Zinc Deficiency—An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke?
Abstract
1. Introduction
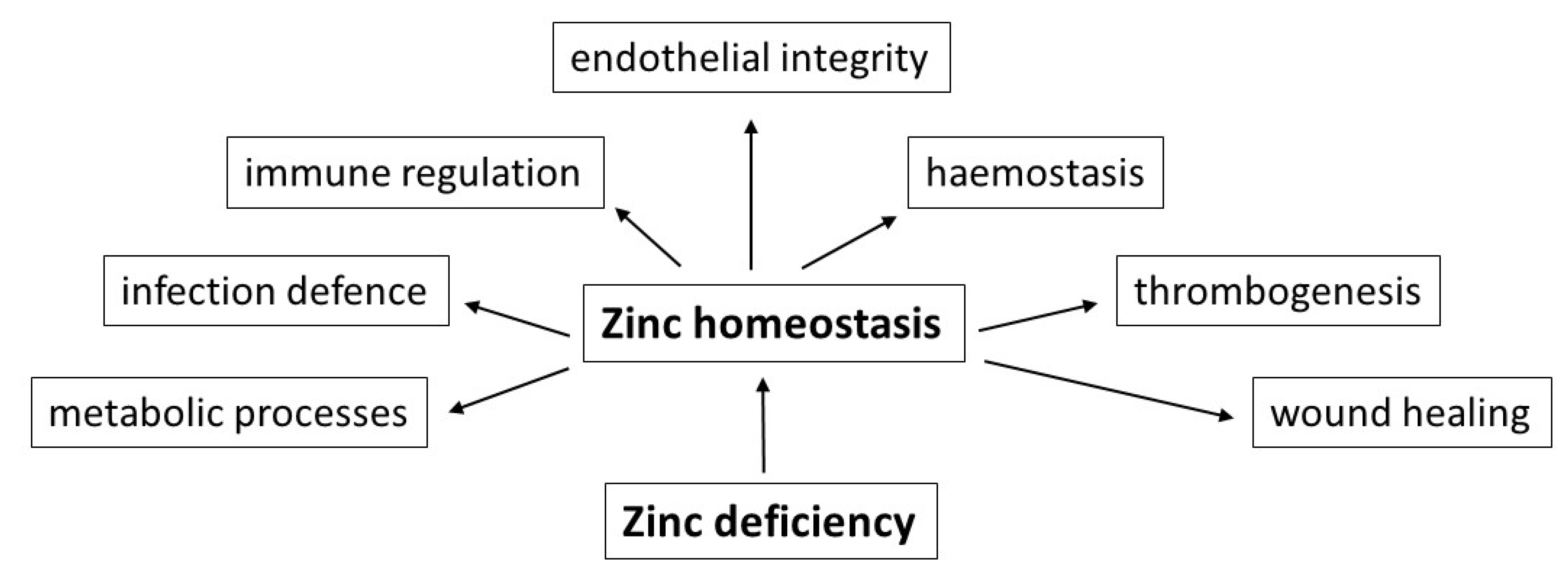
2. Functions of Zinc
3. Resorption of Zinc
4. Zinc and Nutrition (Diet)
5. Zinc Deficiency
6. Zinc Deficiency: Influence on Vessels, Coagulation and Stroke
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tong, Y.N.; Appleby, P.N.; Bradbury, K.; Perez-Cornago, A.; Travis, R.C.; Clarke, R.; Key, T.J. Risks of ischaemic heart disease and stroke, in meat eaters, fish eaters, and vegetarians over 18 years follow-up: Results from the prospetive EPIC-Oxford study. BMJ 2019, 366, I14897. [Google Scholar] [CrossRef]
- Maret, W. Regulation of cellular zinc ions and their signaling functions. In Zinc Signaling, 2nd ed.; Fukada, T., Kambe, T., Eds.; Springer Nature: Singapore, 2019; pp. 5–22. [Google Scholar]
- Maret, W. The redox biology of redox-inert zinc ions. Free Radical Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Reinhold, D. Zinc and Liver. In Zinc in Human Health, 1st ed.; Rink, L., Ed.; IOS Press: Amsterdam, The Netherlands, 2011; pp. 473–495. [Google Scholar]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef]
- Vu, T.T.; Fredenburg, J.C.; Weitz, J.I. Zinc: An important co-factor in hemostasis and thrombosis. Thromb. Hemost. 2013, 109, 421–430. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Braun, A. Zinc homeostasis in platelet-related diseases. Int. J. Mol. Sci. 2019, 20, 5228. [Google Scholar] [CrossRef]
- King, J.C. Yet again, serum zinc concentrations are unrelated to zinc intakes. J. Nutr. 2018, 148, 1399–1401. [Google Scholar] [CrossRef]
- Coverdal, P.C.; Barnett, J.P.; Adamu, A.H.; Griffiths, E.J.; Stewart, A.J.; Blindauer, C.A. A metalloproteomic analysis of interactions between plasma proteins and zinc: Elevated fatty levels affect zinc distribution. Metallomics 2019, 11, 1805–1819. [Google Scholar] [CrossRef]
- Lonergan, Z.R.; Skaar, E.P. Nutrient zinc at the host-pathogen interface. Trends Biochem. Sci. 2019, 44, 1041–1056. [Google Scholar] [CrossRef]
- Markowitz, M.E.; Rosen, J.F.; Mizruchi, M. Circadian variations in serum zinc (Zn) concentrations: Correlation with blood ionized calcium, serum total calcium and phosphate in humans. Am. J. Clin. Nutr. 1985, 41, 689–696. [Google Scholar] [CrossRef]
- Kanabrocki, E.L.; Scheving, L.E.; Olwin, J.H.; Marks, G.E.; McCormick, J.B.; Halberg, F.; Pauly, J.E.; Greco, J.; De Bartolo, M.; Nemchausky, B.A.; et al. Circadian variation in the urinary excretion of electrolytes and trace elements in men. Am. J. Anat. 1983, 166, 121–148. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Goodall, M.J.; Stall, C.; Pritts, J. Post-prandial and daily changes in plasma zinc. J. Trace Elem. Elektrolytes Health Dis. 1989, 3, 55–57. [Google Scholar]
- Henningar, S.R.; Lieberman, H.R.; Fulgoni, V.L.; McClung, J.P. Serum zinc concentrations in the US population are related to sex, age, and time of blood draw but not dietary or supplemental zinc. J. Nutr. 2018, 148, 1341–1351. [Google Scholar] [CrossRef]
- Gröber, U.; Kisters, K.; Classen, H.G. Zinkmangel im Fokus: Ursachen, Symptome, Diagnose und Therapie. EHK 2019, 68, 278–292. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. Zinc-buffering capacity of eukaryotic cells at physiological Zn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef]
- Andrews, G.K. Cellular zinc sensors: MTF-1 regulation of gene expression. BioMetals 2001, 14, 223–237. [Google Scholar] [CrossRef]
- Wessels, I.; Rink, L. Micronutrients in autoimmune diseases: Possible therapeutic benefits of zinc and vitamin D. J. Nutr. Biochem. 2020, 77, 108240. [Google Scholar] [CrossRef]
- Cousins, R.J.; Lichten, L.A. Zinc transporters. In Zinc in Human Health, 1st ed.; Rink, L., Ed.; IOS Press: Amsterdam, The Netherlands, 2011; pp. 163–194. [Google Scholar]
- Ishida, T.; Takechi, S. ß-Naphthoflavone, an exogenous ligand of aryl hydrocarbon receptor, disrupts zinc homeostasis in human hepatoma HepG2 cells. J. Toxicol. Sci. 2019, 44, 711–720. [Google Scholar] [CrossRef]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of nutrition for development (BOND)-Zinc review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef]
- Hamibdge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478S–1483S. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Reinhold, D. Zink: Bedeutung in der Ärztlichen Praxis, 1st ed.; Jürgen Hartmann Verlag: Heßdorf-Klebheim, Germany, 2007; pp. 1–96. [Google Scholar]
- Lönnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Maret, W.; Sandstaedt, H.H. Zinc requirements and risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Maret, W. (King’s College London, London, UK). Personal communication, 2020.
- Saunders, A.V.; Craig, W.J.; Baines, S.K. Zinc in vegetarian diets. Med. J. Aust. 2013, 199, 17–22. [Google Scholar] [CrossRef]
- Gibson, R.S. Zinc nutrition in developing countries. Nutr. Res. Rev. 1994, 7, 151–173. [Google Scholar] [CrossRef]
- Hahn, A.; Ströhle, A.; Wolters, M. Ernährung: Physiologische Grundlagen, Prävention, Therapie, 3rd ed.; Wissenschaftliche Verlagsgesellschaft Stuttgart: Stuttgart, Germany, 2016; pp. 1–1182. [Google Scholar]
- Udechukuwu, M.C.; Collins, S.A.; Udenigwe, C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef]
- Trame, S.; Wessels, I.; Haase, H.; Rink, L. A short 18 items food frequeny questionaire biochemically validated to estimate zinc statur in human. J. Trace Elem. Med. Biol. 2018, 49, 285–295. [Google Scholar] [CrossRef]
- Hunt, J.R. Biovailabilty of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef]
- Turnlund, J.R.; King, J.C.; Keyes, W.R.; Gong, B.; Michel, M.C. A stable isotope study of zinc absorption in young men: Effects of phytate and α-cellulose. Am. J. Clin. Nutr. 1982, 40, 1071–1077. [Google Scholar] [CrossRef]
- Hunt, J.R.; Johnson, L.K.; Lykken, G.I. High- versus low-meat diets: Effects on zinc absorption, iron status, and calcium, copper, iron, magnesium, manganese, nitrogen, phosphorus, and zinc balance in postmenopausal women. Am. J. Clin. Nutr. 1995, 62, 621–632. [Google Scholar] [CrossRef]
- Tran, C.D.; Miller, L.V.; Krebs, N.F.; Lei, S.; Hambidge, K.M. Zinc absorption as a function of the dose of zinc sulfate in aequeous solution. Am. J. Clin. Nutr. 2004, 80, 1570–1573. [Google Scholar] [CrossRef]
- Rose, S.D.; Strombom, A.J. Diverticular disease risk reduced with a plant-based diet. Adv. Res. Gastroenterol. Hepatol. 2019, 14, 32–34. [Google Scholar]
- Skrikumar, T.S.; Johansson, G.K.; Öckerman, P.; Gustafsson, P.; Äkesson, B. Trace element status in healthy subjects switching from a mixed to a lactovegetarian diet for 12 months. Am. J. Clin. Nutr. 1992, 55, 885–890. [Google Scholar] [CrossRef]
- World Health Organization. Trace Elements in Human Nutrition and Health, 1st ed.; WHO: Geneva, Switzerland, 1996; pp. 1–361. [Google Scholar]
- Gibson, R.S.; Hess, S.Y.; Hotz, C.; Brown, K.H. Indicators of zinc status at the population level: A review of the evidence. Br. J. Nutr. 2008, 99, S14–S23. [Google Scholar] [CrossRef]
- Grüngreiff, K. Zink in der Pathogenese chronischer Lebererkrankungen. Z. Gastroenterol. 2018, 56, 1301–1302. [Google Scholar]
- Grüngreiff, K. Zinc and the Liver, 1st ed.; Dr. Falk Pharma GmbH: Freiburg, Germany, 2013; pp. 1–84. [Google Scholar]
- Prasad, A.S.; Miale, A.; Farid, Z.; Sandstaed, H.H.; Schulert, A.R.; Darby, W.J. Biochemical studies of dwarfism, hypogonadism and anemia. Arch. Int. Med. 1963, 111, 407–428. [Google Scholar] [CrossRef]
- Garnica, A.D. Trace elements and hemoglobin metabolism. Ann. Clin. Lab. Sci. 1981, 11, 2220–2228. [Google Scholar]
- Siyame, E.W.; Hurst, R.; Waver, A.A.; Young, S.D.; Broadley, M.R.; Chilimba, A.D.; Ander, L.E.; Watts, M.J.; Chilima, B.; Gondwe, J.; et al. A high prevalence of zinc- but not iron-deficiency among women in rural Malawi: A cross-sectional study. Int. J. Vitam. Nutr. Res. 2013, 83, 176–186. [Google Scholar] [CrossRef]
- Spivak, J.L.; Fischer, J.; Isaacs, M.A.; Hankins, W.D. Protein kinases and phosphatases are involved in erythropoetin-mediated signal transduction. Expt. Hematol. 1992, 20, 500–504. [Google Scholar]
- Clair, J.; Talwakar, M.; McClain, R.J. Selective removal of zinc from cell media. J. Trace Elem. Exp. Med. 1995, 7, 143–150. [Google Scholar]
- Hennig, B.; Toborek, M.; McClain, C.J. Antiatherogenic properties of zinc: Implications in endothelial cell metabolism. J. Clin. Nutr. 1996, 12, 711–717. [Google Scholar] [CrossRef]
- Hennig, B.; Wang, Y.; Ramasamy, S.; McClain, C.J. Zinc deficiency alters barrier function of cultured porcine endothelial cells. J. Nutr. 1992, 122, 1242–1247. [Google Scholar] [CrossRef]
- Hennig, B.; McClain, C.J.; Wang, Y.; Ramasamy, S. Zinc protects against linoleic acid-induced disruption of endothelial barrier function in culture. J. Am. Coll. Nutr. 1990, 9, 535–538. [Google Scholar]
- Connell, P.; Young, V.M.; Toborek, M.; Cohen, D.A.; Barve, S.; McClain, C.J.; Hennig, B. Zinc attenuates tumor necrosis factor-mediated activation of transcription factors in endothelial cells. J. Am. Coll. Nutr. 1997, 16, 411–417. [Google Scholar] [CrossRef]
- Geng, Y.I. Molecular signal transduction in vascular cell apoptosis. Cell Res. 2001, 11, 253–264. [Google Scholar] [CrossRef]
- Beattie, J.H.; Kwun, I.S. Is zinc deficiency a risk factor for atherosclerosis? Br. J. Nutr. 2004, 91, 177–181. [Google Scholar] [CrossRef]
- Nakamura, H.; Sekiguchi, A.; Ogawa, Y.; Kawamura, T.; Akai, R.; Iwawaki, T.; Makiguchi, T.; Yokoo, S.; Ishikawa, O.; Motegi, S.I. Zinc deficiency exacerbates pressure ulcers by increasing oxidative stress and ATP in the skin. J. Dermatol. Sci. 2019, 95, 62–69. [Google Scholar] [CrossRef]
- Karadas, S.; Sayın, R.; Aslan, M.; Gonullu, H.; Katı, C.; Dursun, R.; Duran, L.; Gonullu, E.; Demir, H. Serum levels of trace elements and heavy metals in patients with acute hemorrhagic stroke. J. Membr. Biol. 2014, 247, 175–180. [Google Scholar] [CrossRef]
- Munshi, A.; Babu, S.; Kaul, S.; Shafi, G.; Rajeswar, K.; Alladi, S.; Jyothy, A. Depletion of serum zinc in ischemic stroke patients. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 433–436. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Zhang, Y.; Li, H.; Zhang, H.; Huo, Y.; Li, J.; Liu, X.; Wang, X.; Qin, X.; et al. Baseline plasma zinc and risk of first stroke in hypertensive patients. A nested case-control study. Stroke 2019, 50, 3255–3258. [Google Scholar] [CrossRef]
- Khorsadi, H.; Nikpayam, O.; Yousefi, R.; Parandoosh, M.; Hosseinzadeh, N.; Saidpour, A.; Ghorbani, A. Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: A randomized, placebo-controlled, double-blind trial. Diabetol. Metab. Syndr. 2019, 11, 101–112. [Google Scholar] [CrossRef]
- Grüngreiff, K. Non-alcoholic Fatty Liver Disease, Diabetes mellitus, and Zinc/Zinc Transporters: There is a Connection? In Liver Research and Clinical Management, 1st ed.; Rodrigo, L., Ed.; Tech Open Ltd.: London, UK, 2018; pp. 43–53. [Google Scholar]
- Qi, Z.; Liu, K.J. The interaction of zinc and the blood-brain- barrier under physiological and ischemic conditions. Toxicol. Appl. Pharmacol. 2019, 364, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.B.S.; Severo, J.S.; Beserra, J.B.; Soares de Oiveira, A.R.; Climaco Cruz, K.J.; de Sousa Melo, S.R.; Ribeiro do Nascimento, G.V.; Soares de Macedo, G.F.; do Nascimento Marreiro, D. Association between cortisol, insulin resistance and zinc in obesity: A Mini-review. Biol. Trace Elem. Res. 2019, 191, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Bury, N.R.; Chung, N.J.; Sturm, A.; Walker, P.A.; Hogstrand, C. Cortisol stimulates the zinc signaling pathway and expression of metallothioneins and Znt1 in rainbow trout gill epithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Olechnowitz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Ferro, F.E.D.; de Sousa Lima, V.B.; Mello Soares, N.R.; Ma Francisato Cozzolino, S.; do Nascimento Marreiro, D. Biomarkers of metabolic syndrome and its relashionship with zinc nutrional status in obese women. Nutr. Hosp. 2011, 650–654. [Google Scholar]
- Gu, K.; Xiang, W.; Zhang, Y.; Sun, K.; Jiang, X. The association between zinc level and overweight/obesity: A meta-analysis. Eur. J. Nutr. 2019, 58, 2971–2982. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Stampfer, M.J.; Manson, J.E.; Rexrode, K.; Hu, F.; Hennekens, C.H.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation 2001, 103, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Parlin, R.W. Serum cholesterol response to changes in dietary lipids. Am. J. Clin. Nutr. 1966, 19, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N. Engl. J. Med. 1990, 323, 439–445. [Google Scholar] [CrossRef]
- Arleth, T.; Olsen, M.H.; Orre, M.; Rasmussen, R.; Bache, S.; Eskesen, V.; Frikke-Schmidt, R.; Møller, K. Hypozincaemia is associated with severity of subarachnoid hemorrhage: A retrospective cohort study. Acta Neurochir. 2020, 162, 1417–1424. [Google Scholar]
- Hong, K.H.; Keen, C.L.; Mizuno, Y.; Johnston, K.E.; Tamura, T. Effects of dietary zinc deficiency on homocysteine and folate metabolism. J. Nutr. Biochem. 2000, 11, 165–169. [Google Scholar] [CrossRef]
- Barbato, J.C.; Catanescu, O.; Murray, K.; DiBello, P.M.; Jacobsen, D.W. Targeting of metallothionein by L-homocysteine: A novel mechanism for disruption of zinc and redox homeostasis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Rech, L.; Wu, Y.; Goltz, D.; Taylor, C.G.; House, J.D. Effects of zinc deficiency and zinc supplementation on homocysteine levels and related enzyme expression in rats. J. Trace Elem. Med. Biol. 2015, 30, 77–82. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grüngreiff, K.; Gottstein, T.; Reinhold, D. Zinc Deficiency—An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients 2020, 12, 3548. https://doi.org/10.3390/nu12113548
Grüngreiff K, Gottstein T, Reinhold D. Zinc Deficiency—An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients. 2020; 12(11):3548. https://doi.org/10.3390/nu12113548
Chicago/Turabian StyleGrüngreiff, Kurt, Thomas Gottstein, and Dirk Reinhold. 2020. "Zinc Deficiency—An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke?" Nutrients 12, no. 11: 3548. https://doi.org/10.3390/nu12113548
APA StyleGrüngreiff, K., Gottstein, T., & Reinhold, D. (2020). Zinc Deficiency—An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients, 12(11), 3548. https://doi.org/10.3390/nu12113548




