High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Cell Viability Assay
- εOX = molar extinction coefficient of Alamar blue’s oxidized form (BLUE)
- A = absorbance of test wells
- A° = absorbance of positive growth control well (cells without tested compounds)
- λ1 = 570 nm and λ2 = 600 nm.
2.3. Cell Morphology
2.4. Cell Migration
2.5. Chorioallantoic Membrane Assay (CAM)
2.6. Normal Angiogenesis Assessment of the Chorioallantoic Membrane
2.7. Hen’s Egg Test on Chorioallantoic Membrane (HET-CAM) Assay
2.8. Evaluation of Anti-Irritative Potential
2.9. Statistical Analysis
3. Results
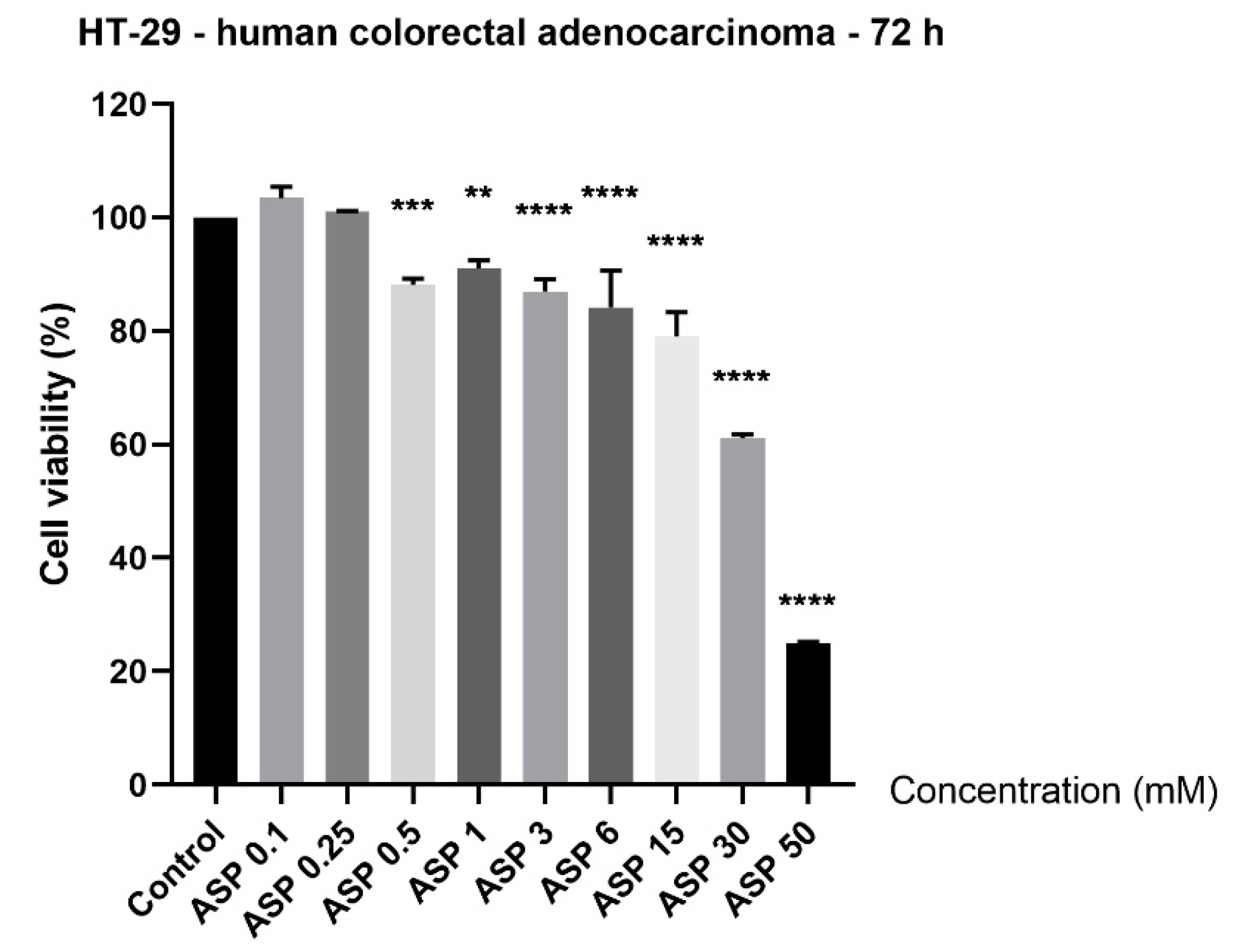
3.1. Aspartame Induced Cytotoxicity in a Concentration-Dependent Manner
3.2. High Concentrations of Aspartame Changed HT-29 Cells’ Morphology and Shape
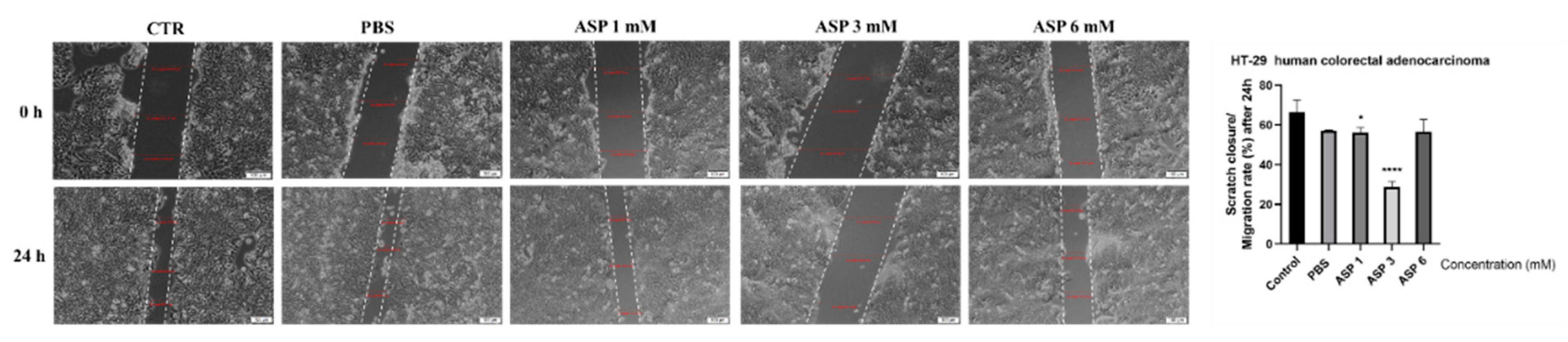
3.3. Cell Migration
3.4. Angiogenic Effect
3.5. Irritant Potential
3.6. Anti-Irritant Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pepin, A.; Stanhope, K.L.; Imbeault, P. Are Fruit Juices Healthier Than Sugar-Sweetened Beverages? A Review. Nutrients 2019, 11, 1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W.L. Sugar addiction: Is it real? A narrative review. Br. J. Sports Med. 2018, 52, 910–913. [Google Scholar] [CrossRef]
- Tandel, K.R. Sugar substitutes: Health controversy over perceived benefits. J. Pharmacol. Pharmacother. 2011, 2, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harpaz, D.; Yeo, L.P.; Cecchini, F.; Koon, T.H.P.; Kushmaro, A.; Tok, A.I.Y.; Marks, R.S.; Eltzov, E. Measuring Artificial Sweeteners Toxicity Using a Bioluminescent Bacterial Panel. Molecules 2018, 23, 2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çadirci, K.; Özdemir Tozlu, Ö.; Türkez, H.; Mardinoğlu, A. The in vitro cytotoxic, genotoxic, and oxidative damage potentials of the oral artificial sweetener aspartame on cultured human blood cells. Turk. J. Med. Sci. 2020, 50, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Pretorius, E. Revisiting the safety of aspartame. Nutr. Rev. 2017, 75, 718–730. [Google Scholar] [CrossRef] [Green Version]
- Haighton, L.; Roberts, A.; Jonaitis, T.; Lynch, B. Evaluation of aspartame cancer epidemiology studies based on quality appraisal criteria. Regul. Toxicol. Pharmacol. 2019, 103, 352–362. [Google Scholar] [CrossRef]
- Haighton, L.; Roberts, A.; Walters, B.; Lynch, B. Systematic review and evaluation of aspartame carcinogenicity bioassays using quality criteria. Regul. Toxicol. Pharmacol. 2019, 103, 332–344. [Google Scholar] [CrossRef]
- Pandurangan, M.; Enkhtaivan, G.; Kim, D.H. Cytotoxic effects of aspartame on human cervical carcinoma cells. Toxicol. Res. 2015, 5, 45–52. [Google Scholar] [CrossRef]
- Costea, T.; Hudiță, A.; Ciolac, O.A.; Gălățeanu, B.; Ginghină, O.; Costache, M.; Ganea, C.; Mocanu, M.M. Chemoprevention of Colorectal Cancer by Dietary Compounds. Int. J. Mol. Sci. 2018, 19, 3787. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Blanco, S.; Fernández, J.; Gutiérrez-Del-Río, I.; Villar, C.J.; Lombó, F. New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Front. Pharmacol. 2017, 8, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeber, A.; Gunsilius, E.; Gastl, G.; Pircher, A. Anti-Angiogenics: Their Value in Colorectal Cancer Therapy. Oncol. Res. Treat. 2018, 41, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yu, J. The association of diet, gut microbiota and colorectal cancer: What we eat may imply what we get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef] [Green Version]
- Pietrzyk, L. Food properties and dietary habits in colorectal cancer prevention and development. Int. J. Food Prop. 2017, 20, 2323–2343. [Google Scholar] [CrossRef] [Green Version]
- Goljat, M.; Niewiadomski, P.; Lazarek, M.; Flegiel, E.; Graczykowska, K.; Denkiewicz, M.; Rozmarynowicz, E.; Walczak, M.; Cybulska, M.; Husejko, J.; et al. The impact of artificial sweeteners on the risk and course of large intestinal adenocarcinoma in the elderly. J. Educ. Health Sport 2019, 9, 992–1008. [Google Scholar] [CrossRef]
- Van Eyk, A.D. The effect of five artificial sweeteners on Caco-2, HT-29 and HEK293 cells. Drug Chem. Toxicol. 2015, 38, 318–327. [Google Scholar] [CrossRef]
- Takayama, S.; Renwick, A.G.; Johansson, S.L.; Thorgeirsson, U.P.; Tsutsumi, M.; Dalgard, D.W.; Sieber, S.M. Long-term toxicity and carcinogenicity study of cyclamate in nonhuman primates. Toxicol. Sci. 2000, 53, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Guercio, B.J.; Zhang, S.; Niedzwiecki, D.; Li, Y.; Babic, A.; Morales-Oyarvide, V.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; et al. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). PLoS ONE 2018, 13, e0199244. [Google Scholar] [CrossRef]
- Yesildal, F.; Aydin, F.N.; Deveci, S.; Tekin, S.; Aydin, I.; Mammadov, R.; Fermanli, O.; Avcu, F.; Acikel, C.H. Aspartame induces angiogenesis in vitro and in vivo models. Hum. Exp. Toxicol. 2015, 34, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Soica, C.; Oprean, C.; Borcan, F.; Danciu, C.; Trandafirescu, C.; Coricovac, D.; Crăiniceanu, Z.; Dehelean, C.A.; Munteanu, M. The synergistic biologic activity of oleanolic and ursolic acids in complex with hydroxypropyl-γ-cyclodextrin. Molecules 2014, 19, 4924–4940. [Google Scholar] [CrossRef] [Green Version]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Jorge Murillo, G.; Perez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Macașoi, I.; Pavel, I.Z.; Moacă, A.E.; Avram, Ș.; David, V.L.; Coricovac, D.; Mioc, A.; Spandidos, D.A.; Tsatsakis, A.; Șoica, C.; et al. Mechanistic investigations of antitumor activity of a Rhodamine B-oleanolic acid derivative bioconjugate. Oncol. Rep. 2020, 44, 1169–1183. [Google Scholar] [CrossRef]
- Aspartame—What Is It? Get the Facts. Available online: https://aspartame.org/ (accessed on 30 September 2020).
- Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States. Available online: https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states (accessed on 28 August 2020).
- Magnuson, B.A.; Burdock, G.A.; Doull, J.; Kroes, R.M.; Marsh, G.M.; Pariza, M.W.; Spencer, P.S.; Waddell, W.J.; Walker, R.; Williams, G.M. Aspartame: A safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit. Rev. Toxicol. 2007, 37, 629–727. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J. Biol. Med. 2010, 83, 101–108. [Google Scholar] [PubMed]
- Ashok, I.; Sheeladevi, R.; Wankhar, D. Effect of long-term aspartame (artificial sweetener) on anxiety, locomotor activity and emotionality behavior in Wistar Albino rats. Biomed. Prev. Nutr. 2014, 4, 39–43. [Google Scholar] [CrossRef]
- Abhilash, M.; Paul, M.V.S.; Varghese, M.V.; Nair, R.H. Effect of long term intake of aspartame on antioxidant defense status in liver. Food Chem. Toxicol. 2011, 49, 1203–1207. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Devi, R.S. Serum biochemical responses under oxidative stress of aspartame in wistar albino rats. Asian Pac. J. Trop. Dis. 2014, 4, S403–S410. [Google Scholar] [CrossRef]
- Mourad, M. Effect of aspartame on some oxidative stress parameters in liver and kidney of rats. Afr. J. Pharm. Pharm. 2011, 5, 678–862. [Google Scholar]
- Choudhary, A.K.; Sundareswaran, L.; Sheela Devi, R. Effects of aspartame on the evaluation of electrophysiological responses in Wistar albino rats. J. Taibah Univ. Sci. 2016, 10, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Soffritti, M.; Padovani, M.; Tibaldi, E.; Falcioni, L.; Manservisi, F.; Belpoggi, F. The carcinogenic effects of aspartame: The urgent need for regulatory re-evaluation. Am. J. Ind. Med. 2014, 57, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, E.M.; Sadek, R.R.; Abdel-Latief, W.M.; Mosallem, F.A.; Hassan, E.E. The role of dietary and lifestyle factors in the development of colorectal cancer: Case control study in Minia, Egypt. Cent Eur. J. Public Health 2014, 22, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millstone, E. EFSA on Aspartame December 2013. Available online: https://www.sussex.ac.uk/webteam/gateway/file.php?name=millstone-on-efsa-on-aspartame-16dec2013.pdf&site=25 (accessed on 28 August 2020).
- Millstone, E.P.; Dawson, E. EFSA’s toxicological assessment of aspartame: Was it even-handedly trying to identify possible unreliable positives and unreliable negatives? Arch. Public Health 2019, 77, 34. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA J. 2013, 11, 3496. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.P.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza-Diaz, J.; Pastor-Villaescusa, B.; Rueda-Robles, A.; Abadia-Molina, F.; Ruiz-Ojeda, F.J. Plausible Biological Interactions of Low- and Non-Calorie Sweeteners with the Intestinal Microbiota: An Update of Recent Studies. Nutrients 2020, 12, 1153. [Google Scholar] [CrossRef]
- Palmnäs, M.S.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Lobach, A.R.; Roberts, A.; Rowland, I.R. Assessing the in vivo data on low/no-calorie sweeteners and the gut microbiota. Food Chem. Toxicol. 2019, 124, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical. Adv. Nutr. 2019, 10 (Suppl. 1), S31–S48. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Saus, E.; Iraola-Guzmán, S.; Willis, J.R.; Brunet-Vega, A.; Gabaldón, T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol. Asp. Med. 2019, 69, 93–106. [Google Scholar] [CrossRef]
- Sawadsopanon, T.; Meksawan, K.; Chanvorachote, P. Aspartame inhibits migration of human intestinal epithelial cells. J. Food Biochem. 2017, 41, e12341. [Google Scholar] [CrossRef]
- Horio, Y.; Sun, Y.; Liu, C.; Saito, T.; Kurasaki, M. Aspartame-induced apoptosis in PC12 cells. Environ. Toxicol. Pharmacol. 2014, 37, 158–165. [Google Scholar] [CrossRef]
- Pandurangan, M.; Enkhtaivan, G.; Mistry, B.; Chandrasekaran, M.; Noorzai, R.; Kim, D.H. Investigation of role of aspartame on apoptosis process in HeLa cells. Saudi J. Biol. Sci. 2016, 23, 503–506. [Google Scholar] [CrossRef] [Green Version]
- Zweibaum, A.; Laburthe, M.; Grasset, E.; Louvard, D. Use of cultured cell lines in studies of intestinal cell differentiation and function. Compr. Physiol. 2011, (Suppl. 19), 223–255. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health; Verhoeckx, K., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef] [Green Version]
- Aharon, L. Correlation between In-vitro and In-vivo Studies based on Pharmacokinetic Considerations. AJBSR 2020, 8, 48–50. [Google Scholar] [CrossRef]
- Schiano, C.; Grimaldi, V.; Franzese, M.; Fiorito, C.; De Nigris, F.; Donatelli, F.; Soricelli, A.; Salvatore, M.; Napoli, C. Non-nutritional sweeteners effects on endothelial vascular function. Toxicol. In Vitro 2020, 62, 104694. [Google Scholar] [CrossRef]
- Ribatti, D. Angiogenesis. In Hughes KBT-BE of G, 2nd ed.; Maloy, S., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 130–132. Available online: http://www.sciencedirect.com/science/article/pii/B9780123749840000656 (accessed on 15 June 2020).
- Aguilar, F.; Charrondiere, U.R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Engel, H.; Gontard, N.; Gott, D.; Grilli, S.; et al. Scientific Opinion of the Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2009, 951, 1–19. [Google Scholar]
- Shalaby, A.M.; Ibrahim, M.A.A.H.; Aboregela, A.M. Effect of aspartame on the placenta of adult albino rat. A histological and immunohistochemical study. Ann. Anat. 2019, 224, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Alleva, R.; Borghi, B.; Santarelli, L.; Strafella, E.; Carbonari, D.; Bracci, M.; Tomasetti, M. In vitro effect of aspartame in angiogenesis induction. Toxicol. In Vitro 2011, 25, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]




| SDS 1% | PBS | ASP 15 mM | ASP 30 mM | |
|---|---|---|---|---|
| IS | 18.7 | 0.07 | 3.02 | 3.13 |
| tH | 55 s | 300 s | 123 s | 116 s |
| tL | 44 s | 300 s | 300 s | 300 s |
| tC | 14 s | 300 s | 300 s | 300 s |
| SDS 0.5% | DEX + SDS | ASP 30 mM + SDS | |
|---|---|---|---|
| IS | 18.68 | 18.48 | 17.57 |
| tH | 55 s | 25 s | 60 s |
| tL | 44 s | 35 s | 58 s |
| tC | 14 s | 45 s | 38 s |
| HAI | 0.98 | 1.09 | |
| LAI | 1.47 | 1.31 | |
| CAI | 3.21 | 2.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maghiari, A.L.; Coricovac, D.; Pinzaru, I.A.; Macașoi, I.G.; Marcovici, I.; Simu, S.; Navolan, D.; Dehelean, C. High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells. Nutrients 2020, 12, 3600. https://doi.org/10.3390/nu12123600
Maghiari AL, Coricovac D, Pinzaru IA, Macașoi IG, Marcovici I, Simu S, Navolan D, Dehelean C. High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells. Nutrients. 2020; 12(12):3600. https://doi.org/10.3390/nu12123600
Chicago/Turabian StyleMaghiari, Anca Laura, Dorina Coricovac, Iulia Andreea Pinzaru, Ioana Gabriela Macașoi, Iasmina Marcovici, Sebastian Simu, Dan Navolan, and Cristina Dehelean. 2020. "High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells" Nutrients 12, no. 12: 3600. https://doi.org/10.3390/nu12123600
APA StyleMaghiari, A. L., Coricovac, D., Pinzaru, I. A., Macașoi, I. G., Marcovici, I., Simu, S., Navolan, D., & Dehelean, C. (2020). High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells. Nutrients, 12(12), 3600. https://doi.org/10.3390/nu12123600








