Effect of Probiotic Supplementation on Newborn Birth Weight for Mother with Gestational Diabetes Mellitus or Overweight/Obesity: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Data Extraction and Quality Assessment
2.3. Data Synthesis and Analysis
3. Results
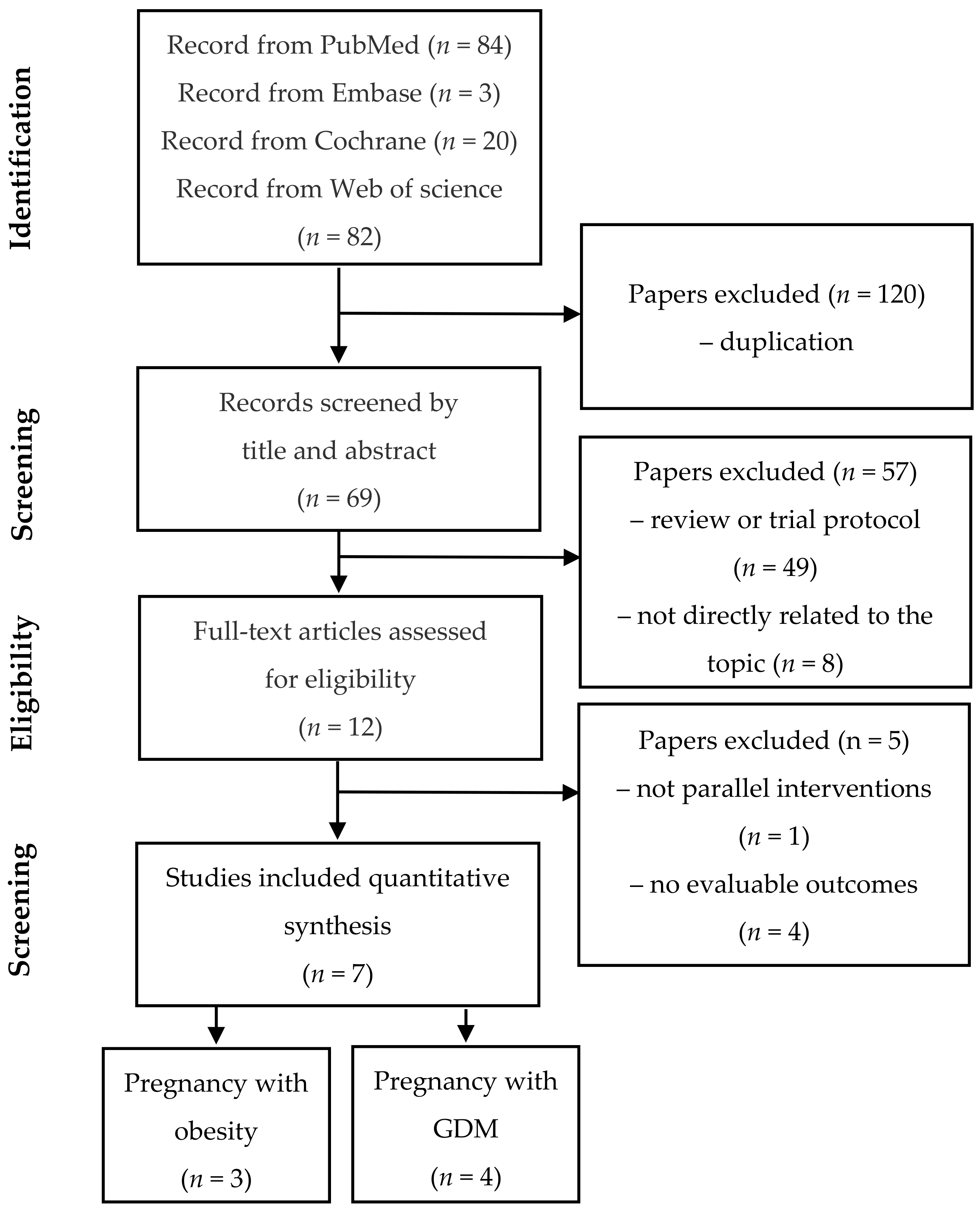
3.1. Search Results and Study Eligibility
3.2. Description of Selected Trials and Study Characteristics
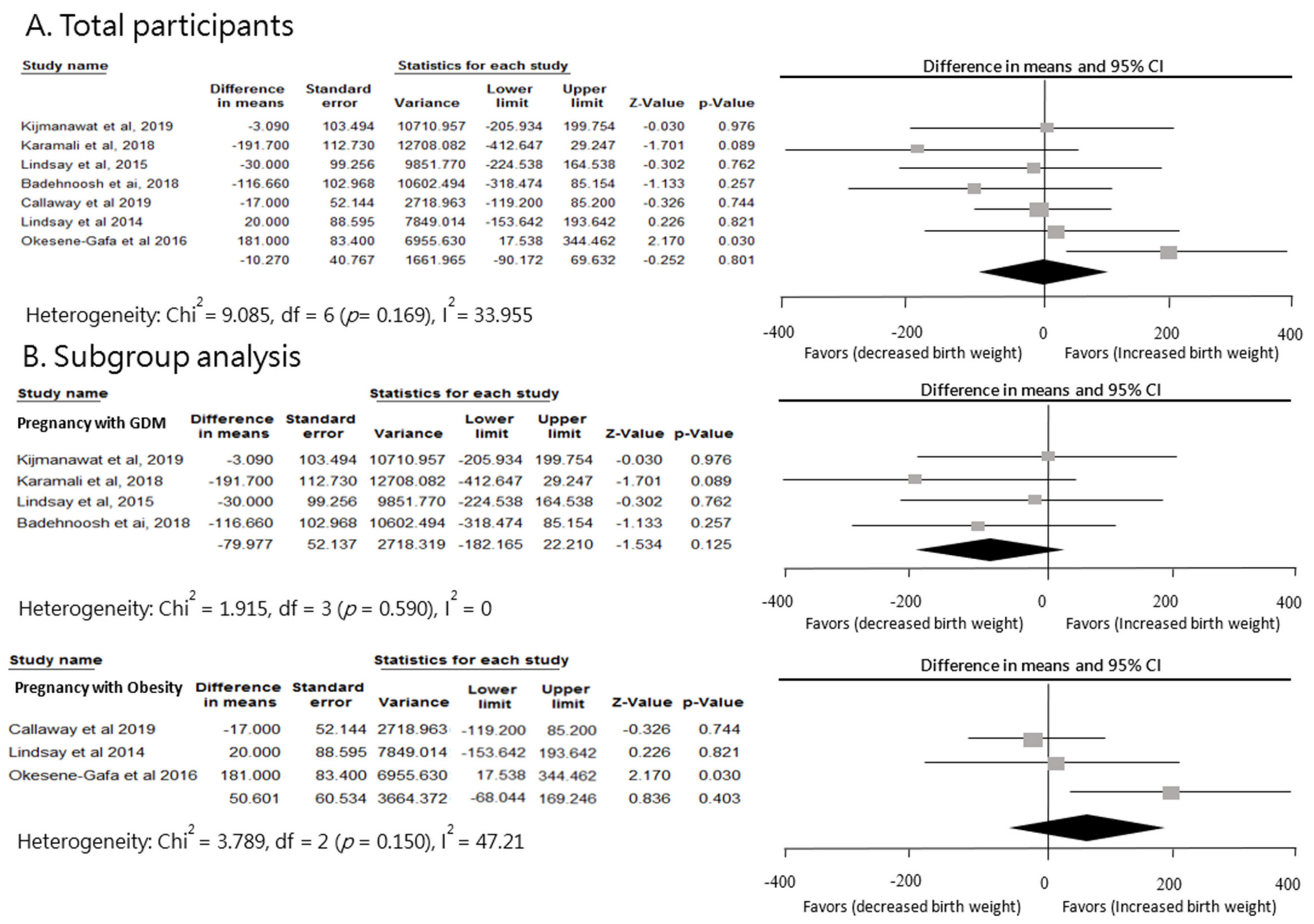
3.3. Effect of Probiotics on Newborn Birth Weight in Pregnant Women with GDM or Overweight
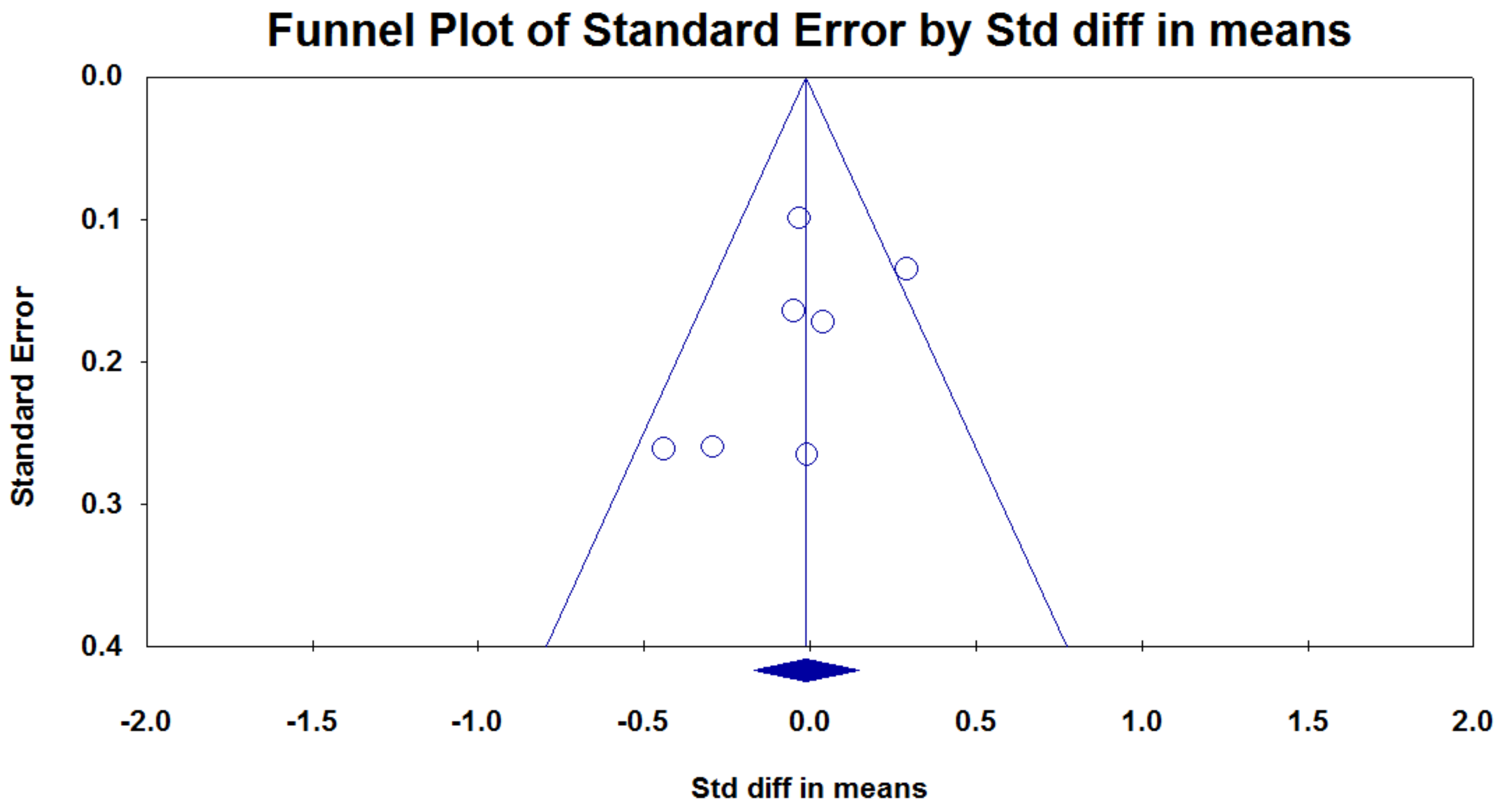
3.4. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnsson, I.W.; Haglund, B.; Ahlsson, F.; Gustafsson, J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr. Obes. 2014, 10, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Braddon, F.E.; Rodgers, B.; Wadsworth, M.E.; Davies, J.M. Onset of obesity in a 36 year birth cohort study. BMJ 1986, 293, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Stettler, N.; Zemel, B.S.; Kumanyika, S.; Stallings, V.A. Infant Weight Gain and Childhood Overweight Status in a Multicenter, Cohort Study. Pediatrics 2002, 109, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Ong, K.; Linné, Y.; Neovius, M.; Brage, S.; Dunger, D.B.; Wareham, N.J.; Rössner, S. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: The Stockholm Weight Development Study (SWEDES). Am. J. Clin. Nutr. 2006, 83, 324–330. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.; Hughes, R.; Tilling, K.; Davies, D.; Smith, G.D.; Ben-Shlomo, Y. Birth weight; postnatal, infant, and childhood growth; and obesity in young adulthood: Evidence from the Barry Caerphilly Growth Study. Am. J. Clin. Nutr. 2007, 86, 907–913. [Google Scholar] [CrossRef]
- Kang, M.; Yoo, J.E.; Kim, K.; Choi, S.; Park, S.M. Associations between birth weight, obesity, fat mass and lean mass in Korean adolescents: The Fifth Korea National Health and Nutrition Examination Survey. BMJ Open 2018, 8, e018039. [Google Scholar] [CrossRef]
- Rooney, B.L.; Mathiason, M.A.; Schauberger, C.W. Predictors of Obesity in Childhood, Adolescence, and Adulthood in a Birth Cohort. Matern. Child Heal. J. 2011, 15, 1166–1175. [Google Scholar] [CrossRef]
- Brumbaugh, D.E.; Tearse, P.; Cree-Green, M.; Fenton, L.Z.; Brown, M.; Scherzinger, A.; Reynolds, R.; Alston, M.; Hoffman, C.; Pan, Z.; et al. Intrahepatic Fat Is Increased in the Neonatal Offspring of Obese Women with Gestational Diabetes. J. Pediatr. 2013, 162, 930–936.e1. [Google Scholar] [CrossRef]
- Werneck, A.O.; Silva, D.R.P.; Collings, P.J.; Fernandes, R.A.; Ronque, E.R.V.; Coelho-E-Silva, M.J.; Sardinha, L.B.; Cyrino, E.S. Birth weight, biological maturation and obesity in adolescents: A mediation analysis. J. Dev. Orig. Health Dis. 2017, 8, 502–507. [Google Scholar] [CrossRef]
- Brumbaugh, D.E.; Friedman, J.E. Developmental origins of nonalcoholic fatty liver disease. Pediatr. Res. 2014, 75, 140–147. [Google Scholar] [CrossRef]
- Weng, S.F.; Redsell, S.A.; Nathan, D.; Swift, J.A.; Yang, M.; Glazebrook, C. Estimating Overweight Risk in Childhood from Predictors During Infancy. Pediatrics 2013, 132, e414–e421. [Google Scholar] [CrossRef]
- Malcolm, J.C. Through the looking glass: Gestational diabetes as a predictor of maternal and offspring long-term health. Diabetes/Metabolism Res. Rev. 2012, 28, 307–311. [Google Scholar] [CrossRef]
- Hoppu, U.; Isolauri, E.; Laakso, P.; Matomäki, J.; Laitinen, K. Probiotics and dietary counselling targeting maternal dietary fat intake modifies breast milk fatty acids and cytokines. Eur. J. Nutr. 2011, 51, 211–219. [Google Scholar] [CrossRef]
- Kim, C. Gestational diabetes mellitus and risk of future maternal cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2010, 8, 1639–1641. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The Hyperglycemia and Adverse Pregnancy Outcome Study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Tanvig, M. Offspring body size and metabolic profile—effects of lifestyle intervention in obese pregnant women. Dan. Med. J. 2014, 61. [Google Scholar]
- Dodd, J.M.; For the LIMIT Randomised Trial Group; McPhee, A.J.; Turnbull, D.; Yelland, L.N.; Deussen, A.R.; Grivell, R.M.; Crowther, C.A.; Wittert, G.; Owens, J.A.; et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on neonatal health outcomes: The LIMIT randomised trial. BMC Med. 2014, 12, 163. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef]
- Myrex, P.; Harper, L.M.; Gould, S. An Evaluation of Birth Outcomes in Overweight and Obese Pregnant Women Who Exercised during Pregnancy. Sports 2018, 6, 138. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.W.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ 2012, 344, e2088. [Google Scholar] [CrossRef]
- Du, M.; Ouyang, Y.-Q.; Nie, X.; Huang, Y.; Redding, S.R. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: A meta-analysis. Birth 2018, 46, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.K.S.; Dietz, P.M.; Galavotti, C.; England, L.J. Weight-Management Interventions for Pregnant or Postpartum Women. Am. J. Prev. Med. 2008, 34, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Streuling, I.; Beyerlein, A.; Rosenfeld, E.; Hofmann, H.; Schulz, T.; Von Kries, R. Physical activity and gestational weight gain: A meta-analysis of intervention trials. BJOG Int. J. Obstet. Gynaecol. 2010, 118, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, J.; Julania, S.; Lam, L. Antenatal Dietary Interventions in Obese Pregnant Women to Restrict Gestational Weight Gain to Institute of Medicine Recommendations. Obstet. Gynecol. 2011, 118, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Oteng-Ntim, E.; Varma, R.; Croker, H.; Poston, L.; Doyle, P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: Systematic review and meta-analysis. BMC Med. 2012, 10, 47. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Pawlak, D.B.; Ludwig, D.S. Childhood obesity: Public-health crisis, common sense cure. Lancet 2002, 360, 473–482. [Google Scholar] [CrossRef]
- Druet, C.; Ong, K.K. Early childhood predictors of adult body composition. Best Pr. Res. Clin. Endocrinol. Metab. 2008, 22, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G.; Forsén, T.; Osmond, C.; Barker, D. Obesity from cradle to grave. Int. J. Obes. 2003, 27, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.L.; Schooling, C.M.; Leung, S.S.L.; Mak, K.H.; Ho, L.M.; Lam, T.H.; Leung, G.M. Birth Weight, Infant Growth, and Childhood Body Mass Index. Arch. Pediatr. Adolesc. Med. 2008, 162, 212–218. [Google Scholar] [CrossRef]
- Soderborg, T.K.; Borengasser, S.J.; Barbour, L.A.; Friedman, J.E. Microbial transmission from mothers with obesity or diabetes to infants: An innovative opportunity to interrupt a vicious cycle. Diabetologia 2016, 59, 895–906. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Ângelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and its relation to gestational diabetes. Endocrine 2018, 64, 254–264. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics: Definition, Sources, Selection, and Uses. Clin. Infect. Dis. 2008, 46, S58–S61. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Kalliomaki, M.; Laitinen, K.; Isolauri, E. The impact of perinatal probiotic intervention on the development of overweight and obesity: Follow-up study from birth to 10 years. Int. J. Obes. 2010, 34, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern. Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabasi, Z.; Talebian, P.; Azarbad, Z.; Hydarzadeh, Z.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: A randomized controlled clinical trial. J. Matern. Neonatal Med. 2011, 25, 1552–1556. [Google Scholar] [CrossRef]
- Asemi, Z.; ATLAS Collaboration; Samimi, M.; Tabassi, Z.; Rad, M.N.; Foroushani, A.R.; Khorammian, H.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 67, 71–74. [Google Scholar] [CrossRef]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Mesgari-Abbasi, M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Heal. Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [PubMed]
- Slykerman, R.; Hood, F.; Wickens, K.; Thompson, J.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Nasiri, N.; Shavazi, N.T.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Synbiotic Supplementation on Pregnancy Outcomes in Gestational Diabetes. Probiotics Antimicrob. Proteins 2017, 10, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Okesene-Gafa, K.; Li, M.; McKinlay, C.J.; Taylor, R.S.; Rush, E.C.; Wall, C.R.; Wilson, J.; Murphy, R.; Taylor, R.; Thompson, J.M.; et al. Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial. Am. J. Obstet. Gynecol. 2019, 221, 152.e1–152.e13. [Google Scholar] [CrossRef] [PubMed]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2018, 10, 163–170. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 496.e1–496.e11. [Google Scholar] [CrossRef]
- Callaway, L.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.A.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings from the SPRING Double-blind Randomized Controlled Trial. Diabetes Care 2019, 42, dc182248. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Kennelly, M.; Culliton, M.; Smith, T.; Maguire, O.C.; Shanahan, F.; Brennan, L.; McAuliffe, F.M. Probiotics in obese pregnancy do not reduce maternal fasting glucose: A double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am. J. Clin. Nutr. 2014, 99, 1432–1439. [Google Scholar] [CrossRef]
- Laitinen, K.; Poussa, T.; Isolauri, E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: A randomised controlled trial. Br. J. Nutr. 2008, 101, 1679–1687. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Han, M.-M.; Sun, J.-F.; Su, X.-H.; Peng, Y.-F.; Goyal, H.; Wu, C.-H.; Zhu, X.-Y.; Li, L. Probiotics improve glucose and lipid metabolism in pregnant women: A meta-analysis. Ann. Transl. Med. 2019, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Bueno, A.A.; De Souza, R.G.M.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Invernici, M.; Furlaneto, F.A.C.; Messora, M.R. Effectiveness of Multi-strain Versus Single-strain Probiotics. J. Clin. Gastroenterol. 2018, 52, S35–S40. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.M.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef]
- Mack, L.R.; Tomich, P.G. Gestational Diabetes. Obstet. Gynecol. Clin. North Am. 2017, 44, 207–217. [Google Scholar] [CrossRef]
- Ricart, W.; Lopez, J.; Mozas, J.; Pericot, A.; Sancho, M.A.; Gonzalez, N.; Balsells, M.; Luna, R.; Cortázar, A.; Navarro, P.; et al. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycaemia. Diabetologia 2005, 48, 1736–1742. [Google Scholar] [CrossRef]

| Study | Study Design | Country | Subjects | GA, Weeks | Age, Years | Regimen of Intervention | BW in Interventions, Mean ± SD | BW in Controls, Mean± SD | Adjustments |
|---|---|---|---|---|---|---|---|---|---|
| Kijmanawa 2019 [45] | Db-RCT | Thailand | diet-controlled GDM | 24–28 | 18–45 | I: L. acidophilus, B. bifidum. D: 109 CFU/day S/E:4 weeks | 3120.4 ± 411.1 n = 28 | 3123.5 ± 369.8 n = 29 | N/A |
| Karamali 2018 [43] | Db-RCT | Iran | GDM, BMI: 28.45 ± 3.4 | <34 | 18–40 | I: L. acidophilus strain T16 (IBRC-M10785), L. casei strain T2 (IBRC-M10783), B. bifidum strain T1 (IBRC-M10771) D: 2 × 109 CFU plus 800 mg inulin/day S/E: 6 weeks | 3181.6 ± 459.8 n = 30 | 3373.3 ± 412.1 n = 30 | Maternal BMI Preterm birth |
| Lindsay 2015 [46] | Db-RCT | Ireland | GDM, BMI: 29.00 ± 6.23 | <34 | >18 | I: L. salivarius UCC118 D:109 CFU/day S/E: 4–6 weeks | 3570 ± 640 n = 74 | 3600 ± 570 n = 75 | Maternal BMI |
| Badehnoosh 2018 [38] | Db-RCT | Iran | GDM BMI: 28.4 ± 3.8 | 24–28 | 18–40 | I: L. acidophilus, L. casei, B. bifidum D: 2 × 109 CFU/day S/E: 6 weeks | 3321.7 ± 443.5 n = 30 | 3438.4 ± 348.4 n = 30 | Maternal BMI Preterm birth |
| Callaway 2019 [47] | Db-RCT | Australia | BMI >25 | <20 | >18 | I: L. rhamnosus B. animalis subspecies lactis D: >109 CFU/day S/E: from enrollment until birth | 3524 ± 540 n = 206 | 3541 ± 514 n = 203 | Maternal BMI Preterm birth |
| Lindsay 2014 [48] | Db-RCT | Ireland | BMI: 30.0–39.9 | <20 | >18 | I: L. salivarius UCC118 D: 109 CFU/day S/E: 4 weeks | 3700 ± 520 n = 62 | 3680 ± 510 n = 74 | Maternal BMI |
| Okesene-Gafa 2016 [44] | 2 × 2 factorial, RCT | New Zealand | BMI > 30.0 | 12–17 | >18 | I: L. rhamnosus, B. animalis subspecies lactis D: 6.5 × 109 CFU/day S/E: from enrollment until birth | 3685 ± 565 n = 110 | 3504 ± 672 n = 112 | Maternal BMI Preterm birth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Tung, Y.-T.; Chang, H.-C.; Lin, C.-H.; Chen, Y.-C. Effect of Probiotic Supplementation on Newborn Birth Weight for Mother with Gestational Diabetes Mellitus or Overweight/Obesity: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 3477. https://doi.org/10.3390/nu12113477
Wang C-C, Tung Y-T, Chang H-C, Lin C-H, Chen Y-C. Effect of Probiotic Supplementation on Newborn Birth Weight for Mother with Gestational Diabetes Mellitus or Overweight/Obesity: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(11):3477. https://doi.org/10.3390/nu12113477
Chicago/Turabian StyleWang, Chun-Chi, Yu-Tang Tung, Hua-Ching Chang, Chang-Hsien Lin, and Yang-Ching Chen. 2020. "Effect of Probiotic Supplementation on Newborn Birth Weight for Mother with Gestational Diabetes Mellitus or Overweight/Obesity: A Systematic Review and Meta-Analysis" Nutrients 12, no. 11: 3477. https://doi.org/10.3390/nu12113477
APA StyleWang, C.-C., Tung, Y.-T., Chang, H.-C., Lin, C.-H., & Chen, Y.-C. (2020). Effect of Probiotic Supplementation on Newborn Birth Weight for Mother with Gestational Diabetes Mellitus or Overweight/Obesity: A Systematic Review and Meta-Analysis. Nutrients, 12(11), 3477. https://doi.org/10.3390/nu12113477






