Maternal Diet Quality, Body Mass Index and Resource Use in the Perinatal Period: An Observational Study
Abstract
1. Introduction
- Assess the diet quality of pregnant Australian women attending a public hospital antenatal clinic;
- Estimate the total effect of BMI, adjusted for diet quality, on healthcare-resource use during the delivery admission, including mode of delivery, length of stay, admission to intensive care and midwifery-in-the-home service;
- Estimate the total effect of maternal diet quality on healthcare-resource use during the delivery admission;
- Estimate the direct effect of maternal diet quality on healthcare-resource use during the delivery admission.
2. Materials and Methods
2.1. The Study
2.1.1. Study Population and Setting
2.1.2. The Survey
2.1.3. Study Recruitment
2.2. Statistical and Economic Analyses
2.2.1. Identification and Measurement of Exposure and Outcomes
- Mode of delivery: caesarean versus vaginal (natural, instrumental, breech, compound).
- Maternal length of stay: (count in days).
- Maternal admission to intensive care: (yes or no).
- Midwifery-in-the-home service utilisation: total number of follow-up care visits associated with maternal discharge post-delivery (count).
2.2.2. Development and Use of Causal Diagrams
- Aim (ii) adjustment set: maternal age, maternal education, parity and ARFS.
- Aim (iii) adjustment set: maternal education,
- Aim (iv) adjustment set: maternal age, maternal education and BMI.
2.2.3. Statistical Methods
3. Results
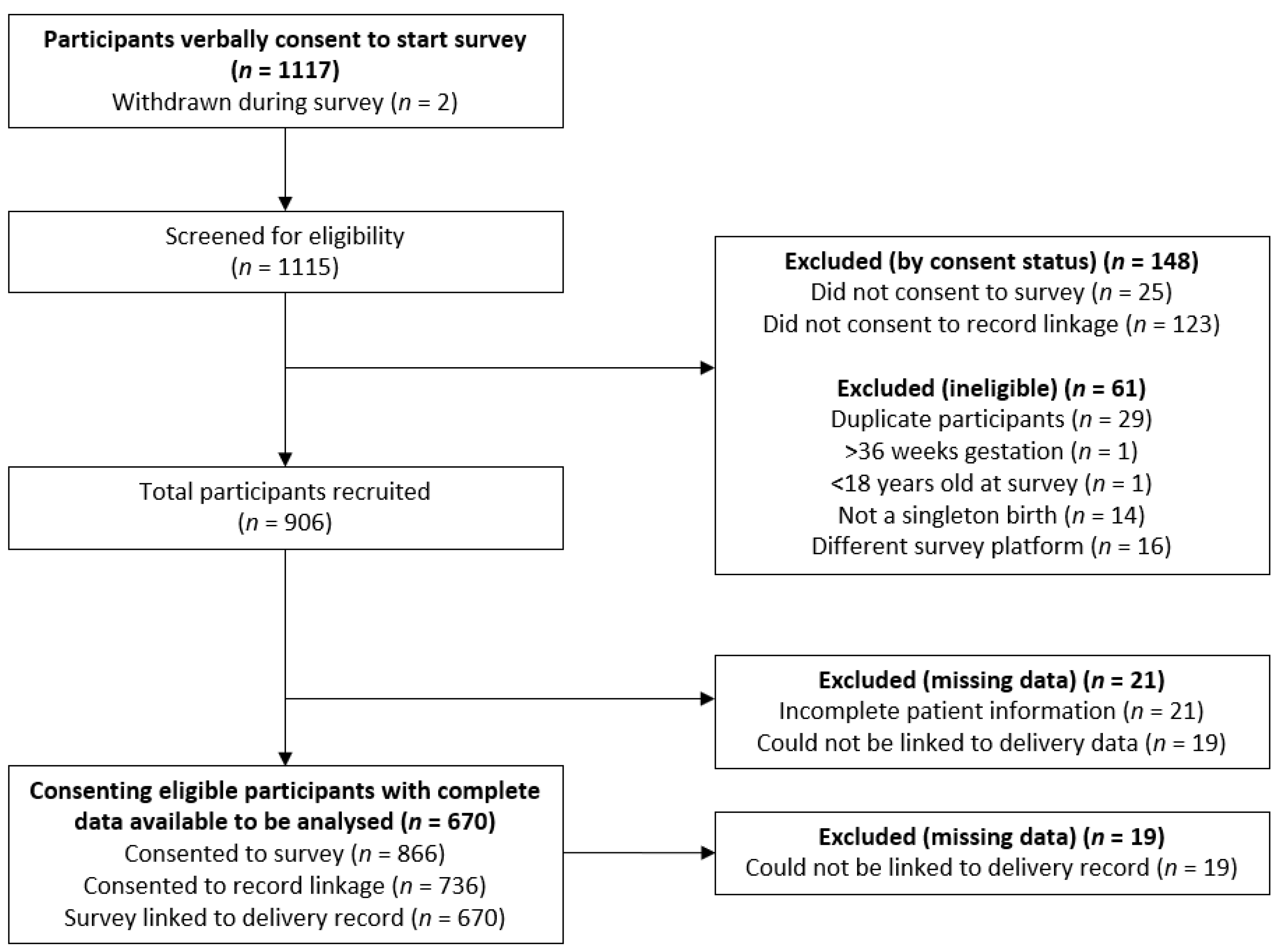
3.1. Study Recruitment
3.2. Participant Demographics
3.3. Aim (i): Diet Quality of Pregnant Women
3.4. Aim (ii): Estimate of the Total Effect of BMI on Healthcare-Resource Use
3.5. Aim (iii): Estimate of the Total Effect of Maternal Diet Quality on Healthcare-Resource Use
3.6. Aim (iv): Estimate of the Direct Effect of Maternal Diet Quality on Healthcare-Resource Use
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Study Data | Description | Preconception Process |
|---|---|---|
| Demographics | ||
| Maternal age | Age in years |
|
| Education | Maternal education level acquired: high school, TAFE, tertiary education, post-graduate | |
| Partner status | Relationship status: single, married, de facto, divorced |
|
| Insurance status | Health insurance status: no insurance, private health insurance, private insurance without obstetrics |
|
| Lifestyle | ||
| Cigarette smoking | Did the mother smoke nicotine during this pregnancy? |
|
| Alcohol consumption | AUDIT-C score |
|
| Diet Quality | Maternal ARFS during current pregnancy |
|
| Previous Medical History | ||
| Body mass index | At booking visit (20 weeks gestation) |
|
| Previous mode of delivery | Total number of previous caesarean sections |
|
| Parity | Number of previous pregnancies |
|
| Assisted reproductive therapy required? (ART) | Did the mother require ART or IVF to conceive this pregnancy? |
|
| Diabetes | Has the mother been diagnosed with type I or type II diabetes? |
|
| Recent Antenatal Period | ||
| Weight change | Did the patient gain an appropriate amount of weight during pregnancy? |
|
| Hypertensive disorders | Was the mother diagnosed with hypertensive disorders? | |
| Gestational diabetes: | Was the mother diagnosed with GDM? |
|
| Plurality | Number of infants born (2, 3, 4, …, x) |
|
| Gestation | Number of weeks at delivery |
|
| Infant birth weight | Infant birth weight in grams |
|
| Mode of delivery | Caesarean; surgical intervention (including internal manoeuvres); vaginal birth |
|
| Mother length of stay | Mothers length of stay in days |
|
| Infant length of stay | Infant length of stay in days | |
| Infant admission to nursery | Neonatal intensive care admission | |
References
- The Royal Australian College of Obstetricians and Gynaecologists. Aust. N. Z. J. Obstet. Gynaecol. 1981, 21, 128. [CrossRef]
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies 2017—In Brief; AIHW: Canberra, Australia, 2019. [Google Scholar]
- Samura, T.; Steer, J.; Michelis, L.D.; Carroll, L.; Holland, E.; Perkins, R. Factors Associated with Excessive Gestational Weight Gain: Review of Current Literature. Glob. Adv. Health Med. 2016, 5, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hure, A.J.; Powers, J.R.; Chojenta, C.; Loxton, D. Rates and Predictors of Caesarean Section for First and Second Births: A Prospective Cohort of Australian Women. Matern. Child Health J. 2017, 21, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Callaway, L.; O’Callaghan, M.; Williams, G.M.; Najman, J.M.; Alati, R.; Clavarino, A.M.; Lawlor, D.A. Associations of maternal pre-pregnancy obesity and excess pregnancy weight gains with adverse pregnancy outcomes and length of hospital stay. BMC Pregnancy Childbirth 2011, 11, 62. [Google Scholar] [CrossRef]
- Department of Health. Clinical Practice Guidelines: Pregnancy Care; Australian Government Department of Health: Canberra, Australia, 2018. [Google Scholar]
- The Royal Hospital for Women. Obesity and Weight Gain in Pregnancy, Labour and Postpartum in Local Operating Procedure: Clinical Guidelines, Procedures and Policies; The Royal Hospital for Women: Brisbane, Australia, 2014. [Google Scholar]
- Hure, A.J.; Young, A.; Smith, R.; Collins, C. Diet and pregnancy status in Australian women. Public Health Nutr. 2009, 12, 853–861. [Google Scholar] [CrossRef]
- Blumfield, M.L.; Hure, A.J.; MacDonald-Wicks, L.K.; Patterson, A.J.; Smith, R.; Collins, C.E. Disparities exist between National food group recommendations and the dietary intakes of women. BMC Women’s Health 2011, 11, 37. [Google Scholar] [CrossRef]
- Szewczyk, Z.; Holliday, E.; Dean, B.; Collins, C.; Reeves, P. A systematic review of economic evaluations of antenatal nutrition and alcohol interventions and their associated implementation interventions. Nutr. Rev. 2020. [Google Scholar] [CrossRef]
- World Health Organization. Promoting Health, Preventing Disease: The Economic Case; McDaid, D., Sasso, F., Merkur, S., Eds.; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Independent Hospital Pricing Authority. Bundled Pricing for Maternity Care, in Final Report of IHPA and the Bundled Pricing Advisory Group; IHPA: Darlinghurst, Australia, 2017. [Google Scholar]
- Australian Institute of Health and Welfare. Australias Health 2018; AIHW: Canberra, Australia, 2018. [Google Scholar]
- Morgan, K.; Rahman, M.A.; Hill, R.A.; Khanom, A.; Lyons, R.A.; Brophy, S.T. Obesity in pregnancy: Infant health service utilisation and costs on the NHS. BMJ Open 2015, 5, e008357. [Google Scholar] [CrossRef]
- Danyliv, A.; Gillespie, P.; O’Neill, C.; Noctor, E.; O’Dea, A.; Tierney, M.; McGuire, B.; Glynn, L.G.; Dunne, F. Short- and long-term effects of gestational diabetes mellitus on healthcare cost: A cross-sectional comparative study in the ATLANTIC DIP cohort. Diabet. Med. 2015, 32, 467–476. [Google Scholar] [CrossRef]
- Elenoir-Wijnkoop, I.; Van Der Beek, E.M.; Egarssen, J.; Nuijten, M.J.C.; Uauy, R.D. Health economic modeling to assess short-term costs of maternal overweight, gestational diabetes, and related macrosomia a pilot evaluation. Front. Pharmacol. 2015, 6, 103. [Google Scholar] [CrossRef]
- Ofman, J.J.; Sullivan, S.D.; Neumann, P.J.; Chiou, C.-F.; Henning, J.M.; Wade, S.W.; Hay, J.W. Examining the Value and Quality of Health Economic Analyses: Implications of Utilizing the QHES. J. Manag. Care Pharm. 2003, 9, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.; Edmunds, K.; Searles, A.; Wiggers, J. Economic evaluations of public health implementation-interventions: A systematic review and guideline for practice. Public Health 2019, 169, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.F. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Oxford Univeristy Press: Oxford, UK, 2015. [Google Scholar]
- Rabarison, K.M.; Bish, C.L.; Massoudi, M.S.; Giles, W.H. Economic Evaluation Enhances Public Health Decision Making. Front. Public Health 2015, 3, 164. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. Efficiency in Health in Productivity Commission Research Paper; Australian Government Productivity Commission: Canberra, Australia, 2015. [Google Scholar]
- Hunter New England Local Health District. Welcome to the John Hunter Hospital Birthing Services. In Information for Women and Their Families; Hunter New England Local Health District: New Lambton, Australia, 2018. [Google Scholar]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Husereau, D. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ Br. Med. J. 2013, 346. [Google Scholar] [CrossRef]
- Collins, C.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Rollo, M.E.; Hutchesson, M.J.; Burrows, T.L. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin. Nutr. 2014, 33, 906–914. [Google Scholar] [CrossRef]
- Ashton, L.M.; Williams, R.L.; Wood, L.G.; Schumacher, T.L.; Burrows, T.L.; Rollo, M.E.; Pezdirc, K.B.; Callister, R.; Collins, C.E. Comparison of Australian Recommended Food Score (ARFS) and Plasma Carotenoid Concentrations: A Validation Study in Adults. Nutrients 2017, 9, 888. [Google Scholar] [CrossRef]
- Collins, C.E.; Burrows, T.L.; Rollo, M.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.B.; Hutchesson, M.J. The Comparative Validity and Reproducibility of a Diet Quality Index for Adults: The Australian Recommended Food Score. Nutrients 2015, 7, 785–798. [Google Scholar] [CrossRef]
- Australian Consortium for Classification Development. AR-DRG. 2019. Available online: https://www.accd.net.au/ArDrg.aspx. (accessed on 9 September 2020).
- Williams, T.C.; Bach, C.C.; Matthiesen, N.B.; Henriksen, T.B.; Gagliardi, L. Directed acyclic graphs: A tool for causal studies in paediatrics. Pediatr. Res. 2018, 84, 487–493. [Google Scholar] [CrossRef]
- Gage, S.H.; Munafò, M.R.; Smith, G.D. Causal Inference in Developmental Origins of Health and Disease (DOHaD) Research. Annu. Rev. Psychol. 2016, 67, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, J.M. Thinking Clearly About Correlations and Causation: Graphical Causal Models for Observational Data. Adv. Methods Pr. Psychol. Sci. 2018, 1, 27–42. [Google Scholar] [CrossRef]
- Textor, J.; Van Der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2017, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Firth, D. Bias Reduction of Maximum Likelihood Estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Australian Consortium for Classification Development. Review of the AR-DRG Classification Case Complexity Process: Final Report 2014; Prepared for the Independent Hospital Pricing Authority: Sydney, Australia, 2014. [Google Scholar]
- Taylor, C.R.; Dominguez, J.E.; Habib, A.S. Obesity and Obstetric Anesthesia: Current Insights. Local Reg. Anesthesia 2019, 12, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.S.; Lamon, A.M. Managing anesthesia for cesarean section in obese patients: Current perspectives. Local Reg. Anesth. 2016, 9, 45–57. [Google Scholar] [CrossRef]
- Schmied, V.; Duff, M.; Dahlen, H.G.; Mills, A.; Kolt, G.S. ‘Not waving but drowning’: A study of the experiences and concerns of midwives and other health professionals caring for obese childbearing women. Midwifery 2011, 27, 424–430. [Google Scholar] [CrossRef]
- Slater, K.; Rollo, M.E.; Szewczyk, Z.; Ashton, L.M.; Schumacher, T.L.; E Collins, C. Do the Dietary Intakes of Pregnant Women Attending Public Hospital Antenatal Clinics Align with Australian Guide to Healthy Eating Recommendations? Nutrients 2020, 12, 2438. [Google Scholar] [CrossRef]
- Bailey, C.; Skouteris, H.; Teede, H.J.; Hill, B.; De Courten, B.; Walker, R.; Liew, D.; Thangaratinam, S.; Ademi, Z. Are Lifestyle Interventions to Reduce Excessive Gestational Weight Gain Cost Effective? A Systematic Review. Curr. Diabetes Rep. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Bailey, C.; Skouteris, H.; Harrison, C.L.; Boyle, J.; Bartlett, R.; Hill, B.; Thangaratinam, S.; Teede, H.; Ademi, Z. Cost Effectiveness of Antenatal Lifestyle Interventions for Preventing Gestational Diabetes and Hypertensive Disease in Pregnancy. Pharm. Econ. Open 2020, 4, 499–510. [Google Scholar] [CrossRef]
- Lee, Y.Q.; Collins, C.E.; Schumacher, T.L.; Weatherall, L.J.; Keogh, L.; Sutherland, K.; Gordon, A.; Rae, K.M.; Pringle, K.G. Disparities exist between the dietary intake of Indigenous Australian women during pregnancy and the Australian dietary guidelines: The Gomeroi gaaynggal study. J. Hum. Nutr. Diet. 2018, 31, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M.S.; Bromiker, R.; Hammerman, C.; Chertman, L.; Ioscovich, A.; Granovsky-Grisaru, S.; Samueloff, A.; Elstein, D. The effects of maternal age and parity on maternal and neonatal outcome. Arch. Gynecol. Obstet. 2014, 291, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Waldenström, U.; Cnattingius, S.; Vixner, L.; Norman, M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: A population-based register study. BJOG Int. J. Obstet. Gynaecol. 2016, 124, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Baser, E.; Seckin, K.D.; Erkilinc, S.; Karsli, M.F.; Yeral, I.M.; Kaymak, O.; Caglar, T.; Danisman, N. The impact of parity on perinatal outcomes in pregnancies complicated by advanced maternal age. J. Turk. Gynecol. Assoc. 2013, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, L.E. Risk of adverse pregnancy outcomes at advanced maternal age. Obstet. Gynecol. 2018, 131, 457–463. [Google Scholar] [CrossRef]
- Baron, R.; Manniën, J.; Velde, S.J.T.; Klomp, T.; Hutton, E.K.; Brug, J. Socio-demographic inequalities across a range of health status indicators and health behaviours among pregnant women in prenatal primary care: A cross-sectional study. BMC Pregnancy Childbirth 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Boudet-Berquier, J.; Salanave, B.; Desenclos, J.-C.; Castetbon, K. Sociodemographic factors and pregnancy outcomes associated with prepregnancy obesity: Effect modification of parity in the nationwide Epifane birth-cohort. BMC Pregnancy Childbirth 2017, 17, 273. [Google Scholar] [CrossRef][Green Version]
- Gresham, E.; Collins, C.E.; Mishra, G.D.; Byles, J.E.; Hure, A.J. Diet quality before or during pregnancy and the relationship with pregnancy and birth outcomes: The Australian Longitudinal Study on Women’s Health. Public Health Nutr. 2016, 19, 2975–2983. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Australia’s Welfare 2017: in Brief; Australian Government: Canberra, Australia, 2017. [Google Scholar]
- Adams, N.; Gibbons, K.; Tudehope, D. Public-private differences in short-term neonatal outcomes following birth by prelabour caesarean section at early and full term. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 176–185. [Google Scholar] [CrossRef]
- Nippita, T.A.; Trevena, J.A.; Patterson, J.; Ford, J.; Morris, J.M.; Roberts, C.L. Inter-hospital variations in labor induction and outcomes for nullipara: An Australian population-based linkage study. Acta Obstet. Gynecol. Scand. 2016, 95, 411–419. [Google Scholar] [CrossRef]
- Robson, S.J.; Laws, P.; Sullivan, E.A. Adverse outcomes of labour in public and private hospitals in Australia: A population-based descriptive study. Med. J. Aust. 2009, 190, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, H.G.; Tracy, S.; Tracy, M.; Bisits, A.; Brown, C.; Thornton, C. Rates of obstetric intervention among low-risk women giving birth in private and public hospitals in NSW: A population-based descriptive study. BMJ Open 2012, 2, e001723. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies 2012. In Perinatal Statistics Series Number 30; Australian Institute of Health and Welfare: Canberra, Australia, 2014. [Google Scholar]
- Blatt, K.; Moore, E.; Chen, A.; Van Hook, J.; DeFranco, E.A. Association of Reported Trimester-Specific Smoking Cessation With Fetal Growth Restriction. Obstet. Gynecol. 2015, 125, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Kingsland, M.; Doherty, E.; Anderson, A.E.; Crooks, K.; Tully, B.; Tremain, D.; Tsang, T.W.; Attia, J.; Wolfenden, L.; Dunlop, A.J.; et al. A practice change intervention to improve antenatal care addressing alcohol consumption by women during pregnancy: Research protocol for a randomised stepped-wedge cluster trial. Implement. Sci. 2018, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Stanesby, O.; Cook, M.; Callinan, S. Examining Trends in Alcohol Consumption during Pregnancy in Australia, 2001 to 2016; Foundation for Alcohol Research and Education: Canberra, Australia, 2017. [Google Scholar]
- Dodd, J.M.; For the LIMIT Randomised Trial Group; McPhee, A.J.; Turnbull, D.; Yelland, L.N.; Deussen, A.R.; Grivell, R.M.; Crowther, C.A.; Wittert, G.; Owens, J.A.; et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on neonatal health outcomes: The LIMIT randomised trial. BMC Med. 2014, 12, 163. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.W.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012, 344, e2088. [Google Scholar] [CrossRef]
- Graves, B.W.; DeJoy, S.; Heath, A.; Pekow, P. Maternal Body Mass Index, Delivery Route, and Induction of Labor in a Midwifery Caseload. J. Midwifery Women’s Health 2006, 51, 254–259. [Google Scholar] [CrossRef]
- Barau, G.; Robillard, P.-Y.; Hulsey, T.; Dedecker, F.; Laffite, A.; Gerardin, P.; Kauffmann, E. Linear association between maternal pre-pregnancy body mass index and risk of caesarean section in term deliveries. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1173–1177. [Google Scholar] [CrossRef]
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Birth after Previous Caesarean Section; Women’s Health Committee Members, Ed.; Royal Australian and New Zealand College of Obstetricians and Gynaecologists: Melbourne, Australia, 2015. [Google Scholar]
- ACOG Committee on Obstetric Practice; ACOG Committee on Gynecologic Practice; ACOG Committee on Genetics. ACOG Committee Opinion #324: Perinatal Risks Associated With Assisted Reproductive Technology. Obstet. Gynecol. 2005, 106, 1143–1146. [Google Scholar] [CrossRef]
- The Royal Australian College of General Practitioners. Pregnancy with pre-existing type 2 diabetes. In General Practice Management of Type 2 Diabetes: 2016–18; The Royal Australian College of General Practitioners: Melbourne, Australia, 2016. [Google Scholar]
- Guelinckx, I.; Devlieger, R.; Beckers, K.; VanSant, G. Maternal obesity: Pregnancy complications, gestational weight gain and nutrition. Obes. Rev. 2008, 9, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; Bowyer, L.; Lust, K.; McMahon, L.P.; Morton, M.R.; North, R.A.; Paech, M.J.; Said, J.M. The SOMANZ Guidelines for the Management of Hypertensive Disorders of Pregnancy 2014. Aust. N. Z. J. Obstet. Gynaecol. 2014, 55, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Dyson, P.; Kelly, T.; Deakin, T.; Duncan, A.; Frost, G.; Harrison, Z.; Khatri, D.; Kunka, D.; McArdle, P.; Mellor, D.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2011, 28, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Multiple pregnancy: Antenatal care for twin and triplet pregnancies. In Clinical Guideline [CG129]; NICE: London, UK, 2011. [Google Scholar]
- Seaton, S.E.; Barker, L.; Draper, E.S.; Abrams, K.R.; Modi, N.; Manktelow, B.N. Estimating neonatal length of stay for babies born very preterm. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 104, F182–F186. [Google Scholar] [CrossRef]
- Hsia, R.Y.; Antwi, Y.A.; Weber, E. Analysis of variation in charges and prices paid for vaginal and caesarean section births: A cross-sectional study. BMJ Open 2014, 4, e004017. [Google Scholar] [CrossRef]
- Ministry of Health. NSW Maternity and Neonatal Service Capability Framework. In Guideline; NSW Government, Ed.; Ministry of Health, NSW: Sydney, Australia, 2016. [Google Scholar]
| Participant Demographic and Health Data | ||
|---|---|---|
| Characteristic | Statistic or Class | Total (N = 670) |
| Age at survey | mean (SD) | 30.3 (5.5) |
| median (min, max) | 30.1 (18.4, 53.0) | |
| Aboriginal or Torres Strait Islander | No | 600 (93%) |
| Yes | 44 (6.8%) | |
| Born in Australia | No | 62 (9.6%) |
| Yes | 581 (90%) | |
| Marital status | Married/de facto | 548 (85%) |
| Divorced/separated | 21 (3.3%) | |
| Single | 73 (11%) | |
| Language spoken at home | English only | 598 (93%) |
| Other | 44 (6.9%) | |
| Highest educational qualification | No formal qualifications | 20 (3.1%) |
| Year 10 or equivalent | 107 (17%) | |
| Year 12 or equivalent | 111 (17%) | |
| Trade/Apprenticeship | 29 (4.5%) | |
| Certificate/Diploma | 176 (27%) | |
| University undergraduate | 151 (23%) | |
| University postgraduate | 50 (7.8%) | |
| Annual household income | Less than $20,800 | 32 (5.1%) |
| $20,800 to less than $41,600 | 44 (7.0%) | |
| $41,600 to less than $65,000 | 68 (11%) | |
| $65,000 to less than $104,000 | 158 (25%) | |
| $104,000 or more | 172 (27%) | |
| Not provided | 153 (24%) | |
| Weeks of gestation at survey | mean (SD) | 32 (3) |
| median (min, max) | 31 (28, 36) | |
| Received pregnancy diet advice from health professional | Yes | 325 (54%) |
| No | 263 (44%) | |
| Unsure | 15 (2.5%) | |
| Pre-pregnancy body mass index (BMI) measured | mean (SD) | 28.8 (8.3) |
| median (min, max) | 26.8 (14.7, 64.0) | |
| Underweight (<18.5 kg/m2) | 30 (4.5%) | |
| Normal (18.5–24.9 kg/m2) | 247 (37%) | |
| Overweight (25.0–29.9 kg/m2) | 139 (21%) | |
| Obese Class I (30.0–34.9 kg/m2) | 116 (17%) | |
| Obese Class II (35.0–39.9 kg/m2) | 64 (9.6%) | |
| Obese class III (≥40 kg/m2) | 74 (11%) | |
| Number ANC visits | mean (SD) | 12.1 (5.3) |
| median (min, max) | 11.0 (1.0, 40.0) | |
| Alcohol risk score | mean (SD) | 0.1 (0.5) |
| median (min, max) | 0.0 (0.0, 9.0) | |
| Number term pregnancies | mean (SD) | 1.3 (1.1) |
| median (min, max) | 1.0 (0.0, 8.0) | |
| Number preterm pregnancies | mean (SD) | 0.1 (0.4) |
| median (min, max) | 0.0 (0.0, 3.0) | |
| Number living children | mean (SD) | 1.3 (1.1) |
| median (min, max) | 1.0 (0.0, 10.0) | |
| History of endocrine disease | No | 534 (80%) |
| Yes | 136 (20%) | |
| History of hypertension | No | 606 (90%) |
| Yes | 64 (9.6%) | |
| Maternal risk factor—diabetes | No | 488 (73%) |
| Yes | 182 (27%) | |
| Maternal risk factor—hypertension | No | 607 (91%) |
| Yes | 63 (9.4%) | |
| Maternal risk factor—anaemia | No | 448 (67%) |
| Yes | 222 (33%) | |
| Maternal risk factor—smoke during pregnancy | No | 568 (85%) |
| Yes | 102 (15%) | |
| Characteristic | Statistic or Class | Underweight (n = 30) | Normal (n = 247) | Overweight (n = 139) | Obese Class I (n = 116) | Obese Class II (n = 64) | Obese Class III (n = 74) |
|---|---|---|---|---|---|---|---|
| Diet quality (ARFS) | mean (SD) | 27.2 (13.8) | 31.2 (13.1) | 27.2 (14.3) | 27.1 (12.7) | 28.3 (9.8) | 28.2 (12.9) |
| median (min, max) | 28.0 (4.0, 56.0) | 34.0 (1.0, 54.0) | 30.0 (1.0, 50.0) | 29.0 (2.0, 52.0) | 29.5 (9.0, 46.0) | 29.0 (1.0, 51.0) | |
| Maternal length of stay (days) | mean (SD) | 1.6 (1.5) | 1.9 (1.6) | 2.1 (1.6) | 2.2 (1.5) | 2.2 (1.6) | 2.2 (1.6) |
| median (min, max) | 1.0 (0.0, 5.0) | 2.0 (0.0, 9.0) | 2.0 (0.0, 7.0) | 2.0 (0.0, 8.0) | 2.0 (0.0, 9.0) | 2.0 (0.0, 7.0) | |
| Number of “midwifery-in- the-home” visits | mean (SD) | 1.8 (1.0) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.7) | 1.5 (0.8) |
| median (min, max) | 2.0 (0.0, 5.0) | 2.0 (0.0, 6.0) | 2.0 (0.0, 4.0) | 2.0 (0.0, 4.0) | 2.0 (0.0, 3.0) | 2.0 (0.0, 3.0) | |
| Delivery mode | Vaginal birth | 21 (70%) | 167 (68%) | 79 (57%) | 65 (56%) | 33 (52%) | 36 (49%) |
| Caesarean section | 9 (30%) | 80 (32%) | 60 (43%) | 51 (44%) | 31 (48%) | 38 (51%) |
| Characteristic | Statistic or Class | Total (N = 670) |
|---|---|---|
| Infant birthweight (grams) | mean (SD) | 3359.4 (515.1) |
| median (min, max) | 3390.0 (1450.0, 4830.0) | |
| Gestational age at birth (weeks) | mean (SD) | 38.4 (1.4) |
| median (min, max) | 38.0 (31.0, 41.0) | |
| Maternal length of stay (days) | mean (SD) | 2.1 (1.6) |
| median (min, max) | 2.0 (0.0, 9.0) | |
| Mode of delivery | Normal vaginal birth | 334 (50%) |
| Caesarean section | 269 (40%) | |
| Abnormal vaginal birth | 67 (10%) | |
| Pre-term birth (<37 weeks) | No | 626 (93%) |
| Yes | 44 (6.6%) | |
| Gender of infant | Male | 326 (49%) |
| Female | 344 (51%) | |
| Birthweight category | Low birth weight (<2500 g) | 35 (5.2%) |
| Normal range | 568 (85%) | |
| Macrosomia (>4000 g) | 67 (10%) | |
| Midwifery-in-the-home care visits | mean (SD) | 1.6 (0.9) |
| median (min, max) | 2.0 (0.0, 6.0) | |
| Maternal admission to higher level care (intensive care) | No | 664 (99%) |
| Yes | 4 (0.6%) |
| Caesarean Delivery | Maternal Length of Stay | MITH Visits | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Rate Ratio (95% CI) | p-Value | Rate Ratio (95% CI) | p-Value | |
| Aim (ii)—total effect of BMI * | ||||||
| Underweight | 0.58 (0.16 to 2.08) | 0.40 | 0.78 (0.49 to 1.23) | 0.28 | 0.95 (0.63 to 1.44) | 0.82 |
| Normal | (ref) | (ref) | (ref) | |||
| Overweight | 1.57 (0.91 to 2.71) | 0.11 | 1.04 (0.86 to 1.26) | 0.71 | 0.95 (0.78 to 1.18) | 0.66 |
| Obese Class I | 1.18 (0.65 to 2.16) | 0.58 | 1.07 (0.87 to 1.32) | 0.51 | 0.89 (0.70 to 1.12) | 0.31 |
| Obese Class II | 2.13 (1.03 to 4.39) | 0.04 | 1.11 (0.87 to 1.42) | 0.41 | 0.99 (0.75 to 1.30) | 0.92 |
| Obese class III | 1.92 (0.98 to 3.73) | 0.06 | 1.10 (0.87 to 1.39) | 0.41 | 0.90 (0.69 to 1.16) | 0.41 |
| Aim (iii)—total effect of ARFS ** | ||||||
| Quintile 1 | 1.08 (0.64 to 1.85) | 0.77 | 1.20 (1.00 to 1.44) | 0.05 | 1.02 (0.83 to 1.26) | 0.85 |
| Quintile 2 | 1.16 (0.69 to 1.96) | 0.58 | 1.10 (0.91 to 1.32) | 0.33 | 1.09 (0.89 to 1.34) | 0.41 |
| Quintile 3 | 0.72 (0.42 to 1.24) | 0.24 | 1.05 (0.87 to 1.27) | 0.60 | 1.01 (0.82 to 1.25) | 0.91 |
| Quintile 4 | 0.93 (0.54 to 1.62) | 0.80 | 1.12 (0.92 to 1.35) | 0.26 | 0.99 (0.79 to 1.22) | 0.90 |
| Quintile 5 | (ref) | (ref) | (ref) | |||
| Aim (iv)—direct effect of ARFS *** | ||||||
| Quintile 1 | 1.23 (0.71 to 2.16) | 0.46 | 1.27 (1.05 to 1.53) | 0.01 | 1.00 (0.81 to 1.24) | 0.99 |
| Quintile 2 | 1.25 (0.72 to 2.17) | 0.43 | 1.14 (0.94 to 1.37) | 0.19 | 1.07 (0.87 to 1.32) | 0.52 |
| Quintile 3 | 0.74 (0.42 to 1.29) | 0.29 | 1.07 (0.88 to 1.29) | 0.50 | 1.00 (0.81 to 1.24) | 0.96 |
| Quintile 4 | 0.97 (0.55 to 1.71) | 0.92 | 1.13 (0.93 to 1.37) | 0.21 | 0.98 (0.78 to 1.21) | 0.83 |
| Quintile 1 | (ref) | . | (ref) | . | (ref) | . |
| AR-DRG | Normal | Obese Class II | Obese Class III | |||||
|---|---|---|---|---|---|---|---|---|
| Code | Description | NWAU Cost | n * = 242 | Cost ($) ** | n = 62 | Cost ($) ** | n = 72 | Cost ($) ** |
| O01A | Caesarean delivery, major complexity | $17,170 | 5 | $85,850 | 2 | $34,340 | 10 | $171,700 |
| O01B | Caesarean delivery, intermediate complexity | $12,310 | 39 | $480,090 | 14 | $172,340 | 15 | $184,650 |
| O01C | Caesarean delivery, minor complexity | $10,074 | 34 | $342,516 | 15 | $151,110 | 13 | $130,962 |
| O02A | Vaginal delivery with operating room procedures, major complexity | $12,691 | 3 | $38,073 | 0 | $0 | 0 | $0 |
| O02B | Vaginal delivery with operating room procedures, minor complexity | $9119 | 6 | $54,714 | 3 | $27,357 | 0 | $0 |
| O60A | Vaginal delivery, major complexity | $8967 | 19 | $170,373 | 9 | $80,703 | 8 | $71,736 |
| O60B | Vaginal delivery, intermediate complexity | $6206 | 82 | $508,892 | 15 | $93,090 | 22 | $136,532 |
| O60C | Vaginal delivery, minor complexity | $4560 | 54 | $246,240 | 4 | $18,240 | 4 | $18,240 |
| Cost per patient *** | $7962 | $9309 | $9914 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, Z.; Weaver, N.; Rollo, M.; Deeming, S.; Holliday, E.; Reeves, P.; Collins, C. Maternal Diet Quality, Body Mass Index and Resource Use in the Perinatal Period: An Observational Study. Nutrients 2020, 12, 3532. https://doi.org/10.3390/nu12113532
Szewczyk Z, Weaver N, Rollo M, Deeming S, Holliday E, Reeves P, Collins C. Maternal Diet Quality, Body Mass Index and Resource Use in the Perinatal Period: An Observational Study. Nutrients. 2020; 12(11):3532. https://doi.org/10.3390/nu12113532
Chicago/Turabian StyleSzewczyk, Zoe, Natasha Weaver, Megan Rollo, Simon Deeming, Elizabeth Holliday, Penny Reeves, and Clare Collins. 2020. "Maternal Diet Quality, Body Mass Index and Resource Use in the Perinatal Period: An Observational Study" Nutrients 12, no. 11: 3532. https://doi.org/10.3390/nu12113532
APA StyleSzewczyk, Z., Weaver, N., Rollo, M., Deeming, S., Holliday, E., Reeves, P., & Collins, C. (2020). Maternal Diet Quality, Body Mass Index and Resource Use in the Perinatal Period: An Observational Study. Nutrients, 12(11), 3532. https://doi.org/10.3390/nu12113532





