Maternal Arsenic Exposure and Gestational Diabetes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Inclusion Criteria
2.3. Data Extraction
2.4. Assessment of Risk of Bias
2.5. Data Analysis
3. Results
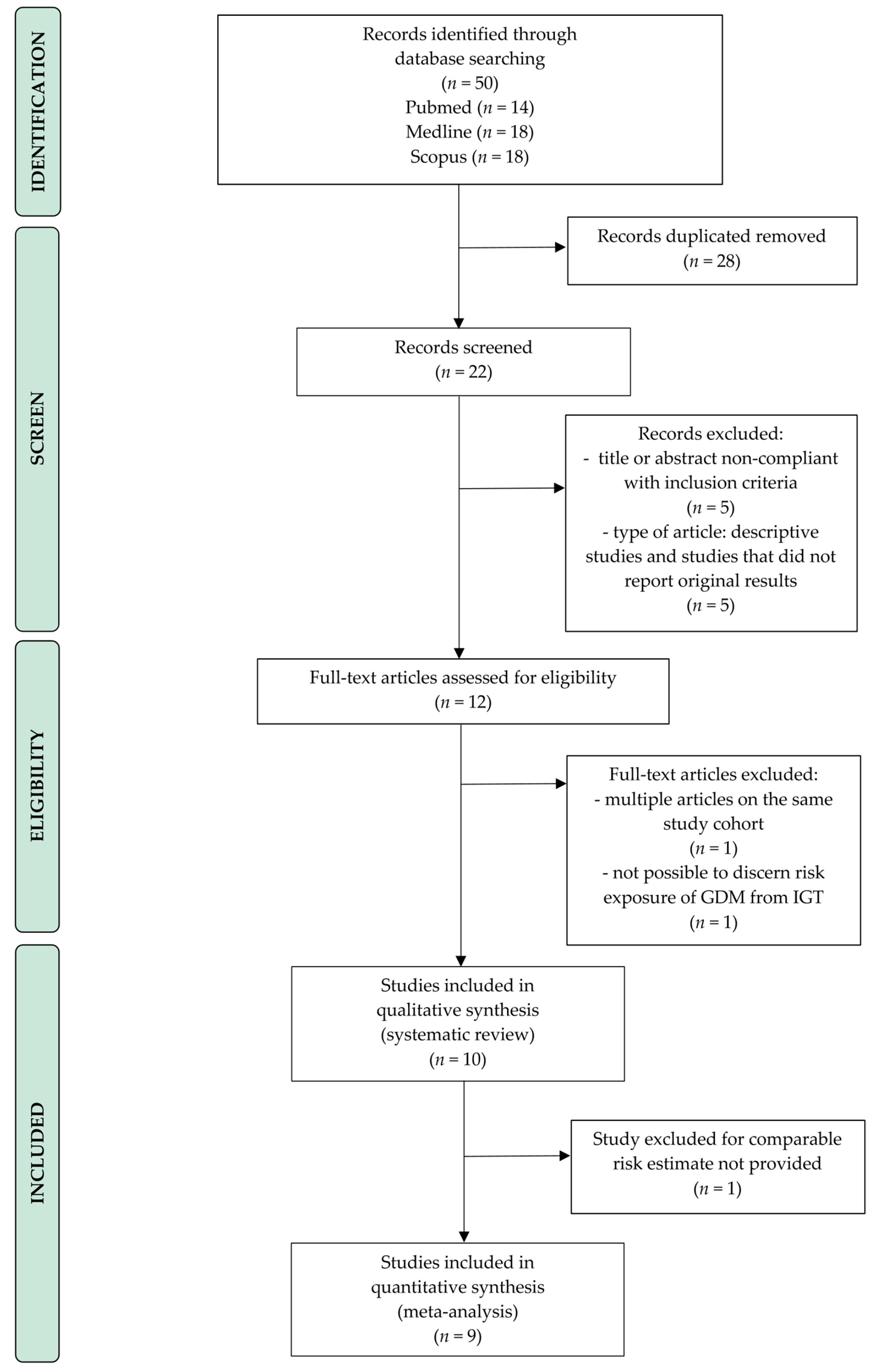
3.1. Literature Search
3.2. Description of Studies
3.2.1. Arsenic in Blood Samples
3.2.2. Arsenic in Urine Samples
3.2.3. Arsenic in Tap Water Samples
3.2.4. Arsenic in Meconium Samples
3.2.5. Arsenic in Toenail Samples
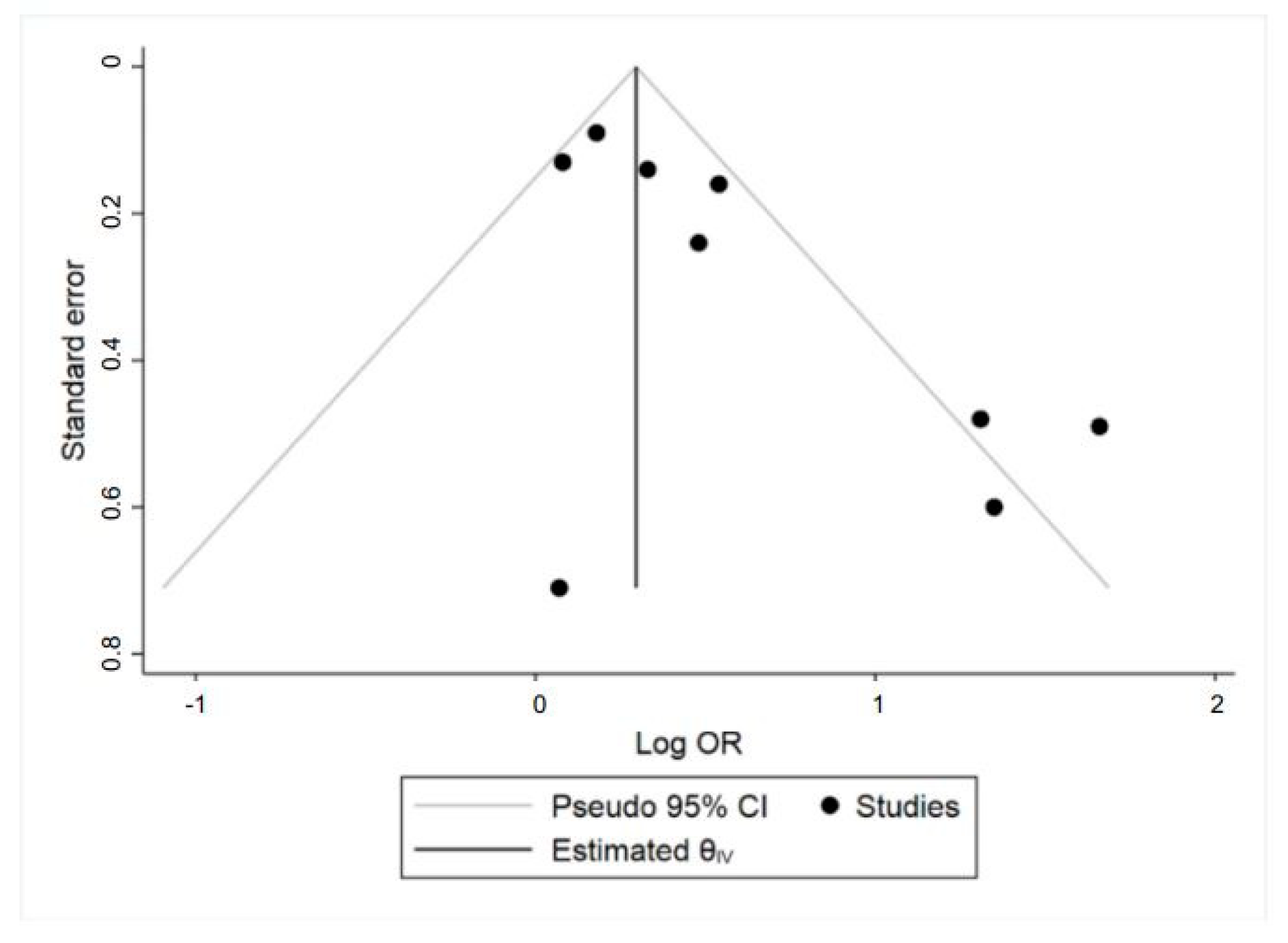
3.3. Meta-Analysis
Subgroups Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American Diabetes Care. Diabetes Advocacy: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S152–S153. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; IDF: Brussels, Belgium, 2017. [Google Scholar]
- Baz, B.; Riveline, J.; Gautier, J. Endocrinology of pregnancy: Gestational diabetes mellitus: Definition, aetiological and clinical aspects. Eur. J. Endocrinol. 2016, 174, R43–R51. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacquemine, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B.; Lowe, L.; Dyer, A.; Contreras, M.; Sacks, D.A.; Watson, W.; Dooley, S.L.; Foderaro, M.; Niznik, C.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Catalano, P.; McIntyre, H.; Cruickshank, J.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Persson, B.; HAPO Study Cooperative Research Group; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef]
- Hwu, L.; Sung, F.; Mou, C.; Wang, I.; Shih, H.; Chang, Y.; Tzeng, Y. Risk of Subsequent Hypertension and Diabetes in Women with Hypertension During Pregnancy and Gestational Diabetes. Mayo Clin. Proc. 2016, 91, 1158–1165. [Google Scholar] [CrossRef]
- Retnakaran, R.; Shah, B.R. Role of Type 2 Diabetes in Determining Retinal, Renal, and Cardiovascular Outcomes in Women With Previous Gestational Diabetes Mellitus. Diabetes Care 2017, 40, 101–108. [Google Scholar] [CrossRef]
- Leybovitz-Haleluya, N.; Wainstock, T.; Landau, D.; Sheiner, E. Maternal gestational diabetes mellitus and the risk of subsequent pediatric cardiovascular diseases of the offspring: A population-based cohort study with up to 18 years of follow up. Acta Diabetol. 2018, 55, 1037–1042. [Google Scholar] [CrossRef]
- Blotsky, A.L.; Rahme, E.; Dahhou, M.; Nakhla, M.; Dasgupta, K. Gestational diabetes associated with incident diabetes in childhood and youth: A retrospective cohort study. CMAJ. 2019, 191, E410–E417. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef]
- Garrison, A. Screening, diagnosis, and management of gestational diabetes mellitus. Am. Fam. Phys. 2015, 91, 460–467. [Google Scholar] [PubMed]
- Zhang, C.; Rawal, S.; Chong, Y.S. Risk factors for gestational diabetes: Is prevention possible? Diabetologia 2016, 59, 1385–1390. [Google Scholar] [CrossRef]
- Shapiro, G.D.; Dodds, L.; Arbuckle, T.E.; Ashley-Martin, J.; Fraser, W.; Fisher, M.; Taback, S.; Keely, E.; Bouchard, M.F.; Dallaire, R.; et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ. Int. 2015, 83, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, Z.; Lin, Y.; Zhang, D. Association of inorganic arsenic exposure with type 2 diabetes mellitus: A meta-analysis. J. Epidemiol. Community Health 2014, 68, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Feseke, S.K.; St-Laurent, J.; Anassour-Sidi, E.; Ayotte, P.; Bouchard, M.; Levallois, P. Arsenic exposure and type 2 diabetes: Results from the 2007–2009 Canadian Health Measures Survey. Health Promot. Chronic Dis. Prev. Can. 2015, 35, 63–72. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U. Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Aylward, L.L.; Ramasamy, S.; Hays, S.M.; Schoeny, R.; Kirman, C.R. Evaluation of urinary speciated arsenic in NHANES: Issues in interpretation in the context of potential inorganic arsenic exposure. Regul. Toxicol. Pharmacol. 2014, 69, 49–54. [Google Scholar] [CrossRef]
- Watanabe, T.; Hirano, S. Metabolism of arsenic and its toxicological relevance. Arch. Toxicol. 2013, 87, 969–979. [Google Scholar] [CrossRef]
- Andra, S.S.; Makris, K.C.; Christophi, C.A.; Ettinger, A.S. Delineating the degree of association between biomarkers of arsenic exposure and type-2 diabetes mellitus. Int. J. Hyg. Environ. Health 2013, 216, 35–49. [Google Scholar] [CrossRef]
- Douillet, C.; Currier, J.; Saunders, J.; Bodnar, W.M.; Matoušek, T.; Stýblo, M. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol. Appl. Pharmacol. 2013, 267, 11–15. [Google Scholar] [CrossRef]
- Padmaja Divya, S.; Pratheeshkumar, P.; Son, Y.; Vinod Roy, R.; Andrew Hitron, J.; Kim, D.; Dai, J.; Wang, L.; Asha, P.; Xu, M.; et al. Arsenic Induces Insulin Resistance in Mouse Adipocytes and Myotubes Via Oxidative Stress-Regulated Mitochondrial Sirt3-FOXO3a Signaling Pathway. Toxicol. Sci. 2015, 146, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol. Appl. Pharmacol. 2004, 197, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Liang, C.; Sheng, J.; Yan, S.; Huang, K.; Li, Z.; Pan, W.; Tao, R.; Hao, J.; Tong, S.; et al. Association between serum arsenic levels and gestational diabetes mellitus: A population-based birth cohort study. Environ. Pollut. 2018, 235, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Chen, X.; Wu, W.; Feng, Y.; Yang, H.; Li, M.; Xie, B.; Guo, P.; Shi, X.; et al. Multiple metal concentrations and gestational diabetes mellitus in Taiyuan, China. Chemosphere 2019, 237, 124412. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Gossai, A.; Chen, Y.; Chasan-Taber, L.; Baker, E.; Karagas, M. Maternal arsenic exposure and gestational diabetes and glucose intolerance in the New Hampshire birth cohort study. Environ. Health 2016, 15, 106. [Google Scholar] [CrossRef]
- Ashley-Martin, J.; Dodds, L.; Arbuckle, T.E.; Bouchard, M.F.; Shapiro, G.D.; Fisher, M.; Monnier, P.; Morisset, A.; Ettinger, A.S. Association between maternal urinary speciated arsenic concentrations and gestational diabetes in a cohort of Canadian women. Environ. Int. 2018, 121, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.P.; Valdes, M.; Munoz-Quezada, M.T.; Lucero, B.; Rubilar, P.; Pino, P.; Iglesias, V. Urinary Inorganic Arsenic Concentration and Gestational Diabetes Mellitus in Pregnant Women from Arica, Chile. Int. J. Environ. Res. Public Health 2018, 15, 1418. [Google Scholar] [CrossRef]
- Khan, M.H.; Ahmad, S.K.A.; Nahar, M.; Faruquee, M.H.; Yasmin, R.; Dutta, S.; Kabir, S.M.N.; Khandker, S. Gestational diabetes among the arsenic exposed women from arsenic contaminated area of Bangladesh. MJPHM 2018, 18, 13–19. [Google Scholar]
- Wang, X.; Gao, D.; Zhang, G.; Zhang, X.; Li, Q.; Gao, Q.; Chen, R.; Xu, S.; Huang, L.; Lin, L.; et al. Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: A prospective cohort study. Environ. Int. 2020, 135, 105370. [Google Scholar] [CrossRef]
- Peng, S.; Liu, L.; Zhang, X.; Heinrich, J.; Zhang, J.; Schramm, K.; Huang, Q.; Tian, M.; Eqani, S.A.M.A.S.; Heqing, S. A nested case-control study indicating heavy metal residues in meconium associate with maternal gestational diabetes mellitus risk. Environ. Health 2015, 14, 19. [Google Scholar] [CrossRef]
- Marie, C.; Léger, S.; Guttmann, A.; Rivière, O.; Marchiset, N.; Lémery, D.; Vendittelli, F.; Sauvant-Rochat, M. Exposure to arsenic in tap water and gestational diabetes: A French semi-ecological study. Environ. Res. 2018, 161, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care 2011, 34 (Suppl. 1), S11–S61. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Collège National des Gynécologues et Obstétriciens Français. Recommandations Pour la Pratique Clinique. Diabète et Grossesse; CNGOF: Paris, France, 1996. [Google Scholar]
- Berger, H.; Crane, J.; Farine, D.; Armson, A.; De La Ronde, S.; Keenan-Lindsay, L.; Leduc, L.; Reid, G.; Van Aerde, J.; Maternal-Fetal Medicine Committee; et al. Screening for gestational diabetes mellitus. J. Obstet. Gynaecol. Can. 2002, 24, 894–912. [Google Scholar] [CrossRef]
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee; Thompson, D.; Berger, H.; Feig, R.; Gagnon, R.; Kader, T.; Keely, E.; Kozak, S.; Ryan, E.; Sermer, M.; et al. Diabetes and Pregnancy. Can. J. Diabetes 2013, 37 (Suppl. 1), S168–S183. [Google Scholar] [CrossRef]
- Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; Rychen, G.; et al. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA J. 2018, 16, e05122. [Google Scholar]
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analysis. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analyzing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated in July 2019); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Oxford, UK, 2019. [Google Scholar]
- Egger, M.; Davey, S.G.; Schneider, M.; Minder, C. Bias in meta-analysis detected bu a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lagakos, S.W.; Ware, J.H.; Hunter, D.J.; Drazen, J.M. Statistics in Medicine—Reporting of Subgroup Analyses in Clinical Trials. N. Eng. J. Med. 2007, 357, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Di Cianni, G.; Miccoli, R.; Volpe, L.; Lencioni, C.; Del Prato, S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab. Res. Rev. 2003, 19, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obstet. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Sung, T.C.; Huang, J.W.; Guo, H.R. Association between Arsenic Exposure and Diabetes: A Meta-Analysis. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Molin, M.; Ulven, M.; Meltzer, H.M.; Alexander, J. Arsenic in the human food chain, biotransformation and toxicology—Review focusing on seafood arsenic. J. Trace Elem. Med. Biol. 2015, 31, 249–259. [Google Scholar] [CrossRef]
- James, K.A.; Marshall, J.A.; Hokanson, J.E.; Meliker, J.R.; Zerbe, G.O.; Byers, T.E. A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ. Res. 2013, 123, 33–38. [Google Scholar] [CrossRef]
- Hall, M.; Chen, Y.; Ahsan, H.; Slavkovich, V.; van Geen, A.; Parvez, F.; Graziano, J. Blood arsenic as a biomarker of arsenic exposure: Results from a prospective study. Toxicology 2006, 225, 225–233. [Google Scholar] [CrossRef]
- National Research Council. Arsenic in Drinking Water; The National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Gilbert-Diamond, D.; Cottingham, K.L.; Gruber, J.F.; Punshon, T.; Sayarath, V.; Gandolfi, A.J.; Baker, E.R.; Jackson, B.P.; Folt, C.L.; Karagas, M.R. Rice consumption contributes to arsenic exposure in US women. Proc. Natl. Acad. Sci. USA 2011, 108, 20656–20660. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Nachman, K.E. Public health responses to arsenic in rice and other foods. JAMA Intern. Med. 2013, 17, 1395–1396. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Tosteson, T.D.; Blum, J.; Klaue, B.; Weiss, J.E.; Stannard, V.; Spate, V.; Morris, J.S. Measurement of low levels of arsenic exposure: A comparison of water and toenail concentrations. Am. J. Epidemiol. 2000, 152, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ostrea, E.M.; Morales, V.; Ngoumgna, E.; Prescilla, R.; Tan, E.; Hernandez, E.; Baens Ramirez, G.; Cifra, H.L.; Manlapaz, M.L. Prevalence of fetal exposure to environmental toxins as determined by meconium analysis. Neurotoxicology 2002, 23, 329–339. [Google Scholar] [CrossRef]
- Jones, M.R.; Tellez-Plaza, M.; Vaidya, D.; Grau-Perez, M.; Post, W.S.; Kaufman, J.D.; Guallar, E.; Francesconi, K.A.; Goessler, W.; Nachman, K.E.; et al. Ethnic, geographic and dietary differences in arsenic exposure in the multi-ethnic study of atherosclerosis (MESA). J. Expo. Sci. Environ. Epidemiol. 2019, 29, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rubio, P.; Klimentidis, Y.C.; Cantu-Soto, E.; Meza-Montenegro, M.M.; Billheimer, D.; Lu, Z.; Chen, Z.; Klimecki, W.T. Indigenous American ancestry is associated with arsenic methylation efficiency in an admixed population of northwest Mexico. J. Toxicol. Environ. Health A 2012, 75, 36–49. [Google Scholar] [CrossRef][Green Version]
- Fu, S.; Wu, J.; Li, Y.; Liu, Y.; Gao, Y.; Yao, F.; Qiu, C.; Song, L.; Wu, Y.; Sun, D.; et al. Urinary arsenic metabolism in a Western Chinese population exposed to high-dose inorganic arsenic in drinking water: Influence of ethnicity and genetic polymorphisms. Toxicol. Appl. Pharm. 2014, 274, 117–123. [Google Scholar] [CrossRef]
- González-Martínez, F.; Sánchez-Rodas, D.; Varela, N.M.; Sandoval, C.A.; Quiñones, L.A.; Johnson-Restrepo, B. As3MT and GST Polymorphisms Influencing Arsenic Metabolism in Human Exposure to Drinking Groundwater. Int. J. Mol. Sci. 2020, 21, 4832. [Google Scholar] [CrossRef]
- Ministerio de Salud: Guía Diabetes y Embarazo. Santiago. 2014. Available online: https://www.minsal.cl/wp-content/uploads/2015/11/GUIA-DIABETES-Y-EMBARAZO_web-14-11-2014.pdf (accessed on 30 July 2020).
- Lapolla, A.; Dalfrà, M.G.; Ragazzi, E.; De Cata, A.P.; Fedele, D. New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria: A retrospective study on pregnancy outcome. Diabet. Med. 2011, 28, 1074–1077. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Colagiuri, S.; Roglic, G.; Hod, M. Diagnosis of GDM: A suggested consensus. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 194–205. [Google Scholar] [CrossRef]
- Hedges, L.; Vevea, J. Fixed-and random-effects models in meta-analysis. Psychol. Methods 1998, 3, 486. [Google Scholar] [CrossRef]
- Rice, K.; Higgins, J.P.T.; Lumley, T. A re-evaluation of fixed effect(s) meta-analysis. J. R. Stat. Soc. Ser. A 2018, 181, 205–227. [Google Scholar] [CrossRef]
- Joober, R.; Schmitz, N.; Annable, L.; Boksa, P. Pubblication bias: What are the challenges and can they be overcome? J. Psychiatry Neurosci. 2012, 37, 149–152. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Study Country | Study Design | Study Period | Sample Size (Cases/ Controls) | Age (Cases/ Controls) | Definition of Cases | Exposure Indicator and When | As Exposure (Cut-Off or LOD) | Confounding Factors Considered |
|---|---|---|---|---|---|---|---|---|---|
| • Blood samples (3 studies) | |||||||||
| Shapiro et al., 2015 [14] | Canada | Cohort study | 2008–2011 | 48/1167 | 18–29 yo: 12.5%/ 24.8% 30–34 yo: 45.8%/ 34.8% ≥35 yo: 41.7%/ 40.2% | CDA-SOGC Criteria a [38,39] | 1st trimester blood samples 1 | LOD: 0.22 µg/L | Maternal age, race, pre-pregnancy BMI, education, parity, race |
| Xia et al., 2018 [25] | China | Cohort study | 05/2013– 09/2014 | 419/2841 | cases: 27.79 ± 4.25 yo controls: 26.18 ± 3.48 yo | ADA Diagnostic Criteria b [35] | 1st,2nd, 3rd trimester serum samples | LOD: 0.0047 µg/L | Maternal age, pre-pregnancy BMI, monthly income, gestational age, parity |
| Wang Y. et al., 2019 [26] | China | Cohort study | 2012–2016 | 776/776 | cases: 31.00 ± 4.53 yo controls: 30.97 ± 4.53 yo | ADA Diagnostic Criteria b [35] | Serum 2 samples the day before delivery | As level: a. Low <10.64 µg/L b. Middle 10.64–21.12 µg/L c. High ≥21.12 µg/L | Maternal age, pre-pregnancy BMI, gestational weight gain, physical activity, family history of diabetes, month of conception, residence, education, monthly income, smoking, fetal gender, parity, gestational age |
| • Urine samples (5 studies) | |||||||||
| Farzan et al., 2016 [27] | USA | Cohort study | 01/2009– 05/2016 | 14/1032 | cases: 32.2 yo controls: 30.9 yo | ADA Diagnostic Criteria b,c [35] | home tap water samples, urine samples at 24–28 gw, toenails samples | LOD (urine): 0.10–0.15 µg/L | Maternal age, pre-pregnancy BMI, pregnancy weight gain, smoking, secondhand smoke exposure, education, gestational week of glucose testing, urinary creatinine |
| Ashley-Martin et al., 2018 [28] | Canada | Cohort study | 2008–2011 | 42/1049 | <29 yo: 19.2%/ 30.3% 30–34 yo: 46.8%/ 36.3% ≥35 yo: 34.0%/ 33.7% | CDA-SOGC Diagnostic Criteria a [38,39] | 1st trimester urinary concentrations of arsenite, arsenate, MMA, DMA and AsB | LOD: 0.75 µg/L | Maternal age, gravidity, race, education, parity, pre-pregnancy BMI, maternal first trimester blood Cd levels |
| Munoz et al., 2018 [29] | Chile | Cross-sectional study | 06/2013– 10/2013 | 21/223 | ≤29 yo: 57.1%/ 74.4% 30–34 yo: 28.6%/ 15.7% ≥35 yo: 14.3%/ 9.9% | WHO Diagnostic Criteria d,e [36] | 2nd trimester urinary levels of arsenite, arsenate, MMA, DMA, T-InAs (calculated by adding values of these species) | LOD: 0.1 µg/L | Maternal age, education, ethnicity, BMI |
| Khan et al., 2018 [30] | Bangladesh | Cross-sectional study | - | 31/169 | cases: 25.19 ± 4.28 yo controls: 23.95 ± 3.92 yo | WHO Diagnostic Criteria d [36] | urine samples (not said when) | Not As exposed: ≤0.100 mg/L As exposed: >0.100 mg/L | Maternal age, gestational age, parity, BMI |
| Wang X. et al., 2020 [31] | China | Cohort study | 07/2014– 07/2016 | 241/1849 | cases: 29.54 ± 4.13 yo controls: 28.25 ± 3.34 yo all sample: 28.40 ± 3.47 yo | ADA Diagnostic Criteria b [35] | urine samples 3 <20 gw | LOD: 0.009 µg/L CAU-As: a. Low <32.11 µg/L b. Middle 32.11–48.11 µg/L c. High ≥48.11 µg/L | Maternal age, pre-pregnancy BMI, gravidity, occupational status, smoking exposure, average personal monthly income, family history of diabetes, physical activity, fetal sex |
| • Tap water samples (2 studies) | |||||||||
| Farzan et al., 2016 [27] | USA | Cohort study | 01/2009– 05/2016 | 14/1032 | cases: 32.2 yo controls: 30.9 yo | ADA Diagnostic Criteria b,c [35] | home tap water samples, urine samples, toenails samples | LOD (water): 0.001–0.07 µg/L | Maternal age, pre-pregnancy BMI, pregnancy weight gain, smoking, secondhand smoke exposure, education, gestational week of glucose testing, urinary creatinine |
| Marie et al., 2018 [33] | France | Semi- ecological study (correlational) | 2003 2006 2010 | 286/4767 | all sample: 29.1 ± 5.6 yo | CNGOF Diagnostic criteria g [37] | water samples during the 12 months before pregnancy | Not As exposed: <10 µg/L As Exposed: a. Low 10–30 µg/L b. High ≥ 30µg/L | Maternal age, family situation, number of inhabitants in commune of residence, geographic origin, employment during pregnancy, paid employment, pre-pregnancy BMI, type of pregnancy, year of delivery |
| • Meconium samples (1 study) | |||||||||
| Peng et al., 2015 [32] | China | Case-control study nested in a cohort | 06/2012– 07/2012 | 137/190 | cases: 27.85 ± 3.87 yo controls: 26.34± 2.64 yo | WHO Diagnostic Criteria d,f [36] | meconium samples during the first 2 postnatal days | LOD: 0.06 µg/L | Maternal age, pre-pregnancy BMI, gravidity, parity, HBV infection, newborn sex |
| • Toenails samples (1 study) | |||||||||
| Farzan et al., 2016 [27] | USA | Cohort study | 01/2009– 05/2016 | 14/1032 | cases: 32.2 yo controls: 30.9 yo | ADA Diagnostic Criteria b,c [35] | home tap water samples, urine samples, toenails samples 2 weeks post-partum | Ln toenails As (µg/g) (not said LOD) | Maternal age, pre-pregnancy BMI, pregnancy weight gain, smoking, secondhand smoke exposure, education, gestational week of glucose testing, urinary creatinine |
| Stratifications | N. Studies | Effect Estimates | Heterogeneity | |||
|---|---|---|---|---|---|---|
| OR | (95% CI) | χ2 | p | I2 | ||
| All included studies a,b [14,25,26,27,28,29,30,31,32,33] | 9 | 1.56 | (1.23, 1.99) | 21.95 | 0.005 | 64% |
| All studies less Peng et al. (2015) | 8 | 1.43 | (1.17, 1.74) | 14.28 | 0.05 | 51% |
| All studies less Farzan et al. (2016) | 8 | 1.73 | (1.27, 2.43) | 19.46 | 0.007 | 64% |
| All studies less Wang Y. et al. (2019) | 8 | 1.72 | (1.30, 2.27) | 18.55 | 0.01 | 62% |
| All studies less Ashley Martin et al. (2018) | 8 | 1.50 | (1.19, 1.89) | 18.88 | 0.009 | 63% |
| All studies less Marie et al. (2018) | 8 | 1.57 | (1.20, 2.05) | 21.32 | 0.003 | 67% |
| All studies less Munoz et al. (2018) | 8 | 1.59 | (1.24, 2.04) | 21.85 | 0.003 | 68% |
| All studies less Shapiro et al. (2015) | 8 | 1.47 | (1.17, 1.84) | 17.56 | 0.01 | 60% |
| All studies less Wang X. et al. (2020) | 8 | 1.66 | (1.23, 2.23) | 21.89 | 0.003 | 68% |
| All studies less Xia et al. (2018) | 8 | 1.55 | (1.18, 2.04) | 19.63 | 0.006 | 64% |
| Study design | ||||||
| Cohort studies | 5 | 1.16 | (1.07, 1.26) | 13.73 | 0.008 | 71% |
| Cross-sectional studies | 2 | 2.28 | (0.92, 5.64) | 1.86 | 0.17 | 46% |
| Nested case-control studies | 1 | / | / | / | / | / |
| Correlational studies | 1 | / | / | / | / | / |
| Exposure indicator | ||||||
| Blood samples | 3 | 1.35 | (1.11, 1.65) | 8.87 | 0.01 | 77% |
| Urine samples | 4 | 1.39 | (1.07, 1.82) | 4.20 | 0.24 | 29% |
| Tap water samples | 2 | 1.11 | (1.02, 1.21) | 2.49 | 0.11 | 60% |
| Meconium samples | 1 | / | / | / | / | / |
| Toenails samples | 1 | / | / | / | / | / |
| Study country | ||||||
| North America | 3 | 1.28 | (1.07, 1.53) | 8.57 | 0.01 | 77% |
| North America less Shapiro et al. (2015) c | 2 | 1.23 | (1.03, 1.48) | 3.65 | 0.06 | 73% |
| Asia | 4 | 1.37 | (1.17, 1.62) | 12.32 | 0.006 | 76% |
| Asia less Peng et al. (2015) d | 3 | 1.32 | (1.12, 1.56) | 4.78 | 0.09 | 58% |
| South America | 1 | / | / | / | / | / |
| Europe | 1 | / | / | / | / | / |
| Diagnostic criteria | ||||||
| ADA | 4 | 1.27 | (1.12, 1.43) | 5.37 | 0.15 | 44% |
| ADA less Farzan et al. (2016) e | 3 | 1.32 | (1.12, 1.56) | 4.78 | 0.09 | 58% |
| WHO | 2 | 3.13 | (1.41, 6.95) | 3.36 | 0.07 | 70% |
| CDA-SOGC c | 2 | 3.76 | (1.79, 7.91) | 0.00 | 0.96 | 0% |
| CNGOF | 1 | / | / | / | / | / |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmeri, N.; Villanacci, R.; Ottolina, J.; Bartiromo, L.; Cavoretto, P.; Dolci, C.; Lembo, R.; Schimberni, M.; Valsecchi, L.; Viganò, P.; et al. Maternal Arsenic Exposure and Gestational Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 3094. https://doi.org/10.3390/nu12103094
Salmeri N, Villanacci R, Ottolina J, Bartiromo L, Cavoretto P, Dolci C, Lembo R, Schimberni M, Valsecchi L, Viganò P, et al. Maternal Arsenic Exposure and Gestational Diabetes: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(10):3094. https://doi.org/10.3390/nu12103094
Chicago/Turabian StyleSalmeri, Noemi, Roberta Villanacci, Jessica Ottolina, Ludovica Bartiromo, Paolo Cavoretto, Carolina Dolci, Rosalba Lembo, Matteo Schimberni, Luca Valsecchi, Paola Viganò, and et al. 2020. "Maternal Arsenic Exposure and Gestational Diabetes: A Systematic Review and Meta-Analysis" Nutrients 12, no. 10: 3094. https://doi.org/10.3390/nu12103094
APA StyleSalmeri, N., Villanacci, R., Ottolina, J., Bartiromo, L., Cavoretto, P., Dolci, C., Lembo, R., Schimberni, M., Valsecchi, L., Viganò, P., & Candiani, M. (2020). Maternal Arsenic Exposure and Gestational Diabetes: A Systematic Review and Meta-Analysis. Nutrients, 12(10), 3094. https://doi.org/10.3390/nu12103094









