Choline Plus Working Memory Training Improves Prenatal Alcohol-Induced Deficits in Cognitive Flexibility and Functional Connectivity in Adulthood in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Choline Administration
2.3. Delayed Non-Matching to Place
2.4. Attentional Set Shifting and Reversal Learning
2.5. MRI Data Acquisition
2.6. Rs-fMRI Data Preprocessing
2.7. Statistical Analysis
3. Results
3.1. All Groups Achieved High Response Accuracy over Training Sessions on the Delayed Non-Matching to Place Task
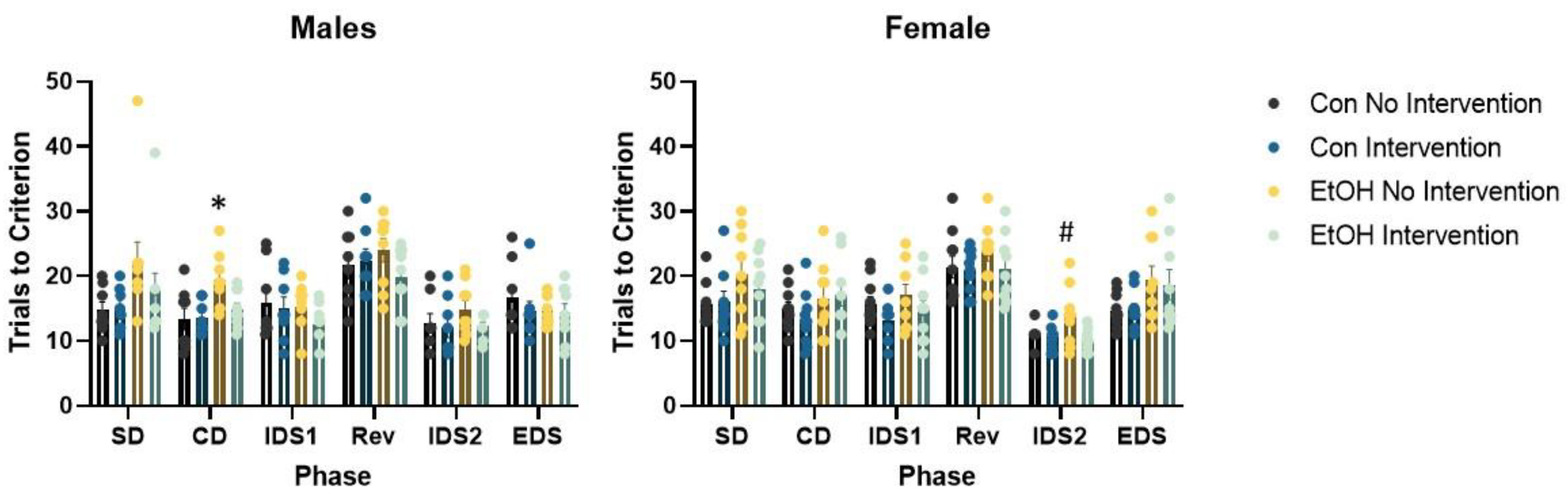
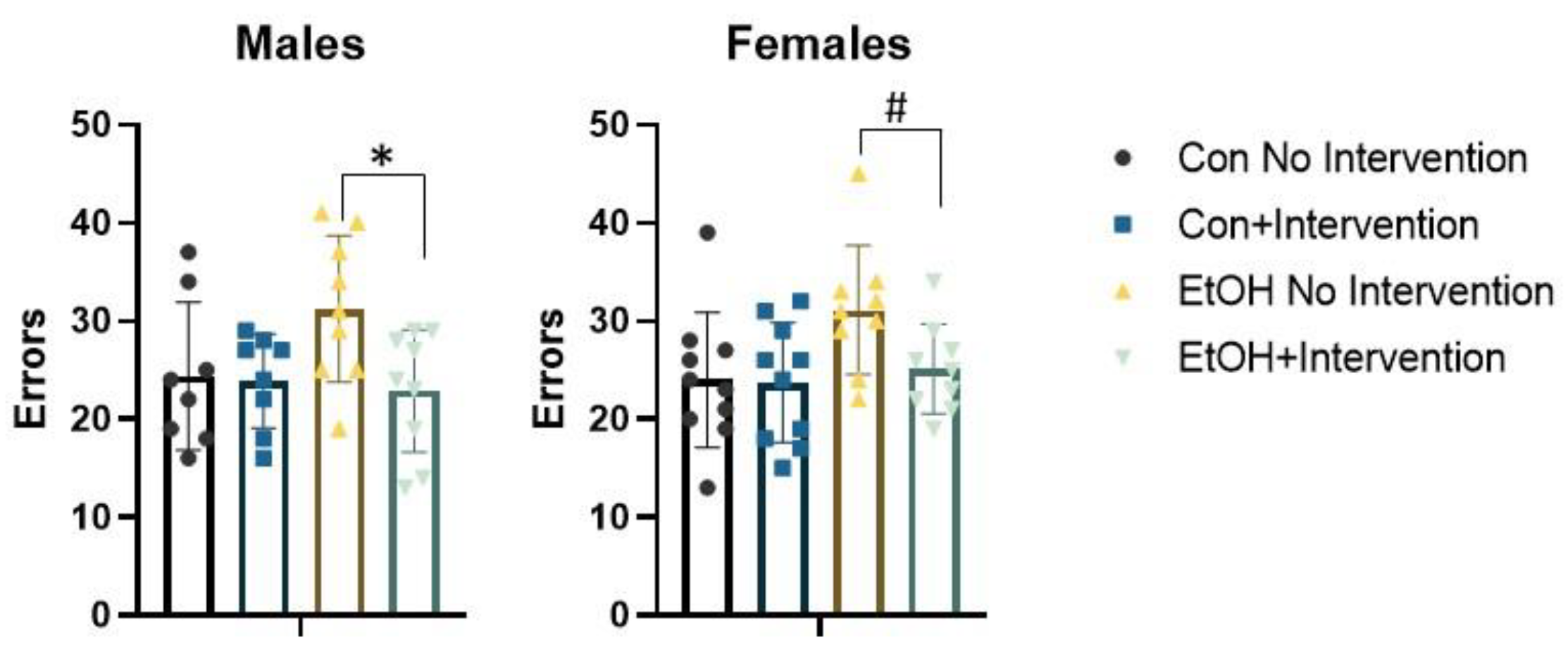
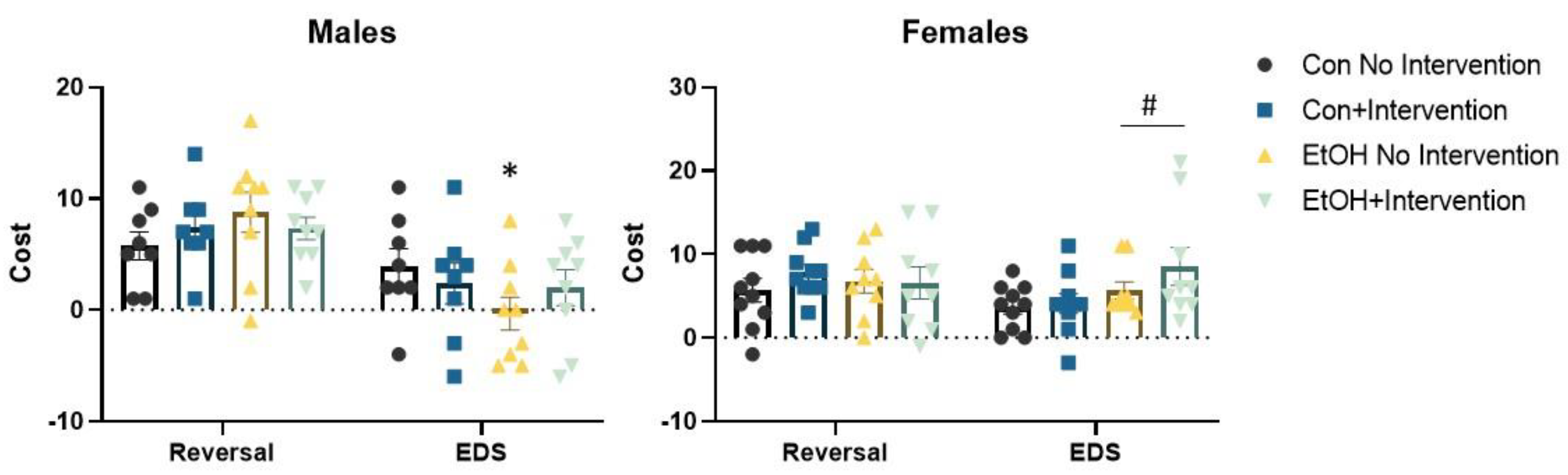
3.2. Prenatal Exposure to a Low Concentration of Ethanol Affected the Formation of an Attentional Set in a Sex-Specific Fashion
3.3. Analysis of Males Alone Revealed a Sub-Group of Ethanol-Exposed Rats Receiving the Intervention that Employed a Normal Strategy to Solve the ASST-R Task
3.4. Analysis of Females Alone Confirmed Effects of Prenatal Ethanol and Intervention
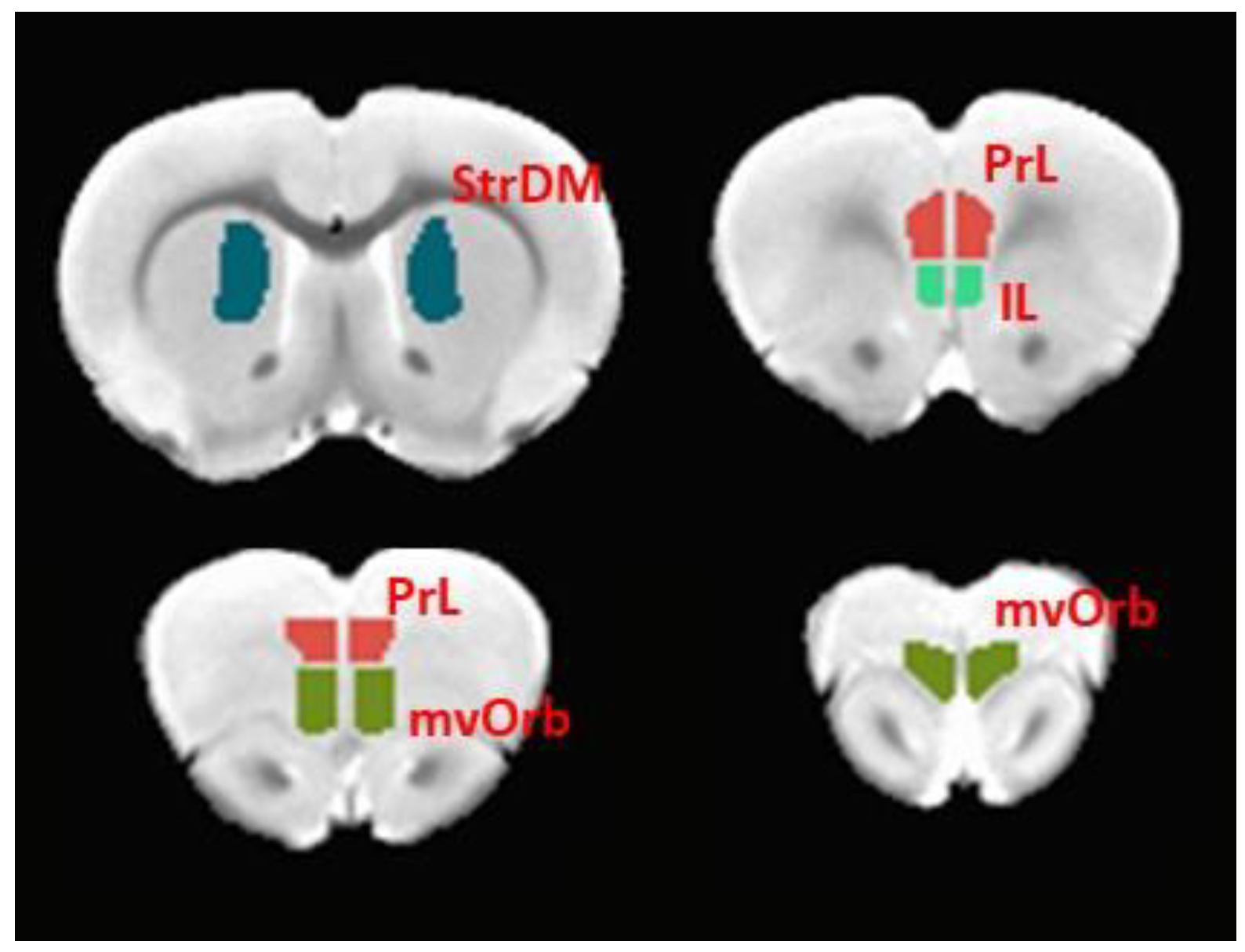
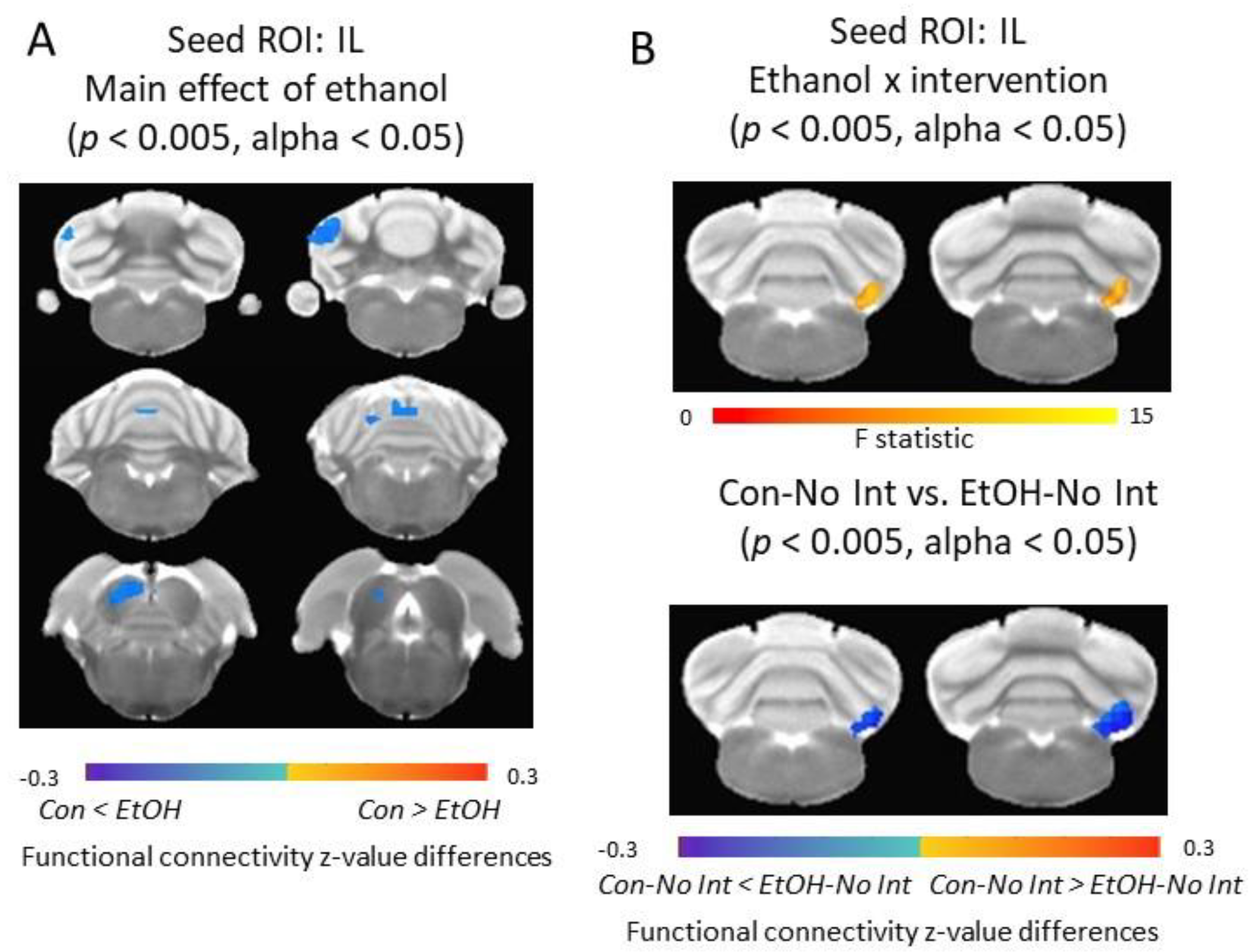
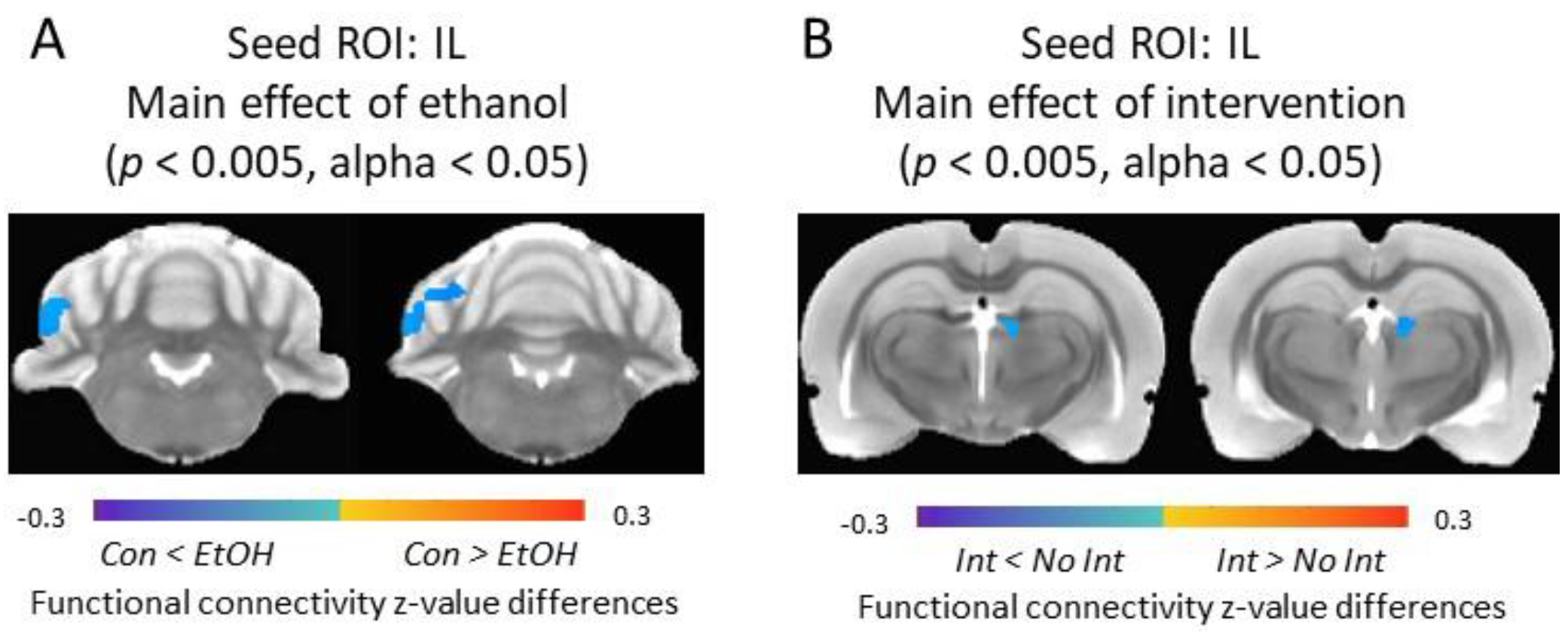
3.5. Functional Connectivity of the Infralimbic Cortex and Cerebellum Was Increased in Ethanol-Exposed Rats
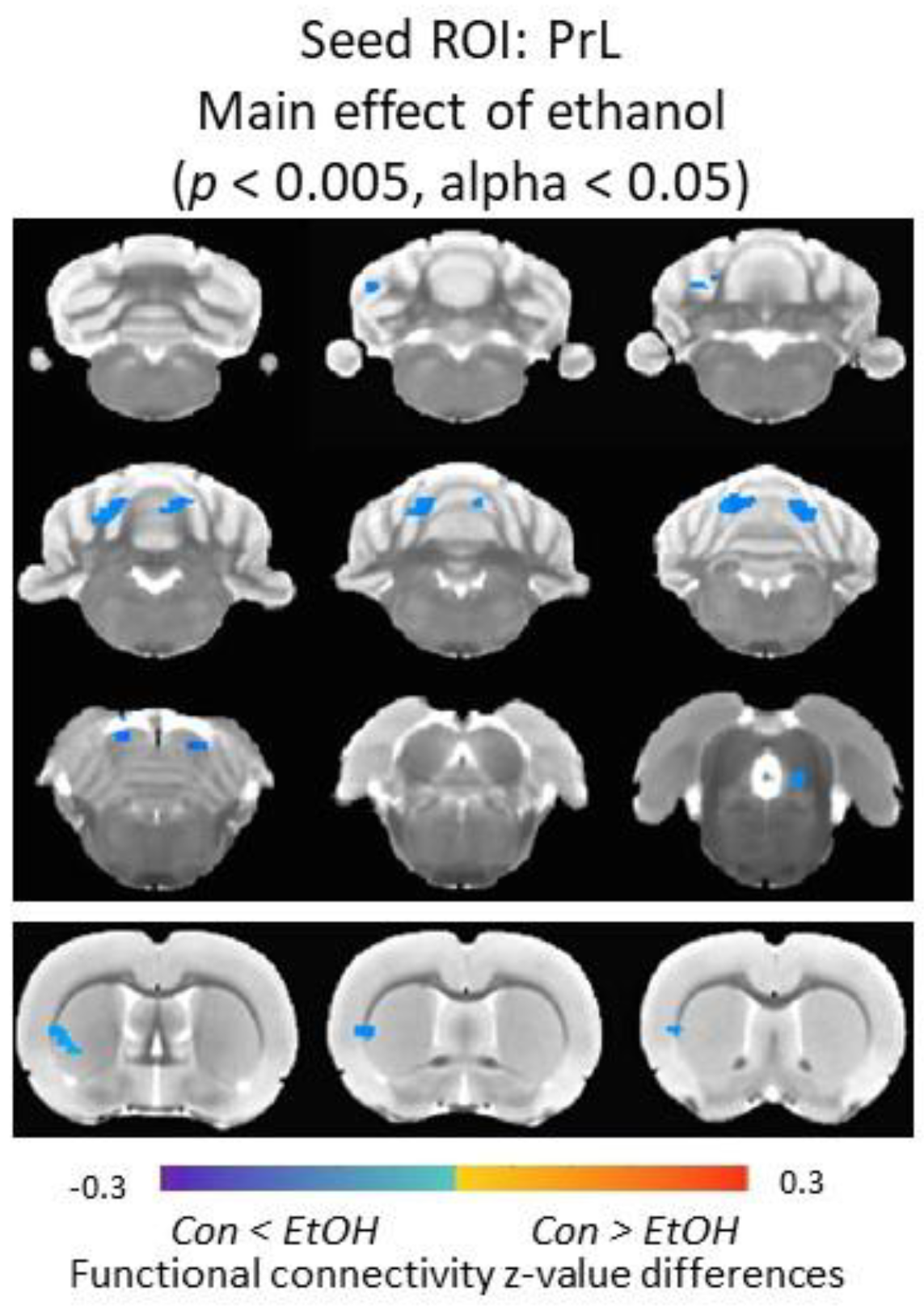
3.6. Functional Connectivity of the Prelimbic Cortex and Cerebellum Was Increased in Ethanol-Exposed Male Rats
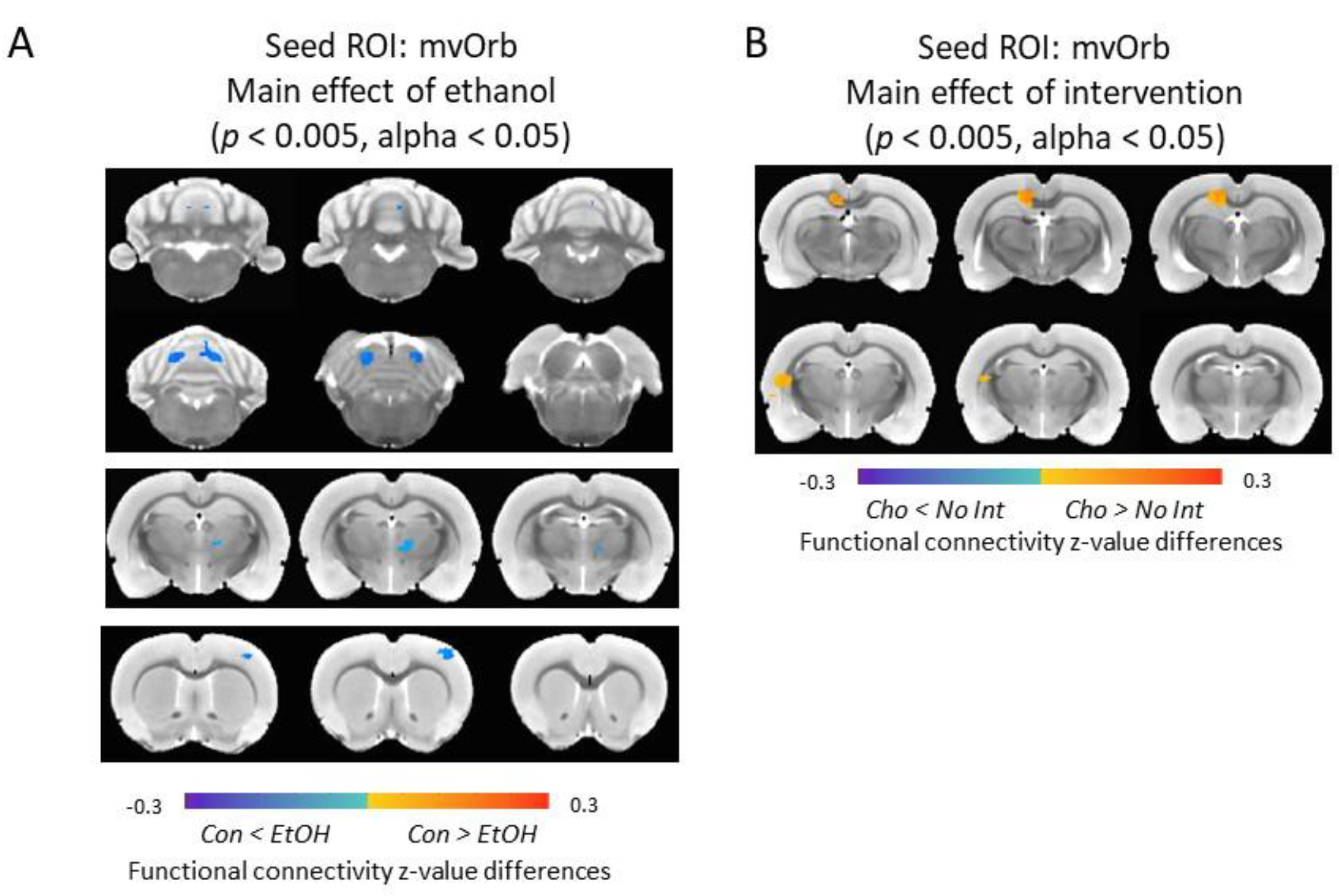
3.7. Functional Connectivity of the Medial-Ventral Orbitofrontal Cortex and Cerebellum Was Increased in Ethanol-Exposed Male Rats
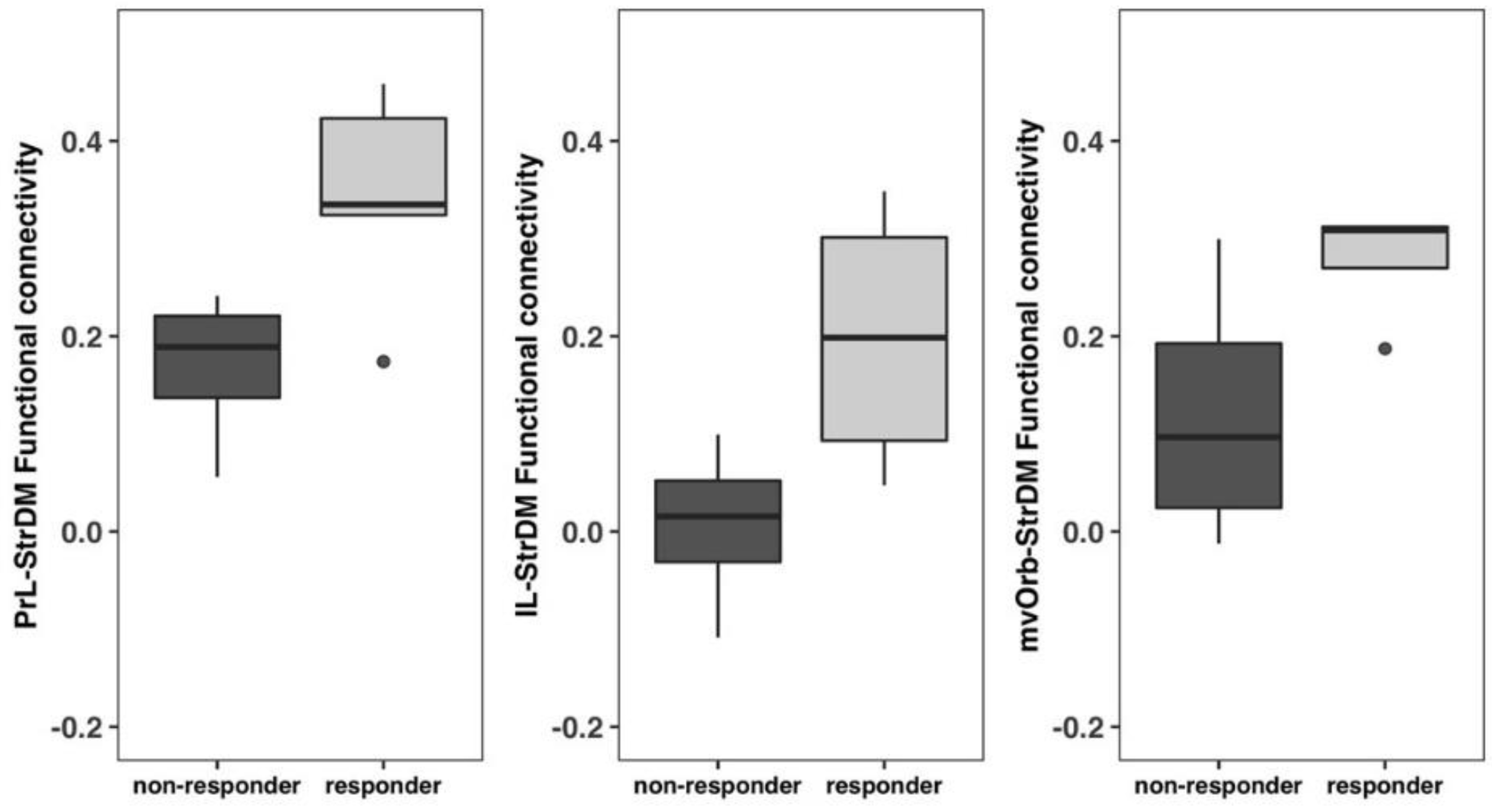
3.8. Non-Responders vs. Responders Exhibited Functional Connectivity Differences between the Frontal Cortices and the Dorsomedial Striatum
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Connor, P.D.; Sampson, P.D.; Bookstein, F.L.; Barr, H.M.; Streissguth, A.P. Direct and Indirect Effects of Prenatal Alcohol Damage on Executive Function. Dev. Neuropsychol. 2000, 18, 331–354. [Google Scholar] [CrossRef] [PubMed]
- Kodituwakku, P.W. Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev. Disabil. Res. Rev. 2009, 15, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Mattson, S.N.; Goodman, A.M.; Caine, C.; Delis, D.C.; Riley, E.P. Executive Functioning in Children With Heavy Prenatal Alcohol Exposure. Alcohol Clin. Exp. Res. 1999, 23, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Mattson, S.N.; Riley, E.P. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcohol Clin. Exp. Res. 2000, 24, 226–231. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Kalberg, W.O.; Robinson, L.K.; Buckley, D.; Manning, M.; Hoyme, H.E. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009, 15, 176–192. [Google Scholar] [CrossRef]
- May, P.A.; Blankenship, J.; Marais, A.-S.; Gossage, J.P.; Kalberg, W.O.; Barnard, R.; De Vries, M.; Robinson, L.K.; Adnams, C.M.; Buckley, D.; et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol. Clin. Exp. Res. 2012, 37, 818–830. [Google Scholar] [CrossRef]
- Thomas, J.D.; La Fiette, M.H.; Quinn, V.R.; Riley, E.P. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicology Teratol. 2000, 22, 703–711. [Google Scholar] [CrossRef]
- Zeisel, S.H. What Choline Metabolism Can Tell Us About the Underlying Mechanisms of Fetal Alcohol Spectrum Disorders. Mol. Neurobiol. 2011, 44, 185–191. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.-A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Cheng, R.-K.; Meck, W.H. Prenatal choline supplementation increases sensitivity to time by reducing non-scalar sources of variance in adult temporal processing. Brain Res. 2007, 1186, 242–254. [Google Scholar] [CrossRef]
- Meck, W.H.; Smith, R.A.; William, C.L. Organizational Changes in Cholinergic Activity and Enhanced Visuospatial Memory as a Function of Choline Administered Prenatally or Postnatally or Both. Behav. Neurosci. 1989, 103, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Tees, R.C.; Mohammadi, E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Dev. Psychobiol. 1999, 35, 226–240. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Fuglestad, A.J.; Eckerle, J.K.; Fink, B.A.; Hoecker, H.L.; Boys, C.J.; Radke, J.P.; Kroupina, M.G.; Miller, N.C.; Brearley, A.M.; et al. Choline supplementation in children with fetal alcohol spectrum disorders: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2015, 102, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Risbud, R.D.; Mattson, S.N.; Chambers, C.D.; Thomas, J.D. Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. Am. J. Clin. Nutr. 2016, 104, 1683–1692. [Google Scholar] [CrossRef]
- Kerns, K.A.; Macoun, S.; Macsween, J.; Pei, J.; Hutchison, M. Attention and working memory training: A feasibility study in children with neurodevelopmental disorders. Appl. Neuropsychol. Child 2016, 6, 120–137. [Google Scholar] [CrossRef]
- Nash, K.; Stevens, S.; Greenbaum, R.; Weiner, J.; Koren, G.; Rovet, J. Improving executive functioning in children with fetal alcohol spectrum disorders. Child Neuropsychol. 2014, 21, 191–209. [Google Scholar] [CrossRef]
- Kodali, V.N.; Jacobson, J.L.; Lindinger, N.M.; Dodge, N.C.; Molteno, C.D.; Meintjes, E.M.; Jacobson, S.W. Differential Recruitment of Brain Regions During Response Inhibition in Children Prenatally Exposed to Alcohol. Alcohol. Clin. Exp. Res. 2017, 41, 334–344. [Google Scholar] [CrossRef]
- Satterthwaite, T.D.; Wolf, D.H.; Erus, G.; Ruparel, K.; Elliott, M.A.; Gennatas, E.D.; Hopson, R.; Jackson, C.; Prabhakaran, K.; Bilker, W.B.; et al. Functional Maturation of the Executive System during Adolescence. J. Neurosci. 2013, 33, 16249–16261. [Google Scholar] [CrossRef]
- Marquardt, K.; Cavanagh, J.F.; Brigman, J.L. Alcohol exposure in utero disrupts cortico-striatal coordination required for behavioral flexibility. Neuropharmacology 2020, 162, 107832. [Google Scholar] [CrossRef]
- Tang, S.; Xu, S.; Waddell, J.; Zhu, W.; Gullapalli, R.P.; Mooney, S.M. Functional Connectivity and Metabolic Alterations in Medial Prefrontal Cortex in a Rat Model of Fetal Alcohol Spectrum Disorder: A Resting-State Functional Magnetic Resonance Imaging and in vivo Proton Magnetic Resonance Spectroscopy Study. Dev. Neurosci. 2019, 41, 67–78. [Google Scholar] [CrossRef]
- Waddell, J.; Mooney, S.M. Choline and Working Memory Training Improve Cognitive Deficits Caused by Prenatal Exposure to Ethanol. Nutrients 2017, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Montez, D.F.; Calabro, F.J.; Luna, B. Working memory improves developmentally as neural processes stabilize. PLoS ONE 2019, 14, e0213010. [Google Scholar] [CrossRef] [PubMed]
- Montez, D.F.; Calabro, F.J.; Luna, B. The expression of established cognitive brain states stabilizes with working memory development. eLife 2017, 6, e25606. [Google Scholar] [CrossRef] [PubMed]
- Peverill, M.R.; McLaughlin, K.A.; Finn, A.S.; Sheridan, M.A. Working memory filtering continues to develop into late adolescence. Dev. Cogn. Neurosci. 2016, 18, 78–88. [Google Scholar] [CrossRef]
- Bissonette, G.B.; Martins, G.J.; Franz, T.M.; Harper, E.S.; Schoenbaum, G.; Powell, E.M. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 2008, 28, 11124–11130. [Google Scholar] [CrossRef]
- Miller, M.W. Circadian rhythm of cell proliferation in the telencephalic ventricular zone: Effect of in utero exposure to ethanol. Brain Res. 1992, 595, 17–24. [Google Scholar] [CrossRef]
- Youngentob, S.L.; Molina, J.C.; Spear, N.E.; Youngentob, L.M. The effect of gestational ethanol exposure on voluntary ethanol intake in early postnatal and adult rats. Behav. Neurosci. 2007, 121, 1306–1315. [Google Scholar] [CrossRef]
- Wang, S.S.-H.; Kloth, A.D.; Badura, A. The Cerebellum, Sensitive Periods, and Autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef]
- Birrell, J.M.; Brown, V.J. Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. J. Neurosci. 2000, 20, 4320–4324. [Google Scholar] [CrossRef]
- Hennig, J.; Nauerth, A.; Friedburg, H. RARE imaging: A fast imaging method for clinical MR. Magn. Reson. Med. 1986, 3, 823–833. [Google Scholar] [CrossRef]
- Valdés-Hernández, P.A.; Sumiyoshi, A.; Nonaka, H.; Haga, R.; Vasquez, E.A.; Ogawa, T.; Iturria-Medina, Y.; Riera, J.; Kawashima, R. An in vivo MRI Template Set for Morphometry, Tissue Segmentation, and fMRI Localization in Rats. Front. Aging Neurosci. 2011, 5, 26. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; 2007, 6th ed.; Elsevier BV: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Bennett, C.M.; Wolford, G.L.; Miller, M.B. The principled control of false positives in neuroimaging. Soc. Cogn. Affect. Neurosci. 2009, 4, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kirschen, M.P.; Chen, S.H.A.; Desmond, J.E. Modality Specific Cerebro-Cerebellar Activations in Verbal Working Memory: An fMRI Study. Behav. Neurol. 2010, 23, 51–63. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, E.D.; Lu, L.H.; Houston, S.M.; Bookheimer, S.Y.; Sowell, E.R. Neurodevelopmental changes in verbal working memory load-dependency: An fMRI investigation. NeuroImage 2008, 42, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Farr, K.L.; Montano, C.Y.; Paxton, L.L.; Savage, D.D. Prenatal ethanol exposure decreases hippocampal 3H-vinylidene kainic acid binding in 45-day-old rats. Neurotoxicology Teratol. 1988, 10, 563–568. [Google Scholar] [CrossRef]
- Bastos, A.M.; Loonis, R.; Kornblith, S.; Lundqvist, M.; Miller, E.K. Laminar recordings in frontal cortex suggest distinct layers for maintenance and control of working memory. Proc. Natl. Acad. Sci. USA 2018, 115, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.W.; Waskom, M.L.; Gabrieli, J.D.E. Intensive Working Memory Training Produces Functional Changes in Large-scale Frontoparietal Networks. J. Cogn. Neurosci. 2016, 28, 575–588. [Google Scholar] [CrossRef]
- Kodituwakku, P.W.; Handmaker, N.S.; Cutler, S.K.; Weathersby, E.K.; Handmaker, S.D. Specific Impairments in Self-Regulation in Children Exposed to Alcohol Prenatally. Alcohol. Clin. Exp. Res. 1995, 19, 1558–1564. [Google Scholar] [CrossRef]
- Gathmann, B.; Brand, M.; Schiebener, J. One executive function never comes alone: Monitoring and its relation to working memory, reasoning, and different executive functions. Cogn. Process. 2016, 18, 13–29. [Google Scholar] [CrossRef]
- McCabe, D.P.; Roediger, H.L.; McDaniel, M.A.; Balota, D.A.; Hambrick, D.Z. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology 2010, 24, 222–243. [Google Scholar] [CrossRef]
- Geier, C.F.; Garver, K.; Terwilliger, R.; Luna, B. Development of Working Memory Maintenance. J. Neurophysiol. 2009, 101, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Macoveanu, J.; Tegnér, J.; Klingberg, T. Brain Activity Related to Working Memory and Distraction in Children and Adults. Cereb. Cortex 2006, 17, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Astle, D.E.; Barnes, J.J.; Baker, K.; Colclough, G.L.; Woolrich, M.W. Cognitive Training Enhances Intrinsic Brain Connectivity in Childhood. J. Neurosci. 2015, 35, 6277–6283. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.J.; Nobre, A.C.; Woolrich, M.W.; Baker, K.; Astle, D.E. Training working memory in childhood enhances couplinbetween frontoparietal control network and task-related regions. J. Neurosci. 2016, 36, 9001–9011. [Google Scholar] [CrossRef] [PubMed]
- Marvel, C.L.; Desmond, J.E. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex 2010, 46, 880–895. [Google Scholar] [CrossRef]
- Marvel, C.L.; Desmond, J.E. Functional Topography of the Cerebellum in Verbal Working Memory. Neuropsychol. Rev. 2010, 20, 271–279. [Google Scholar] [CrossRef]
- Moore, D.M.; D’Mello, A.M.; McGrath, L.M.; Stoodley, C.J. The developmental relationship between specific cognitive domains and grey matter in the cerebellum. Dev. Cogn. Neurosci. 2017, 24, 1–11. [Google Scholar] [CrossRef]
- Bernard, J.A.; Leopold, D.R.; Calhoun, V.D.; Mittal, V.A. Regional cerebellar volume and cognitive function from adolescence to late middle age. Hum. Brain Mapp. 2015, 36, 1102–1120. [Google Scholar] [CrossRef]
- Diwadkar, V.A.; Meintjes, E.M.; Goradia, D.; Dodge, N.C.; Warton, C.; Molteno, C.D.; Jacobson, S.W.; Jacobson, J.L. Differences in cortico-striatal-cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Hum. Brain Mapp. 2012, 34, 1931–1945. [Google Scholar] [CrossRef]
- O’Hare, E.D.; Lu, L.H.; Houston, S.M.; Bookheimer, S.Y.; Mattson, S.N.; O’Connor, M.J.; Sowell, E.R. Altered frontal-parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Hum. Brain Mapp. 2009, 30, 3200–3208. [Google Scholar] [CrossRef]
- Cheng, D.T.; Meintjes, E.M.; Stanton, M.E.; Dodge, N.C.; Pienaar, M.; Warton, C.M.; Desmond, J.E.; Molteno, C.D.; Peterson, B.S.; Jacobson, J.L.; et al. Functional MRI of Human Eyeblink Classical Conditioning in Children with Fetal Alcohol Spectrum Disorders. Cereb. Cortex 2017, 27, 3752–3767. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.W.; Stanton, M.E.; Dodge, N.C.; Pienaar, M.; Fuller, D.S.; Molteno, C.D.; Meintjes, E.M.; Hoyme, H.E.; Robinson, L.K.; Khaole, N.; et al. Impaired Delay and Trace Eyeblink Conditioning in School-Age Children With Fetal Alcohol Syndrome. Alcohol. Clin. Exp. Res. 2010, 35, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Spottiswoode, B.S.; Meintjes, E.M.; Anderson, A.W.; Molteno, C.D.; Stanton, M.E.; Dodge, N.C.; Gore, J.C.; Peterson, B.S.; Jacobson, J.L.; Jacobson, S.W. Diffusion tensor imaging of the cerebellum and eyeblink conditioning in fetal alcohol spectrum disorder. Alcohol. Clin. Exp. Res. 2011, 35, 2174–2183. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Wurtman, R.J. Choline and Cholinergic Neurons. Science 1983, 221, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Janiesch, P.C.; Krüger, H.-S.; Pöschel, B.; Hanganu-Opatz, I.L. Cholinergic Control in Developing Prefrontal-Hippocampal Networks. J. Neurosci. 2011, 31, 17955–17970. [Google Scholar] [CrossRef]
- Meck, W.H.; Williams, C.L. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Dev. Brain Res. 1999, 118, 51–59. [Google Scholar] [CrossRef]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef]
- Davison, J.M.; Mellott, T.J.; Kovacheva, V.P.; Blusztajn, J.K. Gestational Choline Supply Regulates Methylation of Histone H3, Expression of Histone Methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA Methylation of Their Genes in Rat Fetal Liver and Brain. J. Biol. Chem. 2009, 284, 1982–1989. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline, Other Methyl-Donors and Epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef]
- Mehedint, M.G.; Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Choline deficiency alters global histone methylation and epigenetic marking at the Rel site of the calbindin 1 gene. FASEB J. 2010, 24, 184–195. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006, 20, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Broadwater, M.A.; Lee, S.; Yu, Y.; Zhu, H.; Crews, F.T.; Robinson, D.L.; Shih, Y.I. Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addict. Biol. 2018, 23, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, K.; Sigdel, R.; Caldwell, K.; Brigman, J.L. Prenatal ethanol exposure impairs executive function in mice into adulthood. Alcohol. Clin. Exp. Res. 2014, 38, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Ragozzino, M.E. Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol. Learn. Mem. 2003, 80, 257–267. [Google Scholar] [CrossRef]
- Ragozzino, M.E. Dynamic Changes in Acetylcholine Output in the Medial Striatum During Place Reversal Learning. Learn. Mem. 2004, 11, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Lindner, M.; Mullins, P.G.; Christakou, A. Functional neurochemical imaging of the human striatal cholinergic system during reversal learning. Eur. J. Neurosci. 2018, 47, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Hawes, S.L.; Evans, R.C.; Unruh, B.A.; Benkert, E.E.; Gillani, F.; Dumas, T.C.; Blackwell, K.T. Multimodal Plasticity in Dorsal Striatum While Learning a Lateralized Navigation Task. J. Neurosci. 2015, 35, 10535–10549. [Google Scholar] [CrossRef]
- Chase, E.A.; Tait, D.S.; Brown, V.J. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur. J. Neurosci. 2012, 36, 2368–2375. [Google Scholar] [CrossRef]
- McAlonan, K.; Brown, V.J. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 2003, 146, 97–103. [Google Scholar] [CrossRef]
- Dias, R.; Robbins, T.W.; Roberts, A.C. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav. Neurosci. 1996, 110, 872–886. [Google Scholar] [CrossRef]
- Kim, J.; Ragozzino, M.E. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol. Learn. Mem. 2005, 83, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, H.S.; Wickens, R.; Tait, D.S.; Brown, V.J.; Dunnett, S.B. Lesions of the dorsomedial striatum impair formation of attentional set in rats. Neuropharmacology 2013, 71, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.Q.; Karatayev, O.; Halkina, V.; Edelstien, J.; Ramirez, E.; Leibowitz, S.F. Hypothalamic CCL2/CCR2 chemokine system: Role in sexually dimorphic effects of maternal ethanol exposure on melanin-concentrating hormone and behavior in adolescent offspring. J. Neurosci. 2018, 38, 9072–9090. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, C.J.; Patten, A.R.; Sickmann, H.M.; Helfer, J.L.; Christie, B.R. Effects of pre-natal alcohol exposure on hippocampal synaptic plasticity: Sex, age and methodological considerations. Neurosci. Biobehav. Rev. 2016, 64, 12–34. [Google Scholar] [CrossRef]
- Rodriguez, C.I.; Magcalas, C.M.; Barto, D.; Fink, B.C.; Rice, J.P.; Bird, C.W.; Davies, S.; Pentkowski, N.S.; Savage, D.D.; Hamilton, D.A. Effects of sex and housing on social, spatial, and motor behavior in adult rats exposed to moderate levels of alcohol during prenatal development. Behav. Brain Res. 2016, 313, 233–243. [Google Scholar] [CrossRef]
- Ruggiero, M.J.; Boschen, K.E.; Roth, T.L.; Klintsova, A.Y. Sex Differences in Early Postnatal Microglial Colonization of the Developing Rat Hippocampus Following a Single-Day Alcohol Exposure. J. Neuroimmune Pharmacol. 2017, 13, 189–203. [Google Scholar] [CrossRef]
- Varlinskaya, E.I.; Mooney, S.M. Acute exposure to ethanol on gestational day 15 affects social motivation of female offspring. Behav. Brain Res. 2013, 261, 106–109. [Google Scholar] [CrossRef][Green Version]
- Lan, N.; Yamashita, F.; Halpert, A.G.; Ellis, L.; Yu, W.K.; Viau, V.; Weinberg, J. Prenatal Ethanol Exposure Alters the Effects of Gonadectomy on Hypothalamic-Pituitary-Adrenal Activity in Male Rats. J. Neuroendocr. 2006, 18, 672–684. [Google Scholar] [CrossRef]
- Rodriguez, C.I.; Davies, S.; Calhoun, V.; Savage, D.D.; Hamilton, D.A. Moderate Prenatal Alcohol Exposure Alters Functional Connectivity in the Adult Rat Brain. Alcohol. Clin. Exp. Res. 2016, 40, 2134–2146. [Google Scholar] [CrossRef]
- Bottom, R.T.; Abbott, C.W.; Huffman, K.J. Rescue of ethanol-induced FASD-like phenotypes via prenatal co-administration of choline. Neuropharmacology 2020, 168, 107990. [Google Scholar] [CrossRef]
- Panczakiewicz, A.L.; Glass, L.; Coles, C.D.; Kable, J.A.; Sowell, E.R.; Wozniak, J.R.; Jones, K.L.; Riley, E.P.; Mattson, S.N.; Cifasd, T. Neurobehavioral Deficits Consistent Across Age and Sex in Youth with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2016, 40, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Treit, S.; Chen, Z.; Zhou, D.; Baugh, L.; Rasmussen, C.; Andrew, G.; Pei, J.; Beaulieu, C. Sexual dimorphism of volume reduction but not cognitive deficit in fetal alcohol spectrum disorders: A combined diffusion tensor imaging, cortical thickness and brain volume study. NeuroImage Clin. 2017, 15, 284–297. [Google Scholar] [CrossRef] [PubMed]
| Sex | Control | Ethanol | ||
|---|---|---|---|---|
| Choline Trained | Saline Untrained | Choline Trained | Saline Untrained | |
| Male | 8 | 8 | 9 | 9 |
| Female | 10 | 10 | 9 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waddell, J.; Hill, E.; Tang, S.; Jiang, L.; Xu, S.; Mooney, S.M. Choline Plus Working Memory Training Improves Prenatal Alcohol-Induced Deficits in Cognitive Flexibility and Functional Connectivity in Adulthood in Rats. Nutrients 2020, 12, 3513. https://doi.org/10.3390/nu12113513
Waddell J, Hill E, Tang S, Jiang L, Xu S, Mooney SM. Choline Plus Working Memory Training Improves Prenatal Alcohol-Induced Deficits in Cognitive Flexibility and Functional Connectivity in Adulthood in Rats. Nutrients. 2020; 12(11):3513. https://doi.org/10.3390/nu12113513
Chicago/Turabian StyleWaddell, Jaylyn, Elizabeth Hill, Shiyu Tang, Li Jiang, Su Xu, and Sandra M. Mooney. 2020. "Choline Plus Working Memory Training Improves Prenatal Alcohol-Induced Deficits in Cognitive Flexibility and Functional Connectivity in Adulthood in Rats" Nutrients 12, no. 11: 3513. https://doi.org/10.3390/nu12113513
APA StyleWaddell, J., Hill, E., Tang, S., Jiang, L., Xu, S., & Mooney, S. M. (2020). Choline Plus Working Memory Training Improves Prenatal Alcohol-Induced Deficits in Cognitive Flexibility and Functional Connectivity in Adulthood in Rats. Nutrients, 12(11), 3513. https://doi.org/10.3390/nu12113513




