High Fat-High Fructose Diet-Induced Changes in the Gut Microbiota Associated with Dyslipidemia in Syrian Hamsters
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling and Experimental Design
2.2. Experimental Diets
2.3. Fecal, Blood and Tissue Collection
2.4. Fasting Triglycerides and Cholesterol
2.5. 16S rRNA Gene Sequencing
2.6. Statistical Analyses
3. Results
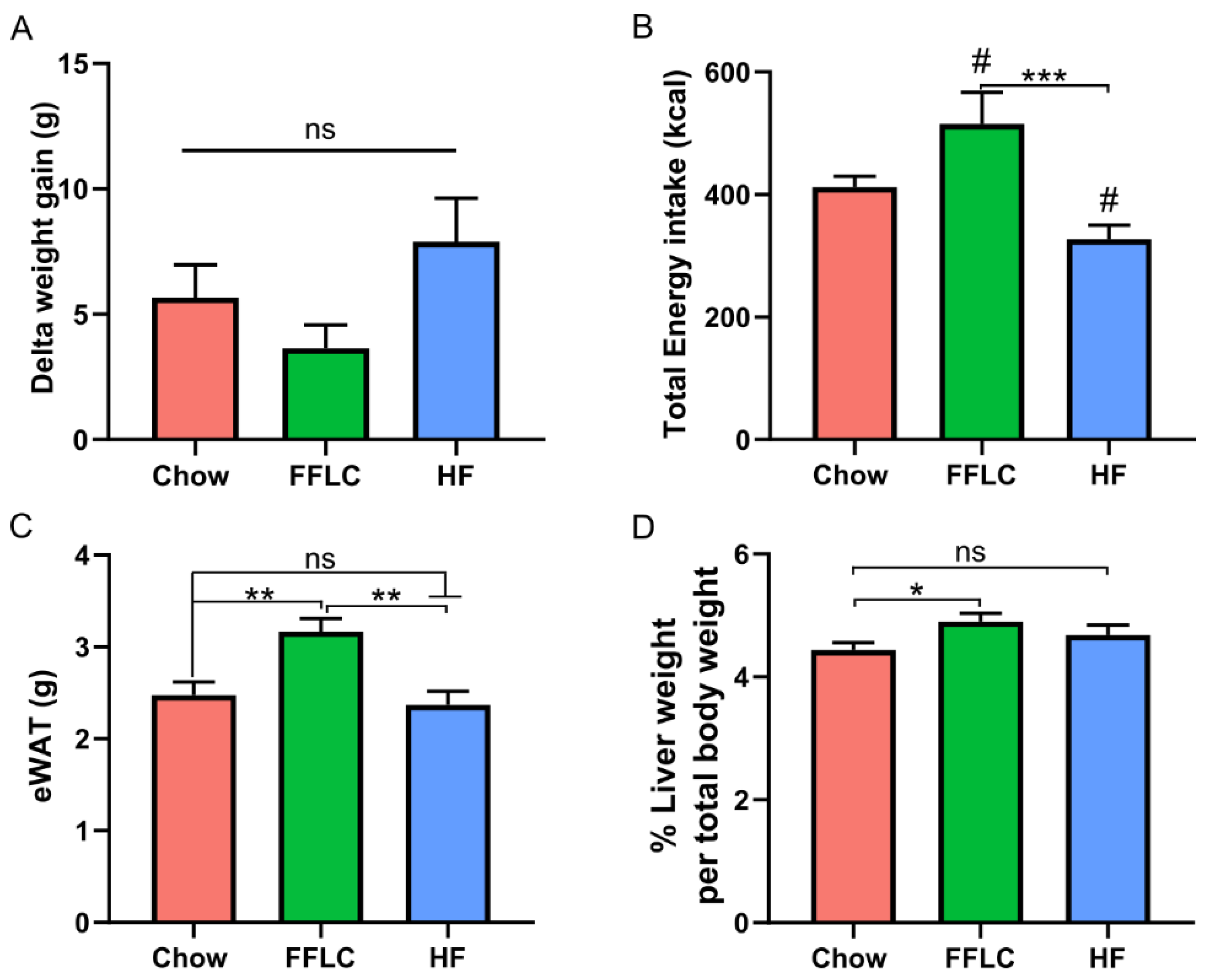
3.1. High-Fructose Diets Do Not Have a Significant Impact on the Weight Gain of Hamsters
3.2. High-Fat/High-Fructose Diet Causes Hepatomegaly and Increased Adipose Tissue
3.3. High-Fat/High-Fructose Diet Induces Dyslipidemia
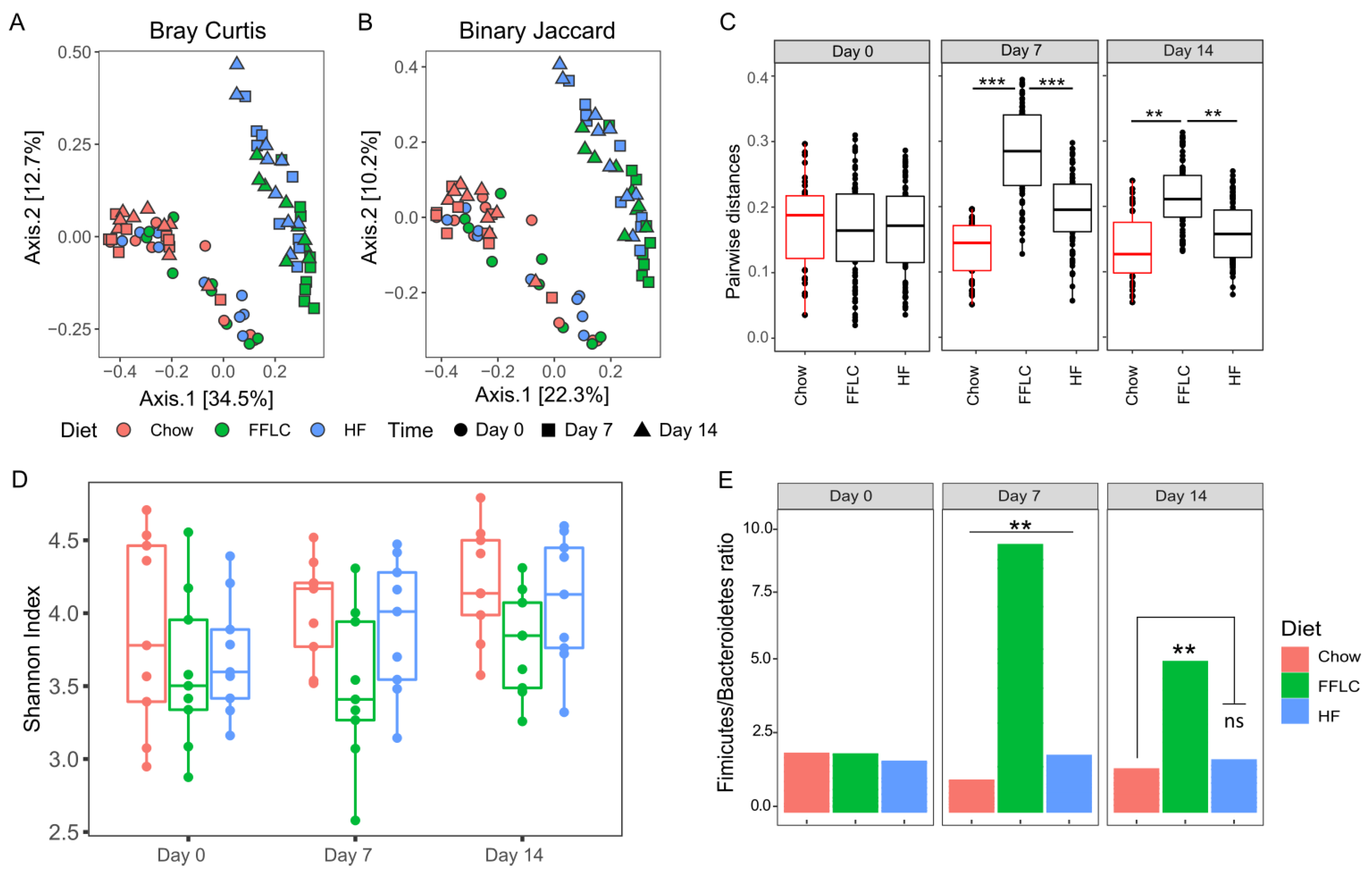
3.4. High-Fat/High-Fructose and Low-Fat/High-Fructose Diets Alter the Intestinal Microbiota
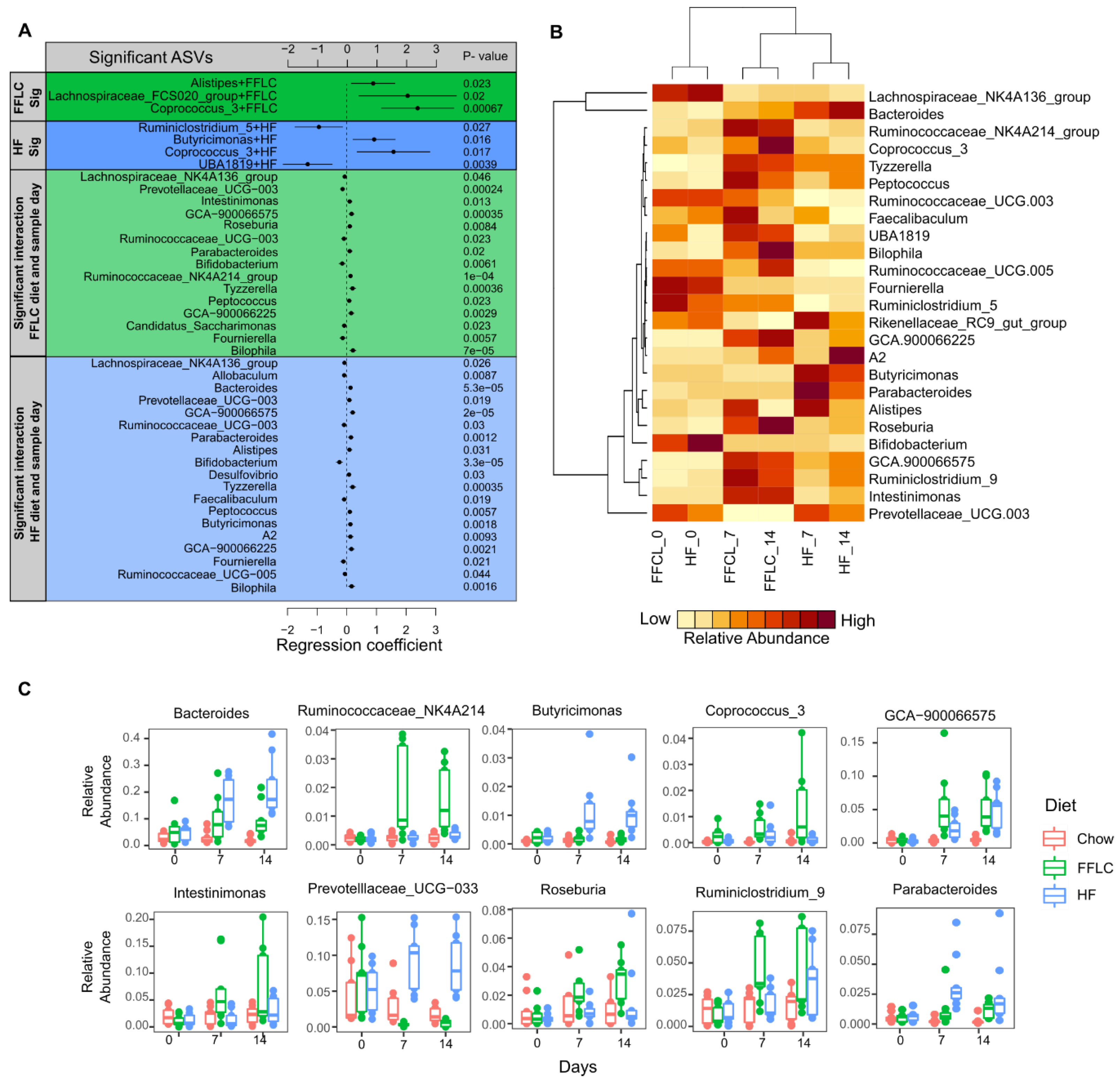
3.5. High-Fat/High-Fructose and Low-Fat/High-Fructose Diets Induce Changes in Bacterial Taxa of Gut Microbiota
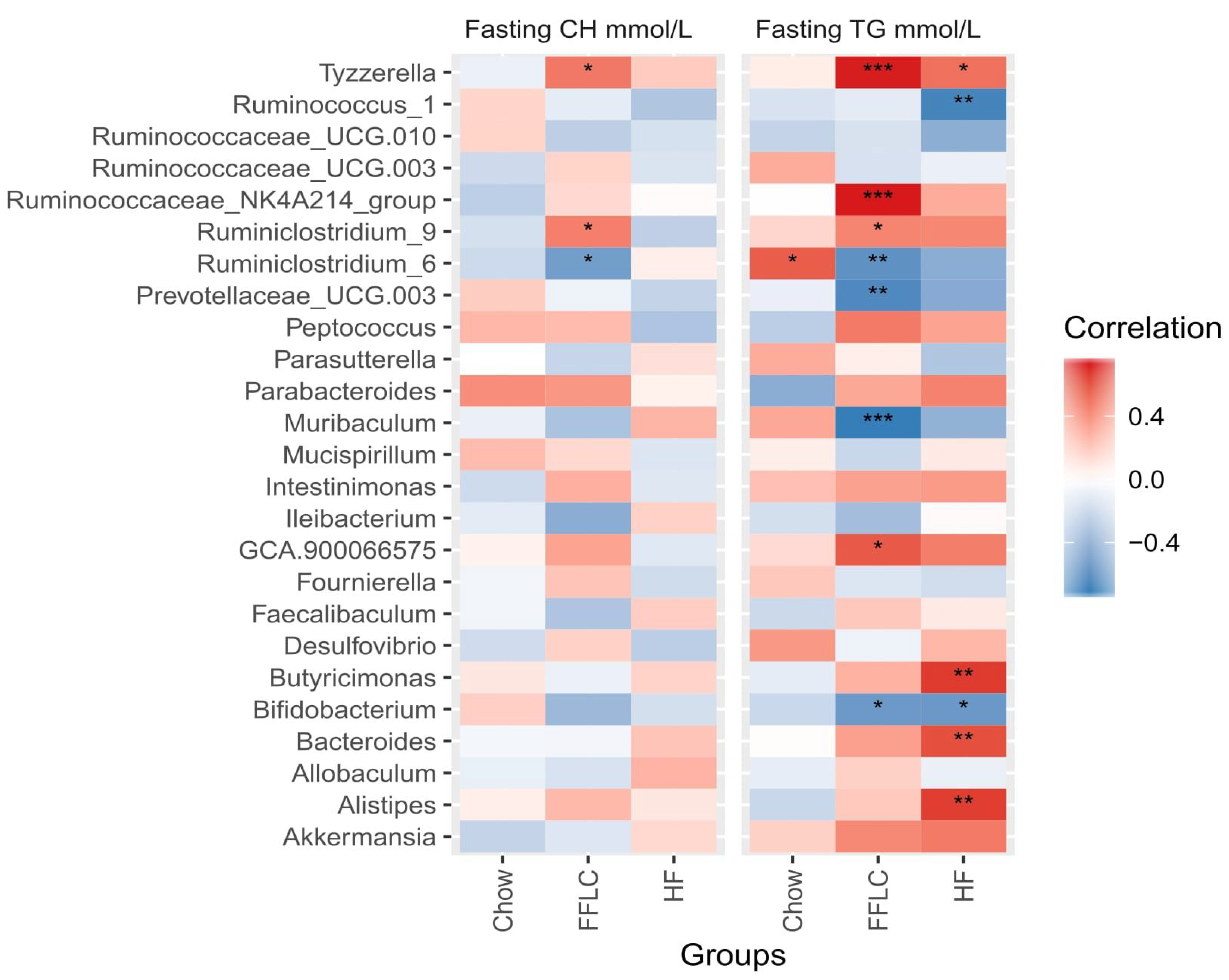
3.6. Dietary-Induced Shifts in Gut Microbiota Composition Correlate with Triglyceride and Cholesterol Levels
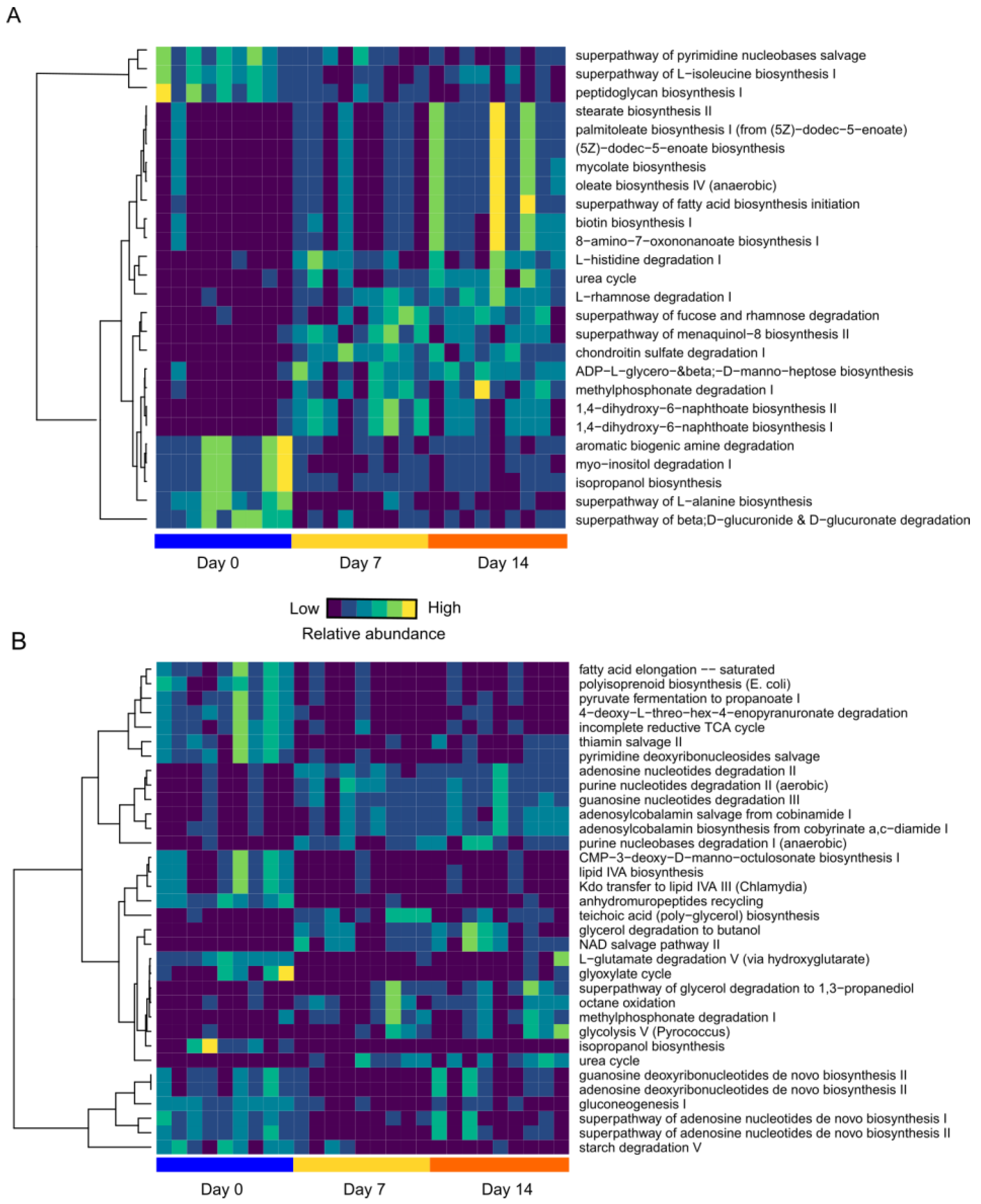
3.7. Dietary Intervention Impacts Functional Changes in the Gut Microbiota
3.8. High-Fat/High-Fructose Diet Leads to Functional Changes in the Gut Microbiome which Contribute to Host Dyslipidemia
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Hooper, L.V.; MacPherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.I.; Davis, C.; Fraser, C.M.; Schneeman, B.O.; Huttenhower, C.; Verbeke, K.; Walter, J.; Latulippe, M.E. Establishing What Constitutes a Healthy Human Gut Microbiome: State of the Science, Regulatory Considerations, and Future Directions. J. Nutr. 2019, 149, 1882–1895. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Lee, L.; Sanders, R.A. Metabolic syndrome. Pediatr. Rev. 2012, 33, 459–466, quiz 467–458. [Google Scholar] [CrossRef]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe 2020, 28, 245–257.e6. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Ahmed, M.I.; Zou, X.; Hussain, M.; Zhang, M.; Zhao, F.; Xu, X.; Zhou, G.; Li, C. Beef, casein, and soy proteins differentially affect lipid metabolism, triglycerides accumulation and gut microbiota of high-fat diet-fed C57BL/6J mice. Front. Microbiol. 2018, 9, 2200. [Google Scholar] [CrossRef] [PubMed]
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef]
- Guerville, M.; Leroy, A.; Sinquin, A.; Laugerette, F.; Michalski, M.C.; Boudry, G. Western-diet consumption induces alteration of barrier function mechanisms in the ileum that correlates with metabolic endotoxemia in rats. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E107–E120. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Flores-Lopez, A.G.; Aguilar-Lopez, M.; Sanchez-Tapia, M.; Medina-Vera, I.; Diaz, D.; Tovar, A.R.; Torres, N. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects With Metabolic Syndrome. J. Am. Heart Assoc. 2019, 8, e012401. [Google Scholar] [CrossRef]
- Roager, H.M.; Dragsted, L.O. Diet-derived microbial metabolites in health and disease. Nutr. Bull. 2019, 44, 216–227. [Google Scholar] [CrossRef]
- Jegatheesan, P.; De Bandt, J.P. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef]
- Jegatheesan, P.; Beutheu, S.; Ventura, G.; Sarfati, G.; Nubret, E.; Kapel, N.; Waligora-Dupriet, A.J.; Bergheim, I.; Cynober, L.; De-Bandt, J.P. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clin. Nutr. 2016, 35, 175–182. [Google Scholar] [CrossRef]
- Oh, J.H.; Alexander, L.M.; Pan, M.; Schueler, K.L.; Keller, M.P.; Attie, A.D.; Walter, J.; van Pijkeren, J.P. Dietary Fructose and Microbiota-Derived Short-Chain Fatty Acids Promote Bacteriophage Production in the Gut Symbiont Lactobacillus reuteri. Cell Host Microbe 2019, 25, 273–284.e6. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrne, S.; Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 2013, 8, e80476. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Fernandez-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein. Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.; Hindlet, P.; Waligora-Dupriet, A.J.; Kapel, N.; Neveux, N.; Mignon, V.; Delomenie, C.; Farinotti, R.; Feve, B.; Buyse, M. Disturbed intestinal nitrogen homeostasis in a mouse model of high-fat diet-induced obesity and glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E668–E680. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Du, Y.; Lv, Z.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of essential amino acids on lipid metabolism in mice and humans. J. Mol. Endocrinol. 2016, 57, 223–231. [Google Scholar] [CrossRef]
- Hsieh, J.; Trajcevski, K.E.; Farr, S.L.; Baker, C.L.; Lake, E.J.; Taher, J.; Iqbal, J.; Hussain, M.M.; Adeli, K. Glucagon-Like Peptide 2 (GLP-2) Stimulates Postprandial Chylomicron Production and Postabsorptive Release of Intestinal Triglyceride Storage Pools via Induction of Nitric Oxide Signaling in Male Hamsters and Mice. Endocrinology 2015, 156, 3538–3547. [Google Scholar] [CrossRef]
- Dalboge, L.S.; Pedersen, P.J.; Hansen, G.; Fabricius, K.; Hansen, H.B.; Jelsing, J.; Vrang, N. A Hamster Model of Diet-Induced Obesity for Preclinical Evaluation of Anti-Obesity, Anti-Diabetic and Lipid Modulating Agents. PLoS ONE 2015, 10, e0135634. [Google Scholar] [CrossRef]
- Wang, P.R.; Guo, Q.; Ippolito, M.; Wu, M.; Milot, D.; Ventre, J.; Doebber, T.; Wright, S.D.; Chao, Y.S. High fat fed hamster, a unique animal model for treatment of diabetic dyslipidemia with peroxisome proliferator activated receptor alpha selective agonists. Eur. J. Pharm. 2001, 427, 285–293. [Google Scholar] [CrossRef]
- Bravo, E.; Cantafora, A.; Calcabrini, A.; Ortu, G. Why prefer the golden Syrian hamster (Mesocricetus auratus) to the Wistar rat in experimental studies on plasma lipoprotein metabolism? Comp. Biochem. Physiol. Part B Comp. Biochem. 1994, 107, 347–355. [Google Scholar] [CrossRef]
- Gaynor, B.J.; Sand, T.; Clark, R.W.; Aiello, R.J.; Bamberger, M.J.; Moberly, J.B. Inhibition of cholesteryl ester transfer protein activity in hamsters alters HDL lipid composition. Atherosclerosis 1994, 110, 101–109. [Google Scholar] [CrossRef]
- Nistor, A.; Bulla, A.; Filip, D.A.; Radu, A. The hyperlipidemic hamster as a model of experimental atherosclerosis. Atherosclerosis 1987, 68, 159–173. [Google Scholar] [CrossRef]
- Shan, K.; Qu, H.; Zhou, K.; Wang, L.; Zhu, C.; Chen, H.; Gu, Z.; Cui, J.; Fu, G.; Li, J.; et al. Distinct Gut Microbiota Induced by Different Fat-to-Sugar-Ratio High-Energy Diets Share Similar Pro-obesity Genetic and Metabolite Profiles in Prediabetic Mice. mSystems 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Whelan, F.J.; Verschoor, C.P.; Stearns, J.C.; Rossi, L.; Luinstra, K.; Loeb, M.; Smieja, M.; Johnstone, J.; Surette, M.G.; Bowdish, D.M. The loss of topography in the microbial communities of the upper respiratory tract in the elderly. Ann. Am. Thorac. Soc. 2014, 11, 513–521. [Google Scholar] [CrossRef]
- Horne, R.; St Pierre, J.; Odeh, S.; Surette, M.; Foster, J.A. Microbe and host interaction in gastrointestinal homeostasis. Psychopharmacology 2019, 236, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, Y.F.; Zhang, L.; Guo, B.; Pendegraft, A.H.; Zhuang, W.; Yi, N. Negative Binomial Mixed Models for Analyzing Longitudinal Microbiome Data. Front. Microbiol. 2018, 9, 1683. [Google Scholar] [CrossRef]
- Fernandez-Mejia, C.; Lazo-de-la-Vega-Monroy, M.-L. Biological Effects of Pharmacological Concentrations of Biotin. J. Evid. Based Integr. Med. 2011, 16, 40–48. [Google Scholar] [CrossRef]
- Revilla-Monsalve, C.; Zendejas-Ruiz, I.; Islas-Andrade, S.; Baez-Saldana, A.; Palomino-Garibay, M.A.; Hernandez-Quiroz, P.M.; Fernandez-Mejia, C. Biotin supplementation reduces plasma triacylglycerol and VLDL in type 2 diabetic patients and in nondiabetic subjects with hypertriglyceridemia. Biomed. Pharm. 2006, 60, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Yoshikawa, T. Propionate Promotes Fatty Acid Oxidation through the Up-Regulation of Peroxisome Proliferator-Activated Receptor alpha in Intestinal Epithelial Cells. J. Nutr. Sci. Vitam. 2015, 61, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.P.; Weller, C.L.; Schlegel, V.L.; Cuppett, S.L.; Guderian, D.M., Jr.; Johnson, K.R. Grain sorghum lipid extract reduces cholesterol absorption and plasma non-HDL cholesterol concentration in hamsters. J. Nutr. 2005, 135, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A.K.; Carr, T.P.; Moriyama, E.N.; Walter, J. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl. Env. Microbiol. 2009, 75, 4175–4184. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.H.; Seo, K.H.; Chon, J.W.; Nah, S.Y.; Bartley, G.E.; Arvik, T.; Lipson, R.; Yokoyama, W. Modulation of the intestinal microbiota is associated with lower plasma cholesterol and weight gain in hamsters fed chardonnay grape seed flour. J. Agric. Food Chem. 2015, 63, 1460–1467. [Google Scholar] [CrossRef]
- Pongking, T.; Haonon, O.; Dangtakot, R.; Onsurathum, S.; Jusakul, A.; Intuyod, K.; Sangka, A.; Anutrakulchai, S.; Cha’on, U.; Pinlaor, S.; et al. A combination of monosodium glutamate and high-fat and high-fructose diets increases the risk of kidney injury, gut dysbiosis and host-microbial co-metabolism. PLoS ONE 2020, 15, e0231237. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Shou, J.W.; Zhao, Z.X.; Li, X.Y.; Zhang, X.F.; Ma, S.R.; He, C.Y.; Lin, Y.; Wen, B.Y.; et al. Gut Microbiota-Mediated Personalized Treatment of Hyperlipidemia Using Berberine. Theranostics 2017, 7, 2443–2451. [Google Scholar] [CrossRef]
- Zinocker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Le, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef]
- Softic, S.; Gupta, M.K.; Wang, G.X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Investig. 2018, 128, 1199. [Google Scholar] [CrossRef] [PubMed]
- Tillman, E.J.; Morgan, D.A.; Rahmouni, K.; Swoap, S.J. Three months of high-fructose feeding fails to induce excessive weight gain or leptin resistance in mice. PLoS ONE 2014, 9, e107206. [Google Scholar] [CrossRef] [PubMed]
- Messier, C.; Whately, K.; Liang, J.; Du, L.; Puissant, D. The effects of a high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in C57BL/6 mice. Behav. Brain Res. 2007, 178, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Simonson, D.C.; Tappy, L.; Jequier, E.; Felber, J.P.; DeFronzo, R.A. Normalization of carbohydrate-induced thermogenesis by fructose in insulin-resistant states. Am. J. Physiol. 1988, 254, E201–E207. [Google Scholar] [CrossRef] [PubMed]
- Mizobe, T.; Nakajima, Y.; Ueno, H.; Sessler, D.I. Fructose administration increases intraoperative core temperature by augmenting both metabolic rate and the vasoconstriction threshold. Anesthesiology 2006, 104, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- DeBosch, B.J.; Chen, Z.; Finck, B.N.; Chi, M.; Moley, K.H. Glucose transporter-8 (GLUT8) mediates glucose intolerance and dyslipidemia in high-fructose diet-fed male mice. Mol. Endocrinol. 2013, 27, 1887–1896. [Google Scholar] [CrossRef]
- Taghibiglou, C.; Carpentier, A.; Van Iderstine, S.C.; Chen, B.; Rudy, D.; Aiton, A.; Lewis, G.F.; Adeli, K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J. Biol Chem. 2000, 275, 8416–8425. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5, 494. [Google Scholar] [CrossRef]
- Wei, S.; Mortensen, M.S.; Stokholm, J.; Brejnrod, A.D.; Thorsen, J.; Rasmussen, M.A.; Trivedi, U.; Bisgaard, H.; Sorensen, S.J. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: A double-blind, randomized, placebo-controlled trial. EBioMedicine 2018, 38, 265–272. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Ulloa-Martinez, M.; Martinez-Rojano, H.; Galvan-Rodriguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Pina-Escobedo, A.; Pizano-Zarate, M.L.; Hoyo-Vadillo, C.; Garcia-Mena, J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of Bile Acids and Gut Microbiota in Obesity Induced by High Fat Diet in Rat Model. J. Agric. Food Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, Y.; Xia, F.; Abudukerimu, B.; Zhang, W.; Guo, Y.; Wang, N.; Lu, Y. A Glucagon-Like Peptide-1 Receptor Agonist Lowers Weight by Modulating the Structure of Gut Microbiota. Front. Endocrinol. 2018, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Kainulainen, V.; Suutarinen, M.; Heini, T.; Bowers, J.R.; Jasso-Selles, D.; Lemmer, D.; Valentine, M.; Barnes, R.; Engelthaler, D.M.; et al. Isolation of Anti-Inflammatory and Epithelium Reinforcing Bacteroides and Parabacteroides Spp. from A Healthy Fecal Donor. Nutrients 2020, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Alang, N.; Kelly, C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis 2015, 2, ofv004. [Google Scholar] [CrossRef]
- Kelly, T.N.; Bazzano, L.A.; Ajami, N.J.; He, H.; Zhao, J.; Petrosino, J.F.; Correa, A.; He, J. Gut Microbiome Associates with Lifetime Cardiovascular Disease Risk Profile among Bogalusa Heart Study Participants. Circ. Res. 2016, 119, 956–964. [Google Scholar] [CrossRef]
- Kuang, Y.S.; Lu, J.H.; Li, S.H.; Li, J.H.; Yuan, M.Y.; He, J.R.; Chen, N.N.; Xiao, W.Q.; Shen, S.Y.; Qiu, L.; et al. Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Rossell, J.; Brindefalk, B.; Baena-Fustegueras, J.A.; Peinado-Onsurbe, J.; Udekwu, K.I. Diet change affects intestinal microbiota restoration and improves vertical sleeve gastrectomy outcome in diet-induced obese rats. Eur. J. Nutr. 2020, 59, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Mendez, A.; Hernandez-Equihua, M.G.; Rueda-Rocha, A.C.; Guajardo-Lopez, C.; Nieto-Aguilar, R.; Serrato-Ochoa, D.; Ruiz Herrera, L.F.; Guzman-Nateras, J.A. Protective effect of supplementation with biotin against high-fructose-induced metabolic syndrome in rats. Nutr. Res. 2018, 57, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Cherbuy, C.; Bellet, D.; Robert, V.; Mayeur, C.; Schwiertz, A.; Langella, P. Modulation of the Caecal Gut Microbiota of Mice by Dietary Supplement Containing Resistant Starch: Impact Is Donor-Dependent. Front. Microbiol. 2019, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Pelgrim, C.E.; Franx, B.A.A.; Snabel, J.; Kleemann, R.; Arnoldussen, I.A.C.; Kiliaan, A.J. Butyrate Reduces HFD-Induced Adipocyte Hypertrophy and Metabolic Risk Factors in Obese LDLr-/-.Leiden Mice. Nutrients 2017, 9, 714. [Google Scholar] [CrossRef]
- Yan, H.; Ajuwon, K.M. Mechanism of Butyrate Stimulation of Triglyceride Storage and Adipokine Expression during Adipogenic Differentiation of Porcine Stromovascular Cells. PLoS ONE 2015, 10, e0145940. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Preiss, B. Regulation of HMG-CoA Reductase in Extrahepatic Tissues. Regul. Hmg-Coa Reductase 1985, 183–200. [Google Scholar]
- Greenspan, M.D.; Yudkovitz, J.B.; Lo, C.Y.; Chen, J.S.; Alberts, A.W.; Hunt, V.M.; Chang, M.N.; Yang, S.S.; Thompson, K.L.; Chiang, Y.C.; et al. Inhibition of hydroxymethylglutaryl-coenzyme A synthase by L-659,699. Proc. Natl. Acad. Sci. USA 1987, 84, 7488–7492. [Google Scholar] [CrossRef] [PubMed]
- Hegardt, F.G. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: A control enzyme in ketogenesis. Biochem. J. 1999, 338, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Alex, S.; Lange, K.; Amolo, T.; Grinstead, J.S.; Haakonsson, A.K.; Szalowska, E.; Koppen, A.; Mudde, K.; Haenen, D.; Al-Lahham, S.; et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor gamma. Mol. Cell Biol. 2013, 33, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X. Microbial management. Science 2020, 369, 153. [Google Scholar] [CrossRef]
- Ottosson, F.; Smith, E.; Melander, O.; Fernandez, C. Altered Asparagine and Glutamate Homeostasis Precede Coronary Artery Disease and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3060–3069. [Google Scholar] [CrossRef]
- Yanni, A.E.; Agrogiannis, G.; Nomikos, T.; Fragopoulou, E.; Pantopoulou, A.; Antonopoulou, S.; Perrea, D. Oral supplementation with L-aspartate and L-glutamate inhibits atherogenesis and fatty liver disease in cholesterol-fed rabbit. Amino Acids 2010, 38, 1323–1331. [Google Scholar] [CrossRef]
- Kujala, U.M.; Makinen, V.P.; Heinonen, I.; Soininen, P.; Kangas, A.J.; Leskinen, T.H.; Rahkila, P.; Wurtz, P.; Kovanen, V.; Cheng, S.; et al. Long-term leisure-time physical activity and serum metabolome. Circulation 2013, 127, 340–348. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Kujala, U.M.; Peltonen, M.; Laine, M.K.; Kaprio, J.; Heinonen, O.J.; Sundvall, J.; Eriksson, J.G.; Jula, A.; Sarna, S.; Kainulainen, H. Branched-Chain Amino Acid Levels Are Related with Surrogates of Disturbed Lipid Metabolism among Older Men. Front. Med. 2016, 3, 57. [Google Scholar] [CrossRef]

| kcal/kg | g/kg | |||||
|---|---|---|---|---|---|---|
| Chow | FFLC | HF | Chow | FFLC | HF | |
| Protein | 872 | 613 | 796 | 218 | 171 | 222 |
| Fat | 819 | 2700 | 540 | 91 | 300 | 60 |
| Carbohydrate | 2072 | 1572 | 2280 | 518 | 414 | 600 |
| Gross Energy | 4600 | 4971 | 3655 | |||
| Predicted Metabolic Pathways | ρ | Diet | AdjP |
|---|---|---|---|
| Superpathway of l-alanine biosynthesis | −0.59 | FFLC | 0.011 |
| l-histidine degradation I | −0.59 | FFLC | 0.012 |
| Anhydromuropeptides recycling | −0.56 | FFLC | 0.019 |
| Superpathway of l-aspartate and l-asparagine biosynthesis | −0.54 | FFLC | 0.024 |
| Pyruvate fermentation to propanoate I | −0.54 | FFLC | 0.025 |
| UDP N-acetylmuramoyl pentapeptide biosynthesis II lysine containing | −0.52 | FFLC | 0.032 |
| Peptidoglycan biosynthesis III mycobacteria | −0.52 | FFLC | 0.034 |
| Peptidoglycan biosynthesis I meso diaminopimelate containing | −0.51 | FFLC | 0.037 |
| CMP 3 deoxy d-manno-octulosonate biosynthesis I | −0.50 | FFLC | 0.042 |
| Superpathway of geranyl-geranyl diphosphate biosynthesis II via MEP. | −0.50 | FFLC | 0.044 |
| Starch degradation V | −0.50 | FFLC | 0.045 |
| Aminoimidazole ribonucleotide biosynthesis I | −0.50 | FFLC | 0.046 |
| UDP N-acetylmuramoyl pentapeptide biosynthesis I meso diaminopimelate containing | −0.49 | FFLC | 0.049 |
| Thiamin salvage II | −0.49 | FFLC | 0.049 |
| Glycolysis II from fructose 6-phosphate | −0.45 | FFLC | 0.049 |
| Purine nucleotides degradation II aerobic | 0.54 | FFLC | 0.027 |
| Guanosine nucleotides degradation III | 0.54 | FFLC | 0.025 |
| Adenosine nucleotides degradation II | 0.56 | FFLC | 0.019 |
| Purine nucleobases degradation I anaerobic | 0.56 | FFLC | 0.018 |
| Anhydromuropeptides recycling | −0.69 | HF | 0.001 |
| TCA cycle VI obligate autotrophs | −0.67 | HF | 0.003 |
| TCA cycle I prokaryotic | −0.66 | HF | 0.003 |
| Reductive TCA cycle I | −0.64 | HF | 0.005 |
| tRNA processing | −0.63 | HF | 0.005 |
| Superpathway of fucose and rhamnose degradation | 0.62 | HF | 0.007 |
| Arginine ornithine and proline interconversion | 0.59 | HF | 0.012 |
| l-rhamnose degradation I | 0.57 | HF | 0.017 |
| TCA cycle V 2-oxoglutarate ferredoxin oxidoreductase | −0.54 | HF | 0.024 |
| Superpathway of l-alanine biosynthesis | −0.54 | HF | 0.026 |
| Fucose degradation | 0.52 | HF | 0.035 |
| l-lysine fermentation to acetate and butanoate | 0.51 | HF | 0.040 |
| Predicted Metabolic Pathways | ρ | Diet | AdjP |
|---|---|---|---|
| Glycolysis II from fructose 6-phosphate | −0.44 | FFLC | 0.049 |
| Superpathway of L-aspartate and L-asparagine biosynthesis | −0.47 | FFLC | 0.034 |
| Teichoic acid poly glycerol biosynthesis | −0.49 | FFLC | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horne, R.G.; Yu, Y.; Zhang, R.; Abdalqadir, N.; Rossi, L.; Surette, M.; Sherman, P.M.; Adeli, K. High Fat-High Fructose Diet-Induced Changes in the Gut Microbiota Associated with Dyslipidemia in Syrian Hamsters. Nutrients 2020, 12, 3557. https://doi.org/10.3390/nu12113557
Horne RG, Yu Y, Zhang R, Abdalqadir N, Rossi L, Surette M, Sherman PM, Adeli K. High Fat-High Fructose Diet-Induced Changes in the Gut Microbiota Associated with Dyslipidemia in Syrian Hamsters. Nutrients. 2020; 12(11):3557. https://doi.org/10.3390/nu12113557
Chicago/Turabian StyleHorne, Rachael G., Yijing Yu, Rianna Zhang, Nyan Abdalqadir, Laura Rossi, Michael Surette, Philip M. Sherman, and Khosrow Adeli. 2020. "High Fat-High Fructose Diet-Induced Changes in the Gut Microbiota Associated with Dyslipidemia in Syrian Hamsters" Nutrients 12, no. 11: 3557. https://doi.org/10.3390/nu12113557
APA StyleHorne, R. G., Yu, Y., Zhang, R., Abdalqadir, N., Rossi, L., Surette, M., Sherman, P. M., & Adeli, K. (2020). High Fat-High Fructose Diet-Induced Changes in the Gut Microbiota Associated with Dyslipidemia in Syrian Hamsters. Nutrients, 12(11), 3557. https://doi.org/10.3390/nu12113557





