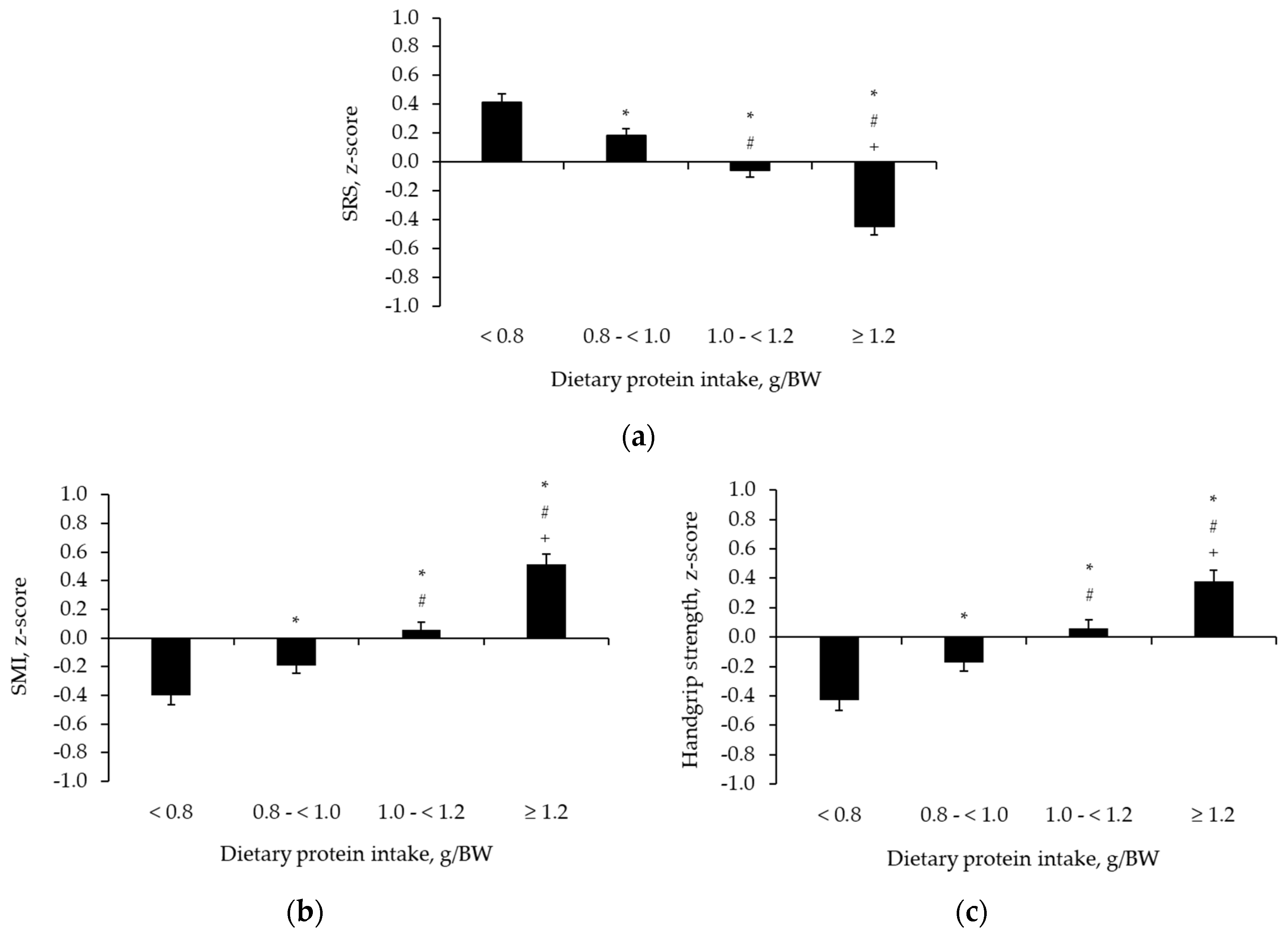
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dodds, R.M.; Roberts, H.C.; Cooper, C.; Sayer, A.A. The epidemiology of sarcopenia. J. Clin. Densitom. 2015, 18, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.; Cooper, C.; Aihie Sayer, A. Nutrition and sarcopenia: A review of the evidence and implications for preventive strategies. J. Aging Res. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Kirk, B.; Mooney, K.; Amirabdollahian, F.; Khaiyat, O. Exercise and dietary-protein as a countermeasure to skeletal muscle weakness: Liverpool hope university—Sarcopenia aging trial (LHU-SAT). Front. Physiol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ortolá, R.; García-Esquinas, E.; García-Varela, G.; Struijk, E.A.; Rodríguez-Artalejo, F.; López-García, E. Influence of changes in diet quality on unhealthy aging: The Seniors-ENRICA cohort. Am. J. Med. 2019, 132, 1091–1102.e9. [Google Scholar] [CrossRef]
- Isanejad, M.; Mursu, J.; Sirola, J.; Kröger, H.; Rikkonen, T.; Tuppurainen, M.; Erkkilä, A.T. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br. J. Nutr. 2016, 115, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Júnior, H.; Milano-Teixeira, L.; Rodrigues, B.; Bacurau, R.; Marzetti, E.; Uchida, M. Relative protein intake and physical function in older adults: A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 1330. [Google Scholar] [CrossRef] [Green Version]
- Farsijani, S.; Morais, J.A.; Payette, H.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K.; Chevalier, S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am. J. Clin. Nutr. 2016, 104, 694–703. [Google Scholar] [CrossRef]
- Gregorio, L.; Brindisi, J.; Kleppinger, A.; Sullivan, R.; Mangano, K.M.; Bihuniak, J.D.; Kenny, A.M.; Kerstetter, J.E.; Insogn, K.L. Adequate dietary protein is associated with better physical performance among post-menopausal women 60–90 years. J. Nutr. Health Aging 2014, 18, 155–160. [Google Scholar] [CrossRef] [Green Version]
- McLean, R.R.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; Sahni, S. Dietary protein intake is protective against loss of grip strength among older adults in the framingham offspring cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The health, aging, and body composition (Health ABC) study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Gaffney-Stomberg, E.; Insogna, K.L.; Rodriguez, N.R.; Kerstetter, J.E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geriatr. Soc. 2009, 57, 1073–1079. [Google Scholar] [CrossRef]
- Takae, R.; Hatamoto, Y.; Yasukata, J.; Kose, Y.; Komiyama, T.; Ikenaga, M.; Yoshimura, E.; Yamada, Y.; Ebine, N.; Higaki, Y.; et al. Physical activity and/or high protein intake maintains fat-free mass in older people with mild disability; the fukuoka island city study: A cross-sectional study. Nutrients 2019, 11, 2595. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Junior, H.J.; Calvani, R.; Gonçalves, I.O.; Rodrigues, B.; Picca, A.; Landi, F.; Bernabei, R.; Uchida, M.C.; Marzetti, E. High relative consumption of vegetable protein is associated with faster walking speed in well-functioning older adults. Aging Clin. Exp. Res. 2019, 31, 837–844. [Google Scholar] [CrossRef]
- Nilsson, A.; Montiel Rojas, D.; Kadi, F. Impact of meeting different guidelines for protein intake on muscle mass and physical function in physically active older women. Nutrients 2018, 10, 1156. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, K.C.H.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein intake and muscle health in old age: From biological plausibility to clinical evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Short, K.R.; Campbell, W.W.; Volpi, E.; Wolfe, R.R. Role of dietary protein in the sarcopenia of aging. Am. J. Clin. Nutr. 2008, 87, 1562S–1566S. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Beasley, J.M.; Deierlein, A.L.; Morland, K.B.; Granieri, E.C.; Spark, A. Is meeting the recommended dietary allowance (RDA) for protein related to body composition among older adults? Results from the Cardiovascular Health of Seniors and Built Environment Study. J. Nutr. Health Aging 2016, 20, 790–796. [Google Scholar] [CrossRef] [Green Version]
- Rafii, M.; Chapman, K.; Elango, R.; Campbell, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G. Dietary Protein requirement of men >65 years old determined by the indicator amino acid oxidation technique is higher than the current estimated average requirement. J. Nutr. 2015, 146, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, S.H.M.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef]
- Phillips, S.M. Nutrient-rich meat proteins in offsetting age-related muscle loss. Meat Sci. 2012, 92, 174–178. [Google Scholar] [CrossRef]
- Bradlee, M.L.; Mustafa, J.; Singer, M.R.; Moore, L.L. High-protein foods and physical activity protect against age-related muscle loss and functional decline. J. Gerontol. Ser. A 2018, 73, 88–94. [Google Scholar] [CrossRef]
- Gazzani, D.; Zamboni, F.; Spelta, F.; Ferrari, P.; Mattioli, V.; Cazzoletti, L.; Zanolin, E.; Tardivo, S.; Ferrari, M. Vegetable but not animal protein intake is associated to a better physical performance: A study on a general population sample of adults. Food Nutr. Res. 2019, 63, 1–7. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J.; Kwok, T. Associations of dietary protein intake on subsequent decline in muscle mass and physical functions over four years in ambulant older Chinese people. J. Nutr. Health Aging 2014, 18, 171–177. [Google Scholar] [CrossRef]
- Behrouzi, P.; Grootswagers, P.; Keizer, P.L.C.; Smeets, E.T.H.C.; Feskens, E.J.M.; de Groot, L.C.P.G.M.; van Eeuwijk, F.A. Dietary intakes of vegetable protein, folate, and vitamins B-6 and B-12 are partially correlated with physical functioning of dutch older adults using copula graphical models. J. Nutr. 2020, 150, 634–643. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Yang, K.-C.; Chang, H.-H.; Lee, L.-T.; Lu, C.-W.; Huang, K.-C. The Association between total protein and vegetable protein intake and low muscle mass among the community-dwelling elderly population in northern Taiwan. Nutrients 2016, 8, 373. [Google Scholar] [CrossRef]
- Sahni, S.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; McLean, R.R. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J. Nutr. 2015, 145, 1569–1575. [Google Scholar] [CrossRef]
- Berendsen, A.; Santoro, A.; Pini, E.; Cevenini, E.; Ostan, R.; Pietruszka, B.; Rolf, K.; Cano, N.; Caille, A.; Lyon-Belgy, N.; et al. Reprint of: A parallel randomized trial on the effect of a healthful diet on inflammageing and its consequences in European elderly people: Design of the NU-AGE dietary intervention study. Mech. Ageing Dev. 2014, 136–137, 14–21. [Google Scholar] [CrossRef]
- Santoro, A.; Pini, E.; Scurti, M.; Palmas, G.; Berendsen, A.; Brzozowska, A.; Pietruszka, B.; Szczecinska, A.; Cano, N.; Meunier, N.; et al. Combating inflammaging through a Mediterranean whole diet approach: The NU-AGE project’s conceptual framework and design. Mech. Ageing Dev. 2014, 136–137, 3–13. [Google Scholar] [CrossRef]
- Ostan, R.; Guidarelli, G.; Giampieri, E.; Lanzarini, C.; Berendsen, A.A.M.; Januszko, O.; Jennings, A.; Lyon, N.; Caumon, E.; Gillings, R.; et al. Cross-sectional analysis of the correlation between daily nutrient intake assessed by 7-day food records and biomarkers of dietary intake among participants of the NU-AGE study. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Berendsen, A.; van de Rest, O.; Feskens, E.; Santoro, A.; Ostan, R.; Pietruszka, B.; Brzozowska, A.; Stelmaszczyk-Kusz, A.; Jennings, A.; Gillings, R.; et al. Changes in dietary intake and adherence to the NU-AGE diet following a one-year dietary intervention among European older adults—Results of the NU-AGE Randomized Trial. Nutrients 2018, 10, 1905. [Google Scholar] [CrossRef] [Green Version]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organ Technical Report Series; WHO: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar]
- Santoro, A.; Guidarelli, G.; Ostan, R.; Giampieri, E.; Fabbri, C.; Bertarelli, C.; Nicoletti, C.; Kadi, F.; de Groot, L.C.P.G.M.; Feskens, E.; et al. Gender-specific association of body composition with inflammatory and adipose-related markers in healthy elderly Europeans from the NU-AGE study. Eur. Radiol. 2019, 29, 4968–4979. [Google Scholar] [CrossRef] [Green Version]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The role of DXA in sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef]
- Santoro, A.; Bazzocchi, A.; Guidarelli, G.; Ostan, R.; Giampieri, E.; Mercatelli, D.; Scurti, M.; Berendsen, A.; Surala, O.; Jennings, A.; et al. A cross-sectional analysis of body composition among healthy elderly from the European NU-AGE study: Sex and country specific features. Front. Physiol. 2018, 9, 1693. [Google Scholar] [CrossRef]
- Montiel-Rojas, D.; Santoro, A.; Nilsson, A.; Franceschi, C.; Capri, M.; Bazzocchi, A.; Battista, G.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.A.M.; et al. Beneficial role of replacing dietary saturated fatty acids with polyunsaturated fatty acids in the prevention of sarcopenia: Findings from the NU-AGE Cohort. Nutrients 2020, 12, 3079. [Google Scholar] [CrossRef]
- Nilsson, A.; Wåhlin-Larsson, B.; Kadi, F. Physical activity and not sedentary time per se influences on clustered metabolic risk in elderly community-dwelling women. PLoS ONE 2017, 12, e0175496. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sport. Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Service. Physical Activity Guidelines Advisory Committee Scientific Report 2018; U.S. Department of Health and Human Service: Washington, DC, USA, 2018.
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Rojas, D.; Nilsson, A.; Santoro, A.; Franceschi, C.; Bazzocchi, A.; Battista, G.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.; Pietruszka, B.; et al. Dietary fibre may mitigate sarcopenia risk: Findings from the NU-AGE cohort of older European adults. Nutrients 2020, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Skilton, M.R.; Laville, M.; Cust, A.E.; Moulin, P.; Bonnet, F. The association between dietary macronutrient intake and the prevalence of the metabolic syndrome. Br. J. Nutr. 2008, 100, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef]
- Millward, D.J. The nutritional value of plant-based diets in relation to human amino acid and protein requirements. Proc. Nutr. Soc. 1999, 58, 249–260. [Google Scholar] [CrossRef]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Dietary protein content for an optimal diet: A clinical view. J. Cachexia Sarcopenia Muscle 2017, 8, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Volek, J.S.; Volk, B.M.; Gómez, A.L.; Kunces, L.J.; Kupchak, B.R.; Freidenreich, D.J.; Aristizabal, J.C.; Saenz, C.; Dunn-Lewis, C.; Ballard, K.D.; et al. Whey protein supplementation during resistance training augments lean body mass. J. Am. Coll. Nutr. 2013, 32, 122–135. [Google Scholar] [CrossRef]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. (Lond.) 2012, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorissen, S.H.M.; Horstman, A.M.H.; Franssen, R.; Crombag, J.J.R.; Langer, H.; Bierau, J.; Respondek, F.; van Loon, L.J.C. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, S.B.; Tarnopolsky, M.A.; MacDonald, M.J.; MacDonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant-versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Cesari, M.; Uchida, M.C.; Calvani, R. Protein intake and frailty: A matter of quantity, quality, and timing. Nutrients 2020, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- de Gavelle, E.; Huneau, J.F.; Bianchi, C.M.; Verger, E.O.; Mariotti, F. Protein adequacy is primarily a matter of protein quantity, not quality: Modeling an increase in plant: Animal protein ratio in French adults. Nutrients 2017, 9, 1333. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, G.M.; Verger, E.O.; Huneau, J.-F.; Carpentier, F.; Dubuisson, C.; Mariotti, F. Plant and animal protein intakes are differently associated with nutrient adequacy of the diet of french adults. J. Nutr. 2013, 143, 1466–1473. [Google Scholar] [CrossRef]
- Halkjær, J.; Olsen, A.; Overvad, K.; Jakobsen, M.U.; Boeing, H.; Buijsse, B.; Palli, D.; Tognon, G.; Du, H.; van der, A.D.L.; et al. Intake of total, animal and plant protein and subsequent changes in weight or waist circumference in European men and women: The Diogenes project. Int. J. Obes. 2011, 35, 1104–1113. [Google Scholar] [CrossRef] [Green Version]
- Ganapathy, A.; Nieves, J.W. Nutrition and sarcopenia—What do we know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Isanejad, M.; Mursu, J.; Sirola, J.; Kröger, H.; Rikkonen, T.; Tuppurainen, M.; Erkkilä, A.T. Association of protein intake with the change of lean mass among elderly women: The Osteoporosis Risk Factor and Prevention—Fracture Prevention Study (OSTPRE-FPS). J. Nutr. Sci. 2015, 4, e41. [Google Scholar] [CrossRef] [Green Version]
- Haub, M.D.; Wells, A.M.; Tarnopolsky, M.A.; Campbell, W.W. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am. J. Clin. Nutr. 2002, 76, 511–517. [Google Scholar] [CrossRef]
- Beasley, J.M.; Wertheim, B.C.; LaCroix, A.Z.; Prentice, R.L.; Neuhouser, M.L.; Tinker, L.F.; Kritchevsky, S.; Shikany, J.M.; Eaton, C.; Chen, Z.; et al. Biomarker-calibrated protein intake and physical function in the women’s health initiative. J. Am. Geriatr. Soc. 2013, 61, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Fang, A.P.; Ma, W.J.; Wu, S.L.; Li, C.L.; Chen, Y.M.; Zhu, H.L. Amount rather than animal vs. plant protein intake is associated with skeletal muscle mass in community-dwelling middle-aged and older Chinese adults: Results from the Guangzhou nutrition and health study. J. Acad. Nutr. Diet. 2019, 119, 1501–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Protein Intake a | |||||
|---|---|---|---|---|---|
| <0.8 | 0.8–<1.0 | 1.0–<1.2 | ≥1.2 | ||
| n | 205 | 296 | 279 | 206 | |
| Basic Characteristics | |||||
| Age, y | 71.7 ± 4.0 | 71.1 ± 4.1 | 70.9 ± 3.8 | 70.4 ± 3.9 * | |
| Weight, kg | 83.3 ± 13.8 | 75.6 ± 11.9 * | 70.9 ± 11.7 * # | 66.9 ± 11.1 * # + | |
| Height, cm | 166.7 ± 9.4 | 165.9 ± 8.8 | 164.5 ± 9.5 | 165 ± 9.2 | |
| Smoking, % never | 46.1 | 45.9 | 55.6 | 58.3 * | |
| Medication, % yes | 85.3 | 82.2 | 70.6 * # | 71.8 * # | |
| PA. guidelines, % yes | 44.1 | 51.0 | 55.2 | 67 * # | |
| MetS, % yes | 63.7 | 45.6 * | 32.3 * # | 27.2 * # | |
| Protein Intake a | ||||
|---|---|---|---|---|
| <0.8 | 0.8–<1.0 | 1.0–<1.2 | ≥1.2 | |
| n | 205 | 296 | 279 | 206 |
| Total energy intake, Kcal | 1510 ± 327 | 1718 ± 310 * | 1878 ± 373 * # | 2143 ± 440 * # + |
| Carbohydrates, E% | 49.5 ± 7.1 | 49.3 ± 6.6 | 48.5 ± 6.2 | 47.9 ± 7.4 |
| Fat, E% | 31.1 ± 5.2 | 30.9 ± 5.4 | 31.2 ± 5.3 | 31.4 ± 6.0 |
| Protein, E% | 15.7 ± 2.6 | 16.3 ± 2.4 | 17.0 ± 2.6 * # | 17.5 ± 2.7 * # |
| Plant Protein, % total protein | 37.4 ± 9.9 | 36.5 ± 8.8 | 36.7 ± 9.6 | 36.7 ± 9.6 |
| Animal Protein, % total protein | 62.6 ± 9.9 | 63.5 ± 8.8 | 63.3 ± 9.6 | 63.3 ± 9.6 |
| Sarcopenia Risk Score | |||
|---|---|---|---|
| Model | β-Coeff. | 95% CI | p-Value |
| Whole Population | |||
| Plant Protein | −0.249 | −0.303 to −0.196 | <0.001 |
| <0.8 g/BW | |||
| Plant Protein | −0.323 | −0.498 to −0.149 | <0.001 |
| ≥0.8–<1.0 g/BW | |||
| Plant Protein | −0.198 | −0.318 to −0.078 | 0.001 |
| ≥1.0–<1.2 g/BW | |||
| Plant Protein | −0.276 | −0.364 to −0.189 | <0.001 |
| ≥1.2 g/BW | |||
| Plant Protein | −0.234 | −0.335 to −0.133 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montiel-Rojas, D.; Nilsson, A.; Santoro, A.; Bazzocchi, A.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.A.M.; Madej, D.; Kaluza, J.; Pietruszka, B.; et al. Fighting Sarcopenia in Ageing European Adults: The Importance of the Amount and Source of Dietary Proteins. Nutrients 2020, 12, 3601. https://doi.org/10.3390/nu12123601
Montiel-Rojas D, Nilsson A, Santoro A, Bazzocchi A, de Groot LCPGM, Feskens EJM, Berendsen AAM, Madej D, Kaluza J, Pietruszka B, et al. Fighting Sarcopenia in Ageing European Adults: The Importance of the Amount and Source of Dietary Proteins. Nutrients. 2020; 12(12):3601. https://doi.org/10.3390/nu12123601
Chicago/Turabian StyleMontiel-Rojas, Diego, Andreas Nilsson, Aurelia Santoro, Alberto Bazzocchi, Lisette C. P. G. M. de Groot, Edith J. M. Feskens, Agnes A. M. Berendsen, Dawid Madej, Joanna Kaluza, Barbara Pietruszka, and et al. 2020. "Fighting Sarcopenia in Ageing European Adults: The Importance of the Amount and Source of Dietary Proteins" Nutrients 12, no. 12: 3601. https://doi.org/10.3390/nu12123601
APA StyleMontiel-Rojas, D., Nilsson, A., Santoro, A., Bazzocchi, A., de Groot, L. C. P. G. M., Feskens, E. J. M., Berendsen, A. A. M., Madej, D., Kaluza, J., Pietruszka, B., Jennings, A., Fairweather-Tait, S., Battista, G., Capri, M., Franceschi, C., & Kadi, F. (2020). Fighting Sarcopenia in Ageing European Adults: The Importance of the Amount and Source of Dietary Proteins. Nutrients, 12(12), 3601. https://doi.org/10.3390/nu12123601










