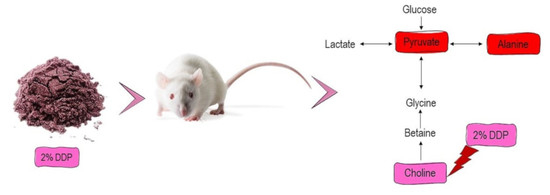
Effect of Defatted Dabai Pulp Extract in Urine Metabolomics of Hypercholesterolemic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Defatted Dabai Pulp
2.2. Nutritional Quality of Defatted Dabai Pulp (DDP)
2.3. Antioxidant Profile in Treatment Diets
2.4. Animal Experiments
2.5. Blood Analysis
2.6. Oxidative Stress Markers Analysis
2.7. Inflammatory Markers Analysis
2.8. 1H-NMR Urinary Metabolomics Analysis
2.9. Statistical Analysis
3. Results
3.1. Nutritional Quality of DDP and DDP Treatment Diet
3.2. Efficacy of 2% DDP Treatment Diet in Hypercholesterolemic Rats
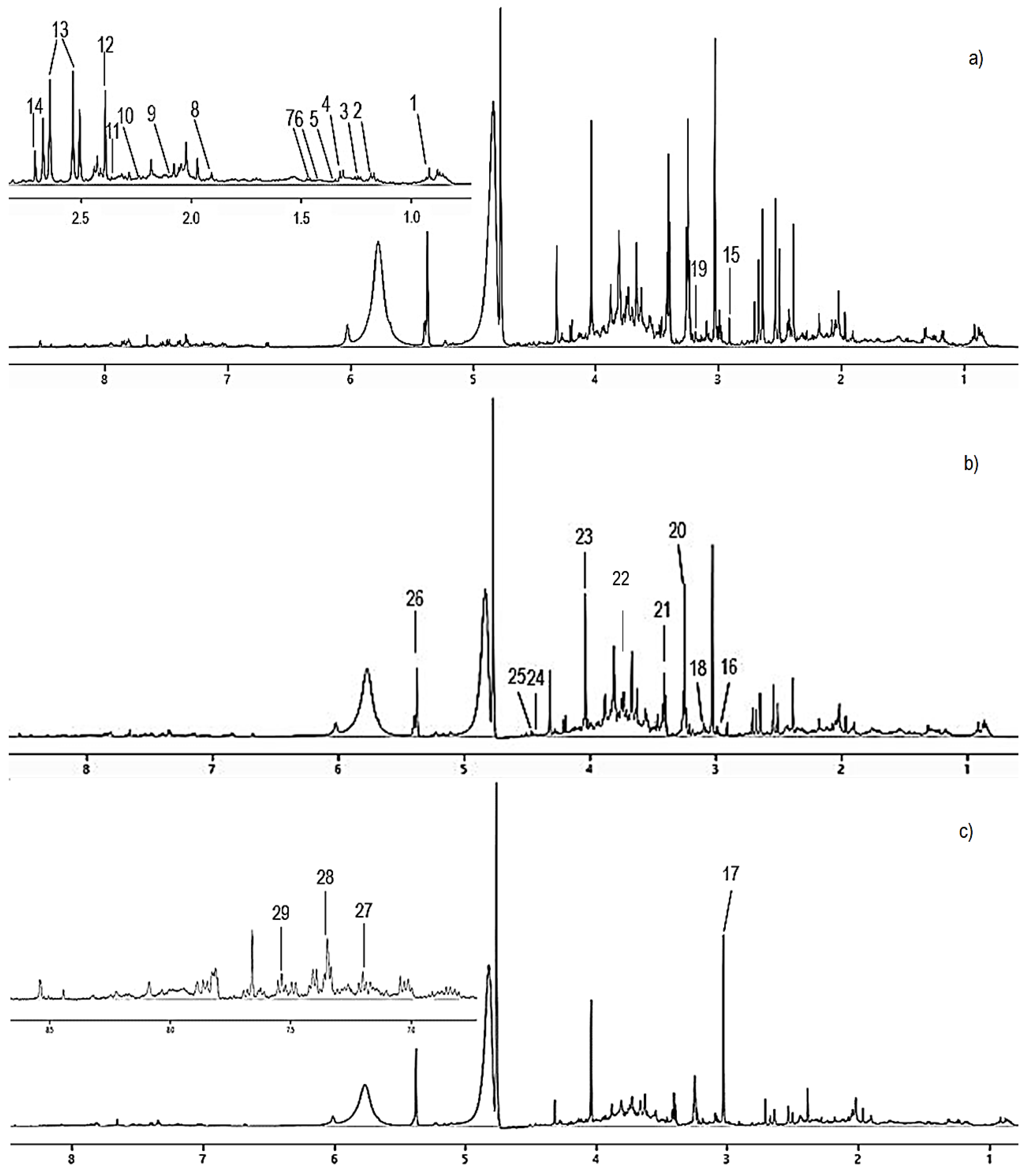
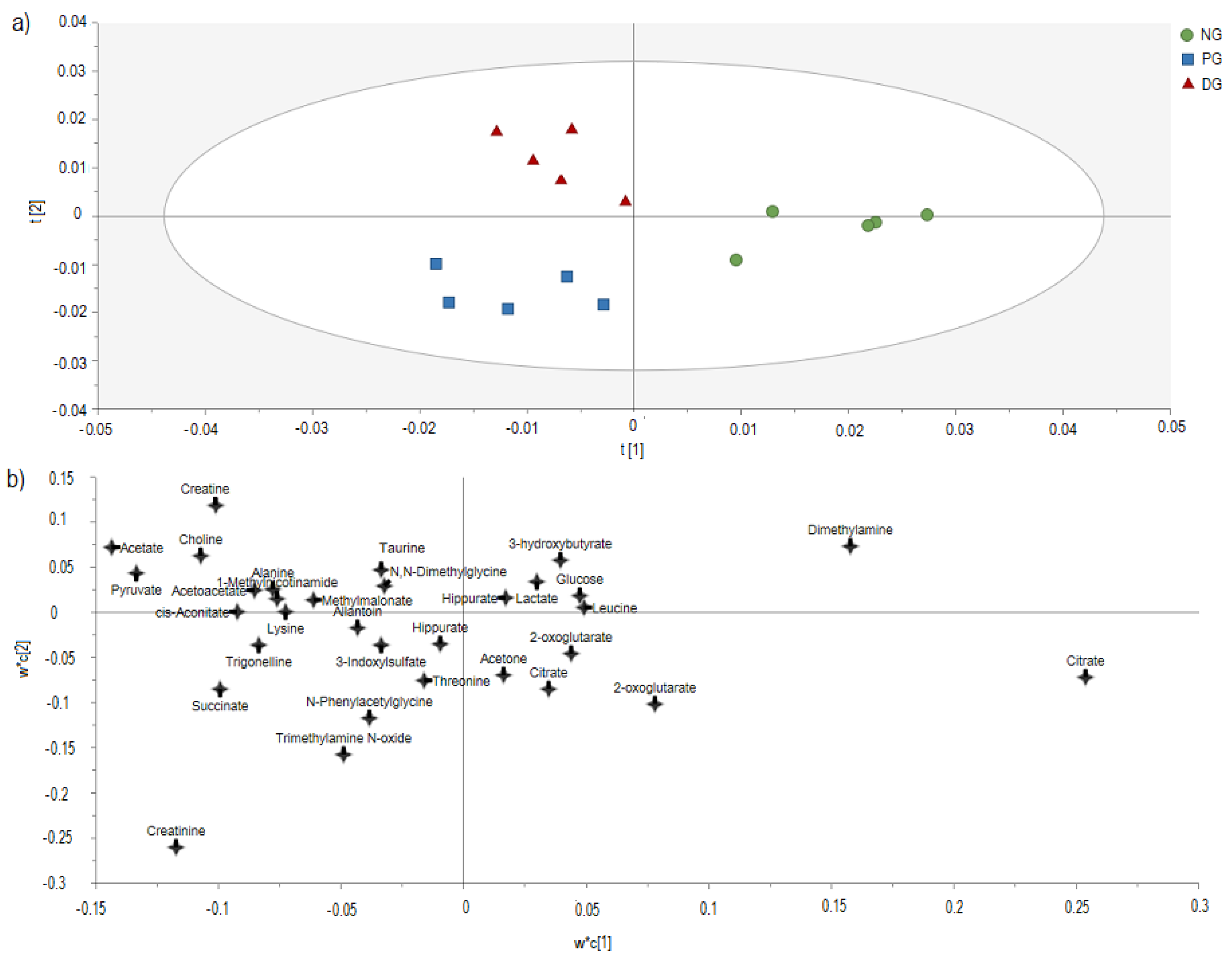
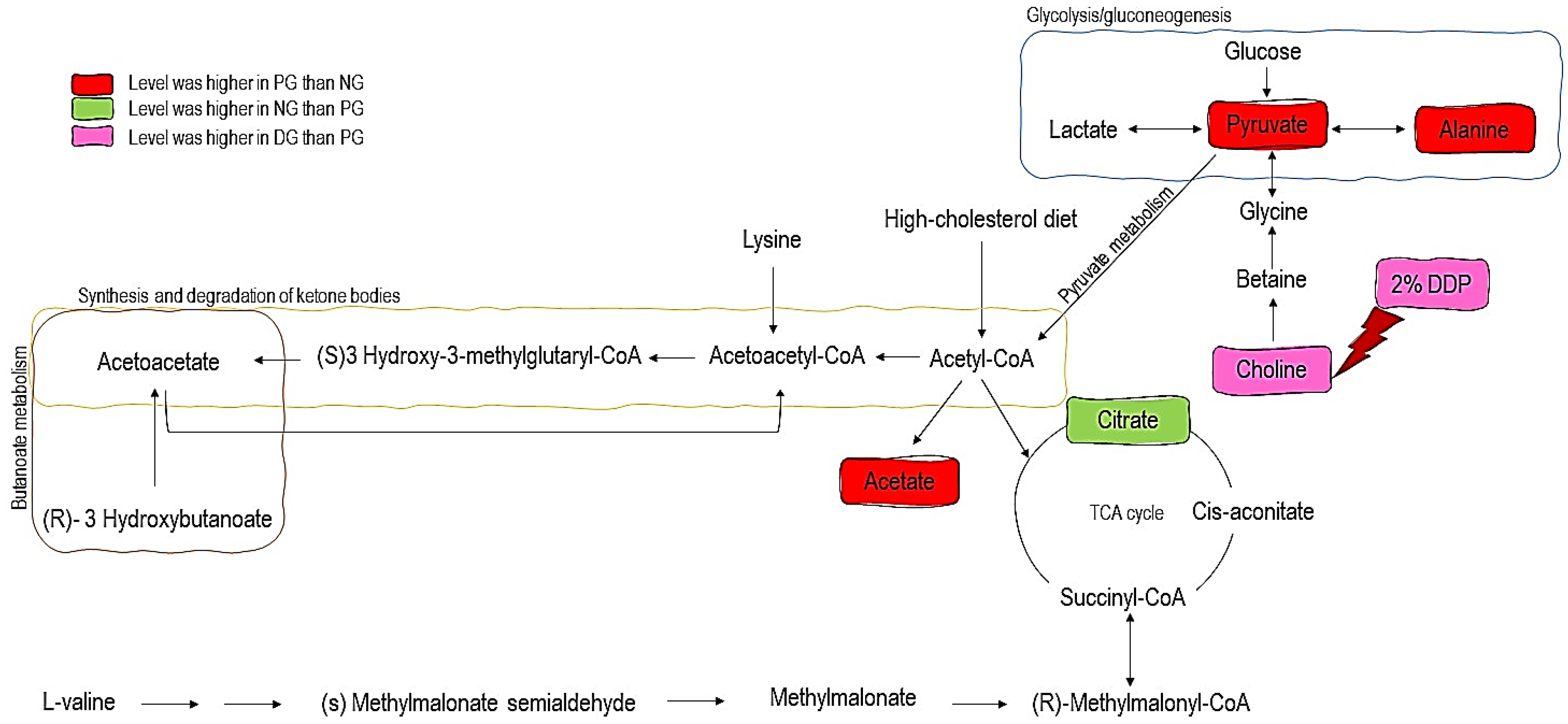
3.3. H-NMR-Based Metabolomics Analysis of Urine from Hypercholesterolemic Rats Treated with 2% DDP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Badimon, L.; Chiva-Blanch, G. Lipid Metabolism in Dyslipidemia and Familial Hypercholesterolemia; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Ž.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [PubMed] [Green Version]
- Institute for Public Health Malaysia. National Health and Morbidity Survey (NHMS) 2019: Non-Communicable Diseases, Healthcare Demand, and Health Literacy—Key Findings; National Institutes of Health (NIH) Ministry of Health Malaysia: Shah Alam, Malaysia, 2020.
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-associated side effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Prasad, K.N.; Khoo, H.E.; Abdul-Aziz, N.; Mohamad, A.; Ismail, A.; Amom, Z. Comparison of fatty acids, vitamin E and physicochemical properties of Canarium odontophyllum Miq. (dabai), olive and palm oils. J. Food Compos. Anal. 2010, 23, 772–776. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Ismail, A.; Abas, F. Antioxidative properties of defatted dabai pulp and peel prepared by solid phase extraction. Molecules 2012, 17, 9754–9773. [Google Scholar] [CrossRef]
- Shakirin, F.H.; Azlan, A.; Ismail, A.; Amom, Z.; Lau, C.Y. Antiatherosclerotic effect of canarium odontophyllum Miq. Fruit parts in rabbits fed high cholesterol diet. Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Nurulhuda, M.H.; Ismail, A.; Abas, F.; Hamid, M.; Roowi, S. Antioxidative and cardioprotective properties of anthocyanins from defatted Dabai extracts. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Kadir, N.A.A.A.; Azlan, A.; Abas, F.; Ismail, I.S. Beneficial effect of supercritical carbon dioxide extracted (SC-CO2) Dabai (Canarium odontophyllum) pulp oil in hypercholesterolemia-induced SPF sprague-dawley rats. Nat. Prod. Commun. 2018, 13, 1583–1586. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Annex G Aoac Official Method 991.43. Total, Soluble, and Insoluble Dietary Fibre in Foods. Cereal Foods 1995, 991, 7–9. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Ismail, A.; Abas, F. Influence of Different Extraction Media on Phenolic Contents and Antioxidant Capacity of Defatted Dabai (Canarium odontophyllum) Fruit. Food Anal. Methods 2012, 5, 339–350. [Google Scholar] [CrossRef]
- Jelani, N.A.A.; Azrina, A.; Khoo, H.E.; Razman, M.R. Fatty acid profile and antioxidant properties of oils extracted from dabai pulp using supercritical carbon dioxide extraction. Int. Food Res. J. 2019, 26, 1587–1598. [Google Scholar]
- Starowicz, M.; Koutsidis, G.; Zieliński, H. Determination of antioxidant capacity, phenolics and volatile Maillard reaction products in rye-buckwheat biscuits supplemented with 3β-D-rutinoside. Molecules 2019, 24, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, A.A.; Padzil, A.M.; Muhamad, I.I. Effect of incorporating purple-fleshed sweet potato in biscuit on antioxidant content, antioxidant capacity and colour characteristics. Malays. J. Anal. Sci. 2018, 22, 667–675. [Google Scholar]
- El Baky, H.H.A.; el Baroty, G.S.; Ibrahem, E.A. Functional characters evaluation of biscuits sublimated with pure phycocyanin isolated from Spirulina and Spirulina biomass. Nutr. Hosp. 2015, 32, 231–241. [Google Scholar]
- Mawatari, S.; Ohnishi, Y.; Kaji, Y.; Maruyama, T.; Murakami, K.; Tsutsui, K.; Fujino, T. High-cholesterol diets induce changes in lipid composition of rat erythrocyte membrane including decrease in cholesterol, increase in alpha-tocopherol and changes in fatty acids of phospholipids. Biosci. Biotechnol. Biochem. 2003, 67, 1457–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Effect of virgin avocado oil on diet-induced hypercholesterolemia in rats via 1 H NMR-based metabolomics approach. Phyther. Res. 2018, 32, 2264–2274. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, C.; Wikström, C.; Wold, S. Multi- and Megavariate Data Analysis Part I. Basic Principles and Applications; Umetrics AB: Umeå, Sweden, 2006. [Google Scholar]
- Mediani, A.; Abas, F.; Maulidiani, M.; Khatib, A.; Tan, C.P.; Ismail, I.S.; Shaari, K.; Ismail, A.; Lajis, N.H. Metabolic and biochemical changes in streptozotocin induced obese-diabetic rats treated with Phyllanthus niruri extract. J. Pharm. Biomed. Anal. 2016, 128, 302–312. [Google Scholar] [CrossRef]
- Yi, L.; Shi, S.; Wang, Y.; Huang, W.; Xia, Z.A.; Xing, Z.; Peng, W.; Wang, Z. Serum Metabolic Profiling Reveals Altered Metabolic Pathways in Patients with Post-traumatic Cognitive Impairments. Sci. Rep. 2016, 6, 21320. [Google Scholar] [CrossRef]
- Singh, U.N.; Kumar, S.; Dhakal, S. Study of Oxidative Stress in Hypercholesterolemia. Int. J. Con-Temp. Med. Res. 2017, 4, 1204–1207. [Google Scholar]
- Al-Muzafar, H.M.; Amin, K.A. Efficacy of functional foods mixture in improving hypercholesterolemia, inflammatory and endothelial dysfunction biomarkers-induced by high cholesterol diet. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.R.; El-Abhar, H.S.; Saleh, S. The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J. Ethnopharmacol. 2002, 79, 1–11. [Google Scholar] [CrossRef]
- Shin, M.J.; Chung, N.; Lee, J.H.; Jang, Y.; Park, E.; Jeon, K.I.; Chung, J.H.; Seo, B.Y. Effects of simvastatin on plasma antioxidant status and vitamins in hypercholesterolemic patients. Int. J. Cardiol. 2007, 118, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Khanum, F.; Swamy, M.S.; Krishna, K.R.S.; Santhanam, K.; Viswanathan, K.R. Dietary fiber content of commonly fresh and cooked vegetables consumed in India. Plant Foods Hum. Nutr. 2000, 55, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Spiller, G.A. CRC Handbook of Dietary Fiber in Human Nutrition, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Norulaini, N.; Ab, N.; Ben, M.M.; Al-rawi, S.S.; Ibrahim, A.H.; Ab, M.O. Comparison of nutritional composition between palm kernel fibre and the effect of the supercritical fluid extraction on its quality. Procedia Food Sci. 2011, 1, 1940–1945. [Google Scholar]
- Guillon, F.; Champ, M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 2000, 33, 233–245. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Nawaz, H. Preparation of fiber and mineral enriched defatted rice bran supplemented cookies. Pakistan J. Nutr. 2009, 8, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Nassar, A.G. Effect of Citrus by-Products Flour Incorporation on Chemical, Rheological and Organolepic Characteristics of Biscuits. Sci. Technol. 2008, 4, 612–616. [Google Scholar]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; di Santo, S.; Carnevale, R.; Violi, F. The polyphenols quercetin and catechin synergize in inhibiting platelet CD40L expression. Thromb. Haemost. 2005, 94, 888–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azlan, A.; Ismail, A.; Amom, Z.; Shakirin, F.H. Effect of defatted Dabai (Canarium odontophyllum Miq.) pulp ingestion on lipid peroxidation and antioxidant status of hypercholesterolemic-induced rabbits. IFMBE Proc. 2013, 40, 137–140. [Google Scholar]
- Mayr, M.; Chung, Y.L.; Mayr, U.; Yin, X.; Ly, L.; Troy, H.; Fredericks, S.; Hu, Y.; Griffiths, J.R.; Xu, Q. Proteomic and metabolomic analyses of atherosclerotic vessels from apolipoprotein E-deficient mice reveal alterations in inflammation, oxidative stress, and energy metabolism. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2135–2142. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wang, J.; Wang, P.; Tian, N.; Yang, M.; Kong, L. 1H NMR-based metabolomics approach to evaluate the effect of Xue-Fu-Zhu-Yu decoction on hyperlipidemia rats induced by high-fat diet. J. Pharm. Biomed. Anal. 2013, 78–79, 202–210. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ding, L.L.; Li, J.M.; Xu, B.L.; Yang, L.; Bi, K.S.; Wang, Z.T. 1H-NMR and MS based metabolomics study of the intervention effect of curcumin on hyperlipidemia mice induced by high-fat diet. PLoS ONE 2015, 10, e0120950. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.Y.; Yang, K.M.; Yang, L.; Miao, Z.X.; Wang, Y.H.; Zhu, H.B. A 1H NMR-Based Metabonomic Investigation of Time-Related Metabolic Trajectories of the Plasma, Urine and Liver Extracts of Hyperlipidemic Hamsters. PLoS ONE 2013, 8, e66786. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Zi, T.; Liu, L.; Feng, R.; Sun, C. A 1H-NMR based metabolomics study of the intervention effect of mangiferin on hyperlipidemia hamsters induced by a high-fat diet. Food Funct. 2017, 8, 2455–2464. [Google Scholar] [CrossRef]
- Akram, M. Citric Acid Cycle and Role of its Intermediates in Metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Wang, M.; Wang, F.; Wang, Y.; Ma, X.; Zhao, M.; Zhao, C. Metabonomics study of the therapeutic mechanism of gynostemma pentaphyllum and atorvastatin for hyperlipidemia in rats. PLoS ONE 2013, 8, e78731. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, Y.; Lu, L.; Yang, Z.; Xue, W.; Tian, X.; Zhang, X. 1H-NMR Based Serum Metabolomics Study to Investigate Hepatoprotective Effect of Qin-Jiao on Carbon Tetrachloride-Induced Acute Hepatotoxicity in Rats. Evid. Based Complement. Altern. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Huo, T.I.; Cheng, H.Y.; Tsai, J.C.; Liao, J.W.; Lee, M.S.; Qin, X.M.; Hsieh, M.T.; Pao, L.H.; Peng, W.H. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE 2014, 9, e96969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Baixauli, J.; Quesada-Vázquez, S.; Mariné-Casadó, R.; Gil Cardoso, K.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Baselga-Escudero, L. Detection of early disease risk factors associated with metabolic syndrome: A new era with the NMR metabolomics assessment. Nutrients 2020, 12, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.O.; Wheatley, A.O.; Hanchard, B.; Gibson, T.N.; McGrowder, D.A.; Dilworth, L.L.; Asemota, H.N. Histopathological alterations in organ structures of hypercholesterolemic rats fed Ortanique peel polymethoxylated flavones. Basic Appl. Pathol. 2011, 4, 71–77. [Google Scholar] [CrossRef]
- Gao, X.; Randell, E.; Zhou, H.; Sun, G. Higher serum choline and betaine levels are associated with better body composition in male but not female population. PLoS ONE 2018, 13, e0193114. [Google Scholar] [CrossRef]
| Ingredients (g) | Treatment Diet | ||
|---|---|---|---|
| ND 1 | HC 2 | HC + 2% DDP 3 | |
| Corn starch | 180 | 170 | 170 |
| Sucrose | 500 | 500 | 500 |
| Casein | 120 | 120 | 120 |
| Vitamin mixture | 10 | 10 | 10 |
| Mineral mixture | 35 | 35 | 35 |
| Cellulose | 50 | 50 | 30 |
| DL-methionine | 3 | 3 | 3 |
| Choline | 2 | 2 | 2 |
| Ghee | 80 | 80 | 80 |
| Corn oil | 20 | 20 | 20 |
| Cholesterol | - | 10 | 10 |
| Defatted dabai pulp | - | - | 20 |
| Total | 1000 | 1000 | 1000 |
| 4 Energy (kcal/100g) | 397 | 423 | 384 |
| Phenolic Compound | Concentration (µg/mL) |
|---|---|
| Gallic acid | 8.73 ± 0.13 |
| 4-hydroxybenzoic acid | 61.46 ± 0.04 |
| Syringic acid | 89.87 ± 15.18 |
| Experimental Diet | ND | HC | 2% DDP |
|---|---|---|---|
| FRAP (mM Fe/g extract) | 7.369 ± 0.05 a | 7.200 ± 0.01 b | 11.197 ± 0.01 a,b |
| TPC (mg GAE/g extract) | 2.842 ± 0.12 a | 2.649 ± 0.05 b | 3.969 ± 0.01 a,b |
| TFC (mg QE/g extract) | 0.776 ± 0.00 | 0.698 ± 0.13 | 1.072 ± 0.00 a,b |
| Group | NG | PG | DG |
|---|---|---|---|
| TC (mmol/L) | 1.57 ± 0.15 a | 2.12 ± 0.65 b | 1.37 ± 0.25 a |
| TG (mmol/L) | 1.97 ± 0.92 | 2.08 ± 0.65 | 1.18 ± 0.38 a |
| LDL-C (mmol/L) | 0.17 ± 0.06 a | 0.50 ± 0.19 b | 0.33 ± 0.11 a,b |
| HDL-C (mmol/L) | 1.36 ± 0.14 | 1.27 ± 0.53 | 1.25 ± 0.19 |
| AST (U/L) | 81.83 ± 4.17 a | 124.33 ± 23.90 b | 88.83 ± 13.73 a |
| ALT (U/L) | 20.33 ± 3.14 a | 30.33 ± 7.66 b | 22.33 ± 3.01 a |
| TAS (U/mL) | 2.24 ± 0.31 a | 1.95 ± 0.22 b | 2.51 ± 0.17 a |
| SOD (ng/mL) | 0.78 ± 0.13 a | 0.58 ± 0.02 b | 0.89 ± 0.12 a |
| GPx (U/L) | 295.98 ± 3.40 a | 282.72 ± 14.98 b | 301.23 ± 2.24 a |
| CAT (U/mL) | 12.96 ± 1.19 a | 4.26 ± 0.69 b | 20.13 ± 5.53 a,b |
| CRP (ng/mL) | 0.85 ± 0.16 a | 1.07 ± 0.20 b | 0.52 ± 0.05 a,b |
| IL-6 (pg/mL) | 315.32 ± 31.28 a | 364.97 ± 49.84 b | 251.11 ± 34.37 a,b |
| α-TNF (pg/mL) | 221.67± 14.36 a | 293.76 ± 20.41 b | 191.88 ± 6.49 a,b |
| Metabolites | VIP | PG/NG | DG/PG |
|---|---|---|---|
| Citrate | 3.79 | 0.62 * | 0.98 |
| Acetate | 2.14 | 1.47 ** | 1.07 |
| Pyruvate | 1.99 | 1.34 ** | 1.03 |
| Choline | 1.60 | 1.25 * | 1.21 * |
| Cis-aconitate | 1.38 | 1.20 | 0.98 |
| Acetoacetate | 1.27 | 1.22 | 1.02 |
| Alanine | 1.16 | 1.19 * | 1.02 |
| Lysine | 1.09 | 1.22 | 0.98 |
| Methylmalonate | 0.91 | 1.13 | 1.01 |
| Metabolic Pathways | p-Value | Impact Value |
|---|---|---|
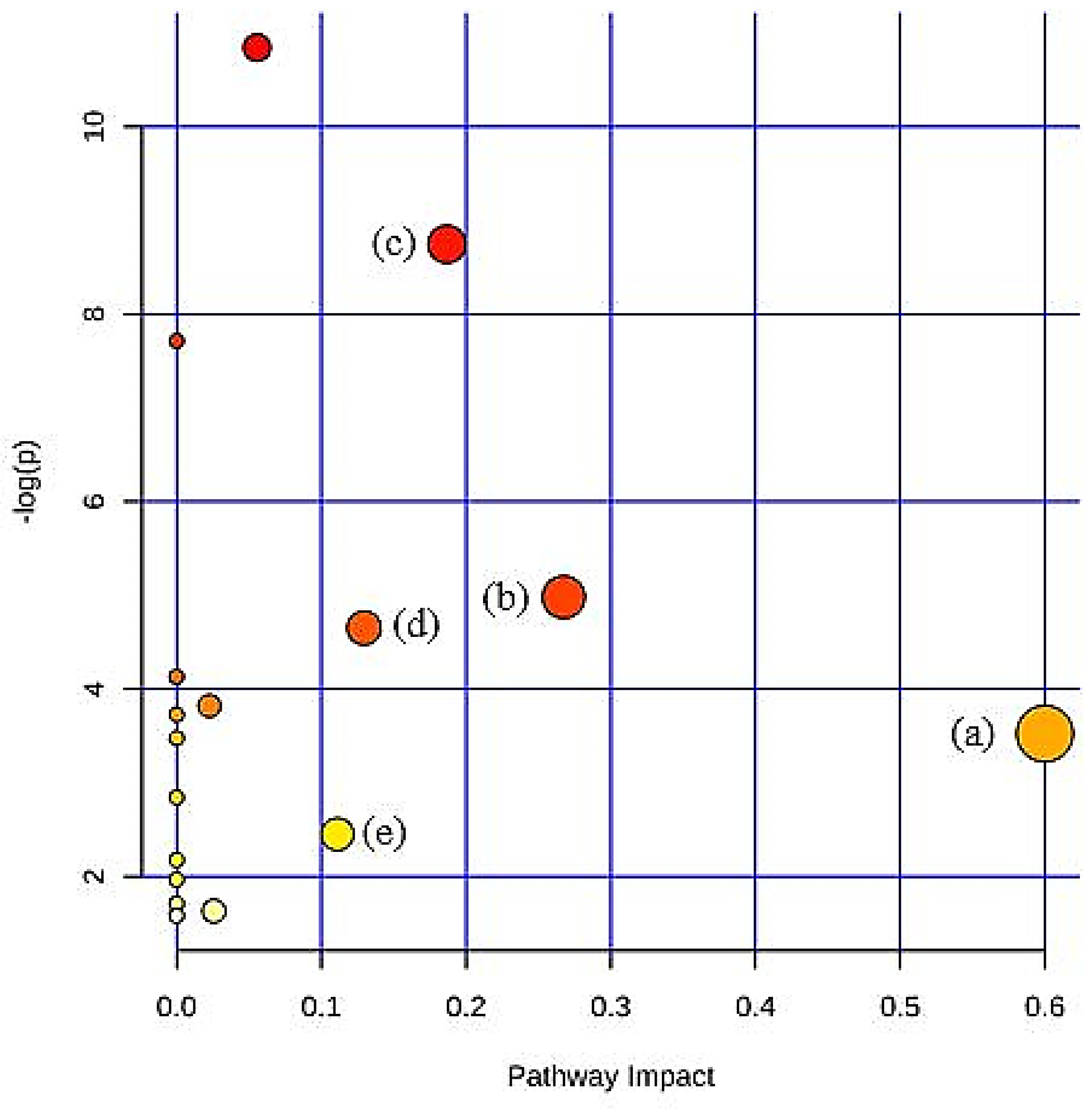
| Citrate cycle (TCA cycle) | 0.0002 | 0.19 |
| Pyruvate metabolism | 0.0085 | 0.27 |
| Glycolysis/Gluconeogenesis | 0.0118 | 0.13 |
| Synthesis and degradation of ketone bodies | 0.0327 | 0.60 |
| Butanoate metabolism | 0.0953 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadir, N.A.A.A.; Azlan, A.; Abas, F.; Ismail, I.S. Effect of Defatted Dabai Pulp Extract in Urine Metabolomics of Hypercholesterolemic Rats. Nutrients 2020, 12, 3511. https://doi.org/10.3390/nu12113511
Kadir NAAA, Azlan A, Abas F, Ismail IS. Effect of Defatted Dabai Pulp Extract in Urine Metabolomics of Hypercholesterolemic Rats. Nutrients. 2020; 12(11):3511. https://doi.org/10.3390/nu12113511
Chicago/Turabian StyleKadir, Noor Atiqah Aizan Abdul, Azrina Azlan, Faridah Abas, and Intan Safinar Ismail. 2020. "Effect of Defatted Dabai Pulp Extract in Urine Metabolomics of Hypercholesterolemic Rats" Nutrients 12, no. 11: 3511. https://doi.org/10.3390/nu12113511
APA StyleKadir, N. A. A. A., Azlan, A., Abas, F., & Ismail, I. S. (2020). Effect of Defatted Dabai Pulp Extract in Urine Metabolomics of Hypercholesterolemic Rats. Nutrients, 12(11), 3511. https://doi.org/10.3390/nu12113511