Effect of Xylo-Oligosaccharides Supplementation by Drinking Water on the Bone Properties and Related Calcium Transporters in Growing Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collection
2.3. Determination of Serum Calcium and Phosphorus
2.4. Determination of Femur Biological Parameters
2.5. Gut Morphology and Immunohistochemistry Analysis
2.6. Statistical Analysis
3. Result
3.1. Weight Gain, Food and Water Intake, Food Efficiency Ratio
3.2. Cecum pH and Cecum Wall Weight
3.3. Serum Ca and P Concentration
3.4. Bone Analysis
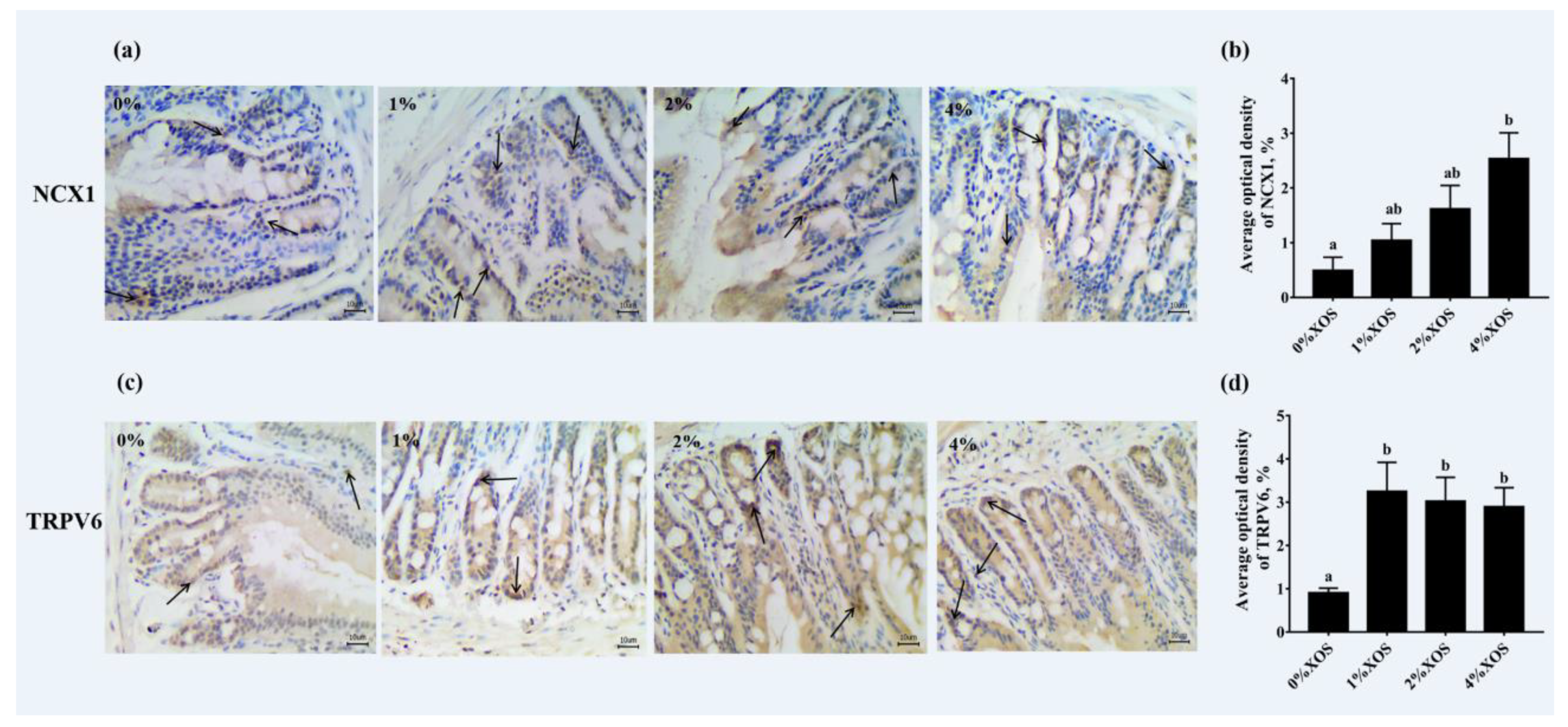
3.5. Gut Morphological Evaluation and Immunohistochemistry Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| BMC | bone mineral content |
| Ca | calcium |
| Ca2+ | calcium ion |
| FOS | fructooligosaccharide |
| FWHM | full width at half maximum |
| GOS | galactooligosaccharide |
| HFD | High-fat diet |
| HMO | human milk oligosaccharides |
| ICR | Institute Cancer Research |
| NCX1 | Na+/Ca2+ exchanger 1 |
| NDOs | non-digestible oligosaccharides |
| P | phosphorus |
| SCFAs | short-chain fatty acids |
| TRPV6 | transient receptor potential vanillin receptor 6 |
| VH:CD | villus height to crypt depth |
| XOS | xylo-oligosaccharides |
References
- Paschalis, E.P.; Gamsjaeger, S.; Hassler, N.; Fahrleitner-Pammer, A.; Dobnig, H.; Stepan, J.J.; Pavo, I.; Eriksen, E.F.; Klaushofer, K. Vitamin D and calcium supplementation for three years in postmenopausal osteoporosis significantly alters bone mineral and organic matrix quality. Bone 2017, 95, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Markiewicz, L.; Lamparski, G.; Juśkiewic, J. Administration of Inulin-Supplemented Gluten-Free Diet Modified Calcium Absorption and Caecal Microbiota in Rats in a Calcium-Dependent Manner. Nutrients 2017, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Seijo, M.; Bryk, G.; Zeni Coronel, M.; Bonanno, M.; Río, M.E.; Pita Martín de Portela, M.L.; Zeni, S.N. Effect of Adding a Galacto-Oligosaccharides/Fructo-Oligosaccharides (GOS/FOS®) Mixture to a Normal and Low Calcium Diet, on Calcium Absorption and Bone Health in Ovariectomy-Induced Osteopenic Rats. Calcif. Tissue Int. 2019, 104, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, J.; Li, X.; Yan, Q.; Ma, J.; Jiang, Z. Curdlan (Alcaligenes faecalis) (1→3)-β-d-Glucan Oligosaccharides Drive M1 Phenotype Polarization in Murine Bone Marrow-Derived Macrophages via Activation of MAPKs and NF-κB Pathways. Molecules 2019, 24, 4251. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Nakamura, S.; Moriyama-Hashiguchi, M.; Kitajima, M.; Ejima, H.; Imori, C.; Oku, T. Dietary Fructooligosaccharide and Glucomannan Alter Gut Microbiota and Improve Bone Metabolism in Senescence-Accelerated Mouse. J. Agric. Food Chem. 2019, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Kivit, S.D.; Kostadinova, A.I.; Kerperien, J.A.; Morgan, M.E.; Willemsen, L.E.M. Dietary, nondigestible oligosaccharides and Bifidobacterium breve M-16V suppress allergic inflammation in intestine via targeting dendritic cell maturation. J. Leukoc. Biol. 2017, 102, 105–115. [Google Scholar] [CrossRef]
- Laura, W.; Marianne, B.S.; Giulio, G.; Vonk, M.M.; Van, E.B.C.A.M.; Knippels, L.M.J.; Johan, G.; Smit, J.J.; Pieters, R.H.H. Dietary Supplementation with Non-Digestible Oligosaccharides Reduces Allergic Symptoms and Supports Low Dose Oral Immunotherapy in a Peanut Allergy Mouse Model. Mol. Nutr. Food Res. 2018, 62, e1800369. [Google Scholar]
- Okazaki, Y.; Katayama, T. Consumption of non-digestible oligosaccharides elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, with increased mucins and microbial fermentation in rats fed a high-fat diet. Br. J. Nutr. 2019, 121, 146–154. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Xylooligosaccharides: Manufacture and applications. Trends Food Sci. Technol. 2000, 11, 387–393. [Google Scholar] [CrossRef]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Ribeiro, T.; Cardoso, V.; Ferreira, L.M.A.; Lordelo, M.M.S.; Coelho, E.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A.; Bedford, M.R.; Fontes, C.M.G.A. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poult. Sci. 2018, 97, 4330–4341. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.M.; Li, D.D.; Bai, S.P.; Wang, J.P.; Zeng, Q.F.; Su, Z.W.; Xuan, Y.; Zhang, K.Y. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 2017, 97, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yin, J.; Zhang, K.; Xie, P.; Kong, X. Dietary xylo-oligosaccharide supplementation alters gut microbial composition and activity in pigs according to age and dose. AMB Express 2019, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Castillo, L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Yasom, S.; Tunapong, W.; Chunchai, T.; Wanchai, K.; Pongchaidecha, A.; Lungkaphin, A.; Sirilun, S.; Chaiyasut, C.; Chattipakorn, N.; et al. Lactobacillus paracasei HII01, xylooligosaccharides and synbiotics reduced gut disturbance in obese rats. Nutrition 2018, 54, 40–47. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, P.; Kong, X.; Xie, S.; Li, Q.; Li, Z.; Zhou, Z. Delicate changes of bioapatite mineral in pig femur with addition of dietary xylooligosaccharide: Evidences from Raman spectroscopy and ICP. Anim. Sci. J. 2017, 88, 1820–1826. [Google Scholar] [CrossRef]
- Raschka, L.; Daniel, H. Mechanisms underlying the effects of inulin-type fructans on calcium absorption in the large intestine of rats. Bone 2005, 37, 728–735. [Google Scholar] [CrossRef]
- Mineo, H.; Hara, H.; Tomita, F. Short-chain fatty acids enhance diffusional Ca transport in the epithelium of the rat cecum and colon. Life Sci. 2001, 69, 517–526. [Google Scholar] [CrossRef]
- Ohta, A.; Motohashi, Y.; Sakai, K.M. Dietary fructooligosaccharides increase calcium absorption and levels of mucosal calbindin-D9k in the large intestine of gastrectomized rats. Scand. J. Gastroenterol. 1998, 33, 1062–1068. [Google Scholar]
- Pascart, T.; Cortet, B.; Olejnik, C.; Paccou, J.; Migaud, H.; Cotton, A.; Delannoy, Y.; During, A.; Hardouin, P.; Penel, G.; et al. Bone Samples Extracted from Embalmed Subjects Are Not Appropriate for the Assessment of Bone Quality at the Molecular Level Using Raman Spectroscopy. Anal. Chem. 2016, 88, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Awonusi, A.; Morris, M.D.; Tecklenburg, M.M.J. Carbonate Assignment and Calibration in the Raman Spectrum of Apatite. Calcif. Tissue Int. 2007, 81, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Carden, A.; Morris, M.D. Application of vibrational spectroscopy to the study of mineralized tissues (review). J. Biomed. Opt. 2000, 5, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Mandair, G.S.; Morris, M.D. Contributions of Raman spectroscopy to the understanding of bone strength. Bonekey Rep. 2015, 4, 620. [Google Scholar] [CrossRef] [PubMed]
- Bryk, G.; Coronel, M.Z.; Pellegrini, G.; Mandalunis, P.; Rio, M.E.; De Portela, M.L.P.M.; Zeni, S.N. Effect of a combination GOS/FOS prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur. J. Nutr. 2015, 54, 913. [Google Scholar] [CrossRef]
- Weaver, C.M.; Martin, B.R.; Nakatsu, C.H.; Armstrong, A.P.; Clavijo, A.; Mccabe, L.D.; Mccabe, G.P.; Duignan, S.; Schoterman, M.H.C.; van den Heuvel, E.G.H.M. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [Google Scholar] [CrossRef]
- Demigné, C.; Jacobs, H.; Moundras, C.; Davicco, M.J.; Horcajada, M.-N.; Bernalier, A.; Coxam, V. Comparison of native or reformulated chicory fructans, or non-purified chicory, on rat cecal fermentation and mineral metabolism. Eur. J. Nutr. 2008, 47, 366–374. [Google Scholar] [CrossRef]
- Lobo, A.R.; Filho, J.M.; Alvares, E.P.; Cocato, M.L.; Colli, C. Effects of dietary lipid composition and inulin-type fructans on mineral bioavailability in growing rats. Nutrition 2009, 25, 216–225. [Google Scholar] [CrossRef]
- Christensen, E.; Licht, T.; Leser, T.; Bahl, M. Dietary Xylo-oligosaccharide stimulates intestinal bifidobacteria and lactobacilli but has limited effect on intestinal integrity in rats. BMC Res. Notes 2014, 7, 1–14. [Google Scholar] [CrossRef]
- Lecerf, J.M.; Dépeint, F.; Clerc, E.; Dugenet, Y.; Niamba, C.N.; Rhazi, L.; Cayzeele, A.; Abdelnour, G.; Jaruga, A.; Younes, H.; et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 2012, 108, 1847–1858. [Google Scholar] [CrossRef]
- Johnson, C.D.; Lucas, E.A.; Hooshmand, S.; Campbell, S.; Arjmandi, B.H. Addition of Fructooligosaccharides and Dried Plum to Soy-Based Diets Reverses Bone Loss in the Ovariectomized Rat. Evid. Based Complement. Alternat. Med. 2010, 2011, 836267. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.C.; Castro, A.S.; Rodrigues, V.C.; Fernandes, S.A.; Fontes, E.A.; de Oliveira, T.T.; Martino, H.S. Yacon Flour and Bifidobacterium longum Modulate Bone Health in Rats. J. Med. Food 2012, 15, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Wu, J.B.; Lu, T.J.; Lin, W.C. The prebiotic effect of Anoectochilus formosanus and its consequences on bone health. Br. J. Nutr. 2013, 109, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- García-Vieyra, M.I.; Del Real, A.; López, M.G. Agave fructans: Their effect on mineral absorption and bone mineral content. J. Med. Food 2014, 17, 1247–1255. [Google Scholar] [CrossRef]
- Suo, H.Q.; Lin, L.U.; Guo-Hui, X.U.; Lin, X.; Chen, X.G.; Xia, R.R.; Zhang, L.Y.; Luo, X.G. Effectiveness of dietary xylo-oligosaccharides for broilers fed a conventional corn-soybean meal diet. J. Integr. Agric. 2015, 14, 2050–2057. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; López, G.; Ros, G. Effect of probiotic prebiotic and synbiotic follow-up infant formulas on large intestine morphology and bone minerlisation in rats. J. Ence Food Agric. 2007, 87, 1059–1068. [Google Scholar] [CrossRef]
- Barboza, G.D.D.; Guizzardi, S.; Talamoni, N.T.D.; Molecular, C.D.B.Y.B.; Médicas, F.D.C.; Córdoba, U.N.D. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015, 21, 7142–7154. [Google Scholar] [CrossRef]
- Glimcher, M.J. Bone: Nature of the Calcium Phosphate Crystals and Cellular, Structural, and Physical Chemical Mechanisms in Their Formation. Rev. Mineral. Geochem. 2006, 64, 223–282. [Google Scholar] [CrossRef]
- Burr, D.B.; Robling, A.G.; Turner, C.H. Effects of biomechanical stress on bones in animals. Bone 2002, 30, 781–786. [Google Scholar] [CrossRef]
- Li, Z.; Pasteris, J.D. Tracing the pathway of compositional changes in bone mineral with age: Preliminary study of bioapatite aging in hypermineralized dolphin’s bulla. BBA Gen. Subj. 2014, 1840, 2331–2339. [Google Scholar] [CrossRef]
| Variables | 0% XOS | 1% XOS | 2% XOS | 4% XOS | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| FI (g/day) | 6.96 | 0.05 | 6.93 | 0.07 | 6.91 | 0.05 | 6.93 | 0.10 |
| WI (mL/day) | 5.96 | 0.07 | 5.98 | 0.14 | 5.94 | 0.16 | 5.85 | 0.12 |
| IBW (g) | 21.38 | 0.08 | 21.25 | 0.10 | 21.99 | 0.13 | 21.44 | 0.13 |
| FBW (g) | 29.04 | 0.68 | 28.99 | 0.71 | 29.65 | 0.67 | 29.13 | 0.60 |
| BG (g) | 7.66 | 0.24 | 7.74 | 0.16 | 7.66 | 0.20 | 7.69 | 0.24 |
| FERs (%) | 3.67 | 0.03 | 3.72 | 0.02 | 3.70 | 0.01 | 3.70 | 0.01 |
| Variables | 0% XOS | 1% XOS | 2% XOS | 4% XOS | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Cecum pH | 7.29 a | 0.01 | 7.11 b | 0.02 | 6.95 c | 0.02 | 6.82 d | 0.03 |
| Cecum Wall Weight (g) | 0.21 a | 0.01 | 0.22 ab | 0.01 | 0.23 ab | 0.01 | 0.24 b | 0.00 |
| Ca (mmol/L) | 2.17 | 0.02 | 2.19 | 0.02 | 2.11 | 0.01 | 2.11 | 0.02 |
| P (mmol/L) | 2.23 | 0.02 | 2.21 | 0.02 | 2.22 | 0.02 | 2.21 | 0.02 |
| Variables | Age (day) | 0% XOS | 1% XOS | 2% XOS | 4% XOS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| Bone length (mm) | 38 | 13.89 | 0.14 | 13.83 | 0.12 | 13.89 | 0.12 | 13.74 | 0.14 |
| 48 | 14.73 | 0.13 | 14.57 | 0.13 | 14.70 | 0.15 | 14.74 | 0.16 | |
| 58 | 14.80 | 0.11 | 15.04 | 0.12 | 15.05 | 0.35 | 15.13 | 0.18 | |
| Bone weight (g) | 38 | 0.54 | 0.01 | 0.53 | 0.00 | 0.55 | 0.00 | 0.60 | 0.01 |
| 48 | 0.64 | 0.01 | 0.60 | 0.02 | 0.57 | 0.00 | 0.60 | 0.01 | |
| 58 | 0.61 | 0.02 | 0.63 | 0.00 | 0.61 | 0.00 | 0.58 | 0.01 | |
| Bone diameter (mm) | 38 | 1.23 | 0.04 | 1.26 | 0.05 | 1.30 | 0.06 | 1.42 | 0.03 |
| 48 | 1.36 | 0.07 | 1.42 | 0.04 | 1.41 | 0.05 | 1.47 | 0.02 | |
| 58 | 1.42 | 0.04 | 1.43 | 0.03 | 1.44 | 0.03 | 1.47 | 0.02 | |
| Bone breaking strength (N) | 38 | 18.87 | 0.59 | 18.43 | 1.00 | 18.97 | 1.12 | 18.94 | 0.88 |
| 48 | 20.32 | 0.64 | 19.89 | 1.09 | 21.35 | 0.64 | 22.70 | 1.23 | |
| 58 | 20.71 a | 0.28 | 21.45 ab | 0.61 | 22.04 ab | 0.71 | 23.29 b | 0.40 | |
| Femur BMD (mg/cm2) | 38 | 92.65 | 3.14 | 90.77 | 2.11 | 95.55 | 3.90 | 95.00 | 3.54 |
| 48 | 102.08 | 2.45 | 105.45 | 3.38 | 106.67 | 3.13 | 111.88 | 3.47 | |
| 58 | 110.52 | 3.50 | 112.14 | 4.01 | 113.91 | 2.17 | 119.55 | 1.25 | |
| Distal femur BMD (mg/cm2) | 38 | 104.77 | 2.31 | 108.36 | 3.94 | 107.05 | 3.24 | 110.97 | 6.47 |
| 48 | 122.27 | 4.38 | 118.68 | 3.97 | 122.97 | 4.40 | 134.68 | 3.24 | |
| 58 | 123.85 a | 2.17 | 132.03 ab | 6.24 | 133.43 ab | 4.83 | 145.10 b | 5.83 | |
| FWHM | 38 | 17.52 | 0.37 | 17.63 | 0.42 | 16.63 | 0.20 | 16.43 | 0.19 |
| 48 | 15.88 | 0.39 | 15.92 | 0.47 | 15.36 | 0.04 | 15.82 | 0.36 | |
| 58 | 16.74 a | 0.15 | 16.12 ab | 0.23 | 16.06 ab | 0.30 | 15.39 b | 0.17 | |
| Variables | 0% XOS | 1% XOS | 2% XOS | 4% XOS | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Intestinal villus height | 568.89 a | 17.85 | 587.21 a | 18.87 | 612.13 a | 25.20 | 699.23 b | 24.88 |
| Crypt depth | 90.80 a | 5.74 | 82.70 ab | 4.11 | 77.91 ab | 4.14 | 71.41 b | 2.51 |
| VH:CD | 6.58 a | 0.32 | 7.19 a | 0.31 | 7.98 ab | 0.38 | 9.28 b | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zhou, Z. Effect of Xylo-Oligosaccharides Supplementation by Drinking Water on the Bone Properties and Related Calcium Transporters in Growing Mice. Nutrients 2020, 12, 3542. https://doi.org/10.3390/nu12113542
Gao H, Zhou Z. Effect of Xylo-Oligosaccharides Supplementation by Drinking Water on the Bone Properties and Related Calcium Transporters in Growing Mice. Nutrients. 2020; 12(11):3542. https://doi.org/10.3390/nu12113542
Chicago/Turabian StyleGao, Hang, and Zhenlei Zhou. 2020. "Effect of Xylo-Oligosaccharides Supplementation by Drinking Water on the Bone Properties and Related Calcium Transporters in Growing Mice" Nutrients 12, no. 11: 3542. https://doi.org/10.3390/nu12113542
APA StyleGao, H., & Zhou, Z. (2020). Effect of Xylo-Oligosaccharides Supplementation by Drinking Water on the Bone Properties and Related Calcium Transporters in Growing Mice. Nutrients, 12(11), 3542. https://doi.org/10.3390/nu12113542




