Variation in Infant Formula Macronutrient Ingredients Is Associated with Infant Anthropometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Infant Formula Nutrition Database
2.2. NHANES Data
2.3. Multivariable Modeling
3. Results
3.1. Cohort Characteristics
3.2. Infant Intake
3.3. Modeling Z-Scores
3.3.1. Infants < 6 Months
3.3.2. Infants 6–12 Months
3.3.3. Infants 0–12 Months
4. Discussion
Funding
Conflicts of Interest
References
- Food and Drug Administration (FDA). CFR—Code of Federal Regulations Title 21—Food and Drugs. Chapter I—Food and Drug Administration. Department of Health and Human Services. Subchapter B—Food for Human Consumption. Part 107 Infant Formula. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=107&showfr=1 (accessed on 18 September 2019).
- Bhatia, J.; Greer, F. Use of soy protein-based formulas in infant feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Body fat and bone mineral content of infants fed breast milk, cow’s milk formula, or soy formula during the first year of life. J. Pediatr. 2013, 163, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Cleves, M.A.; Bellando, J.B.; Pivik, R.T.; Casey, P.H.; Badger, T.M. Developmental status of 1-year-old infants fed breast milk, cow’s milk formula, or soy formula. Pediatrics 2012, 129, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Åkerblom, H.K.; Becker, D.; Dosch, H.M.; Dupre, J.; Fraser, W.; Howard, N.; Ilonen, J.; Krischer, J.P.; Kordonouri, O.; et al. Hydrolyzed infant formula and early beta-cell autoimmunity: A randomized clinical trial. JAMA 2014, 311, 2279–2287. [Google Scholar] [CrossRef]
- Knip, M.; Virtanen, S.M.; Seppä, K.; Ilonen, J.; Savilahti, E.; Vaarala, O.; Reunanen, A.; Teramo, K.; Hämäläinen, A.M.; Paronen, J.; et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N. Engl. J. Med. 2010, 363, 1900–1908. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Munasir, Z.; Hegar, B.; Kumarawati, D.; Suryawan, A.; Kadim, M.; Djais, J.T.; Basrowi, R.W.; Krisnamurti, D. A perspective on partially hydrolyzed protein infant formula in nonexclusively breastfed infants. Korean J. Pediatr. 2019, 62, 149–154. [Google Scholar] [CrossRef]
- Sauser, J.; Nutten, S.; de Groot, N.; Pecquet, S.; Simon, D.; Simon, H.U.; Spergel, J.M.; Koletzko, S.; Blanchard, C. Partially Hydrolyzed Whey Infant Formula: Literature Review on Effects on Growth and the Risk of Developing Atopic Dermatitis in Infants from the General Population. Int. Arch. Allergy Immunol. 2018, 177, 123–134. [Google Scholar] [CrossRef]
- Boyle, R.J.; Ierodiakonou, D.; Khan, T.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Afxentiou, T.; Reeves, T.; Cunha, S.; et al. Hydrolysed formula and risk of allergic or autoimmune disease: Systematic review and meta-analysis. BMJ 2016, 352, i974. [Google Scholar] [CrossRef]
- von Berg, A.; Filipiak-Pittroff, B.; Krämer, U.; Hoffmann, B.; Link, E.; Beckmann, C.; Hoffmann, U.; Reinhardt, D.; Grübl, A.; Heinrich, J.; et al. Allergies in high-risk schoolchildren after early intervention with cow’s milk protein hydrolysates: 10-year results from the German Infant Nutritional Intervention (GINI) study. J. Allergy Clin. Immunol. 2013, 131, 1565–1573. [Google Scholar] [CrossRef]
- Mennella, J.A.; Ventura, A.K.; Beauchamp, G.K. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics 2011, 127, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Inamdar, L.; Pressman, N.; Schall, J.I.; Papas, M.A.; Schoeller, D.; Stallings, V.A.; Trabulsi, J.C. Type of infant formula increases early weight gain and impacts energy balance: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Inamdar, L.; Pressman, N.; Schall, J.I.; Papas, M.A.; Schoeller, D.; Stallings, V.A.; Trabulsi, J.C. Early rapid weight gain among formula-fed infants: Impact of formula type and maternal feeding styles. Pediatr. Obes. 2019, 14, e12503. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Foong, R.X.M.; Thapar, N.; Kritas, S.; Shah, N. Systematic review of the impact of feed protein type and degree of hydrolysis on gastric emptying in children. BMC Gastroenterol. 2015, 15, 137. [Google Scholar] [CrossRef]
- Billeaud, C.; Guillet, J.; Sandler, B. Gastric emptying in infants with or without gastro-oesophageal reflux according to the type of milk. Eur. J. Clin. Nutr. 1990, 44, 577–583. [Google Scholar]
- Tsabouri, S.; Douros, K.; Priftis, K.N. Cow’s milk allergenicity. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 16–26. [Google Scholar] [CrossRef]
- Wal, J.M. Cow’s milk proteins/allergens. Ann. Allergy Asthma Immunol. 2002, 89 (Suppl. 1), 3–10. [Google Scholar] [CrossRef]
- Koo, W.W.; Hammami, M.; Margeson, D.P.; Nwaesei, C.; Montalto, M.B.; Lasekan, J.B. Reduced bone mineralization in infants fed palm olein-containing formula: A randomized, double-blinded, prospective trial. Pediatrics 2003, 111 Pt 1, 1017–1023. [Google Scholar] [CrossRef]
- Koo, W.W.; Hockman, E.M.; Dow, M. Palm olein in the fat blend of infant formulas: Effect on the intestinal absorption of calcium and fat, and bone mineralization. J. Am. Coll. Nutr. 2006, 25, 117–122. [Google Scholar] [CrossRef]
- Leite, M.E.; Lasekan, J.; Baggs, G.; Ribeiro, T.; Menezes-Filho, J.; Pontes, M.; Druzian, J.; Barreto, D.L.; de Souza, C.O.; Mattos, Â.; et al. Calcium and fat metabolic balance, and gastrointestinal tolerance in term infants fed milk-based formulas with and without palm olein and palm kernel oils: A randomized blinded crossover study. BMC Pediatr. 2013, 13, 215. [Google Scholar] [CrossRef]
- Souza, C.O.; Leite, M.E.Q.; Lasekan, J.; Baggs, G.; Pinho, L.S.; Druzian, J.I.; Ribeiro, T.C.M.; Mattos, Â.P.; Menezes-Filho, J.A.; Costa-Ribeiro, H. Milk protein-based formulas containing different oils affect fatty acids balance in term infants: A randomized blinded crossover clinical trial. Lipids Health Dis. 2017, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Slupsky, C.M.; He, X.; Hernell, O.; Andersson, Y.; Rudolph, C.; Lönnerdal, B.; West, C.E. Postprandial metabolic response of breast-fed infants and infants fed lactose-free vs. regular infant formula: A randomized controlled trial. Sci. Rep. 2017, 7, 3640. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA. Available online: https://wwwn.cdc.gov/nchs/nhanes/ (accessed on 30 September 2018).
- Centers for Disease Control and Prevention (CDC). A SAS Program for the WHO Growth Charts (Ages 0 to <2 Years). Available online: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm (accessed on 8 September 2019).
- Abbott Nutrition. Similac for Diarrhea. Infant Formula. Available online: https://abbottnutrition.com/similac-for-diarrhea (accessed on 18 September 2019).
- Miles, E.A.; Calder, P.C. The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutr. Res. 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, K.M.; Borschel, M.W.; Westcott, J.E.; Richardson, K.S.; Krebs, N.F. Lower calcium absorption in infants fed casein hydrolysate- and soy protein-based infant formulas containing palm olein versus formulas without palm olein. J. Am. Coll. Nutr. 2002, 21, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, M.; Basaranoglu, G.; Sabuncu, T.; Sentürk, H. Fructose as a key player in the development of fatty liver disease. World J. Gastroenterol. 2013, 19, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef]
- Mortera, R.R.; Bains, Y.; Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. (Landmark. Ed.) 2019, 24, 186–211. [Google Scholar]
- Chiu, S.; Sievenpiper, J.L.; De Souza, R.J.; Cozma, A.I.; Mirrahimi, A.; Carleton, A.J.; Ha, V.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014, 68, 416–423. [Google Scholar] [CrossRef]
- Mager, D.R.; Iñiguez, I.R.; Gilmour, S.; Yap, J. The Effect of a Low Fructose and Low Glycemic Index/Load (FRAGILE) Dietary Intervention on Indices of Liver Function, Cardiometabolic Risk Factors, and Body Composition in Children and Adolescents With Nonalcoholic Fatty Liver Disease (NAFLD). J. Parenter. Enter. Nutr. 2015, 39, 73–84. [Google Scholar] [CrossRef]
- Bainbridge, R.R.; Mimouni, F.; Tsang, R.C. Bone mineral content of infants fed soy-based formula. J. Pediatr. 1988, 113 Pt 2, 205–207. [Google Scholar] [CrossRef]
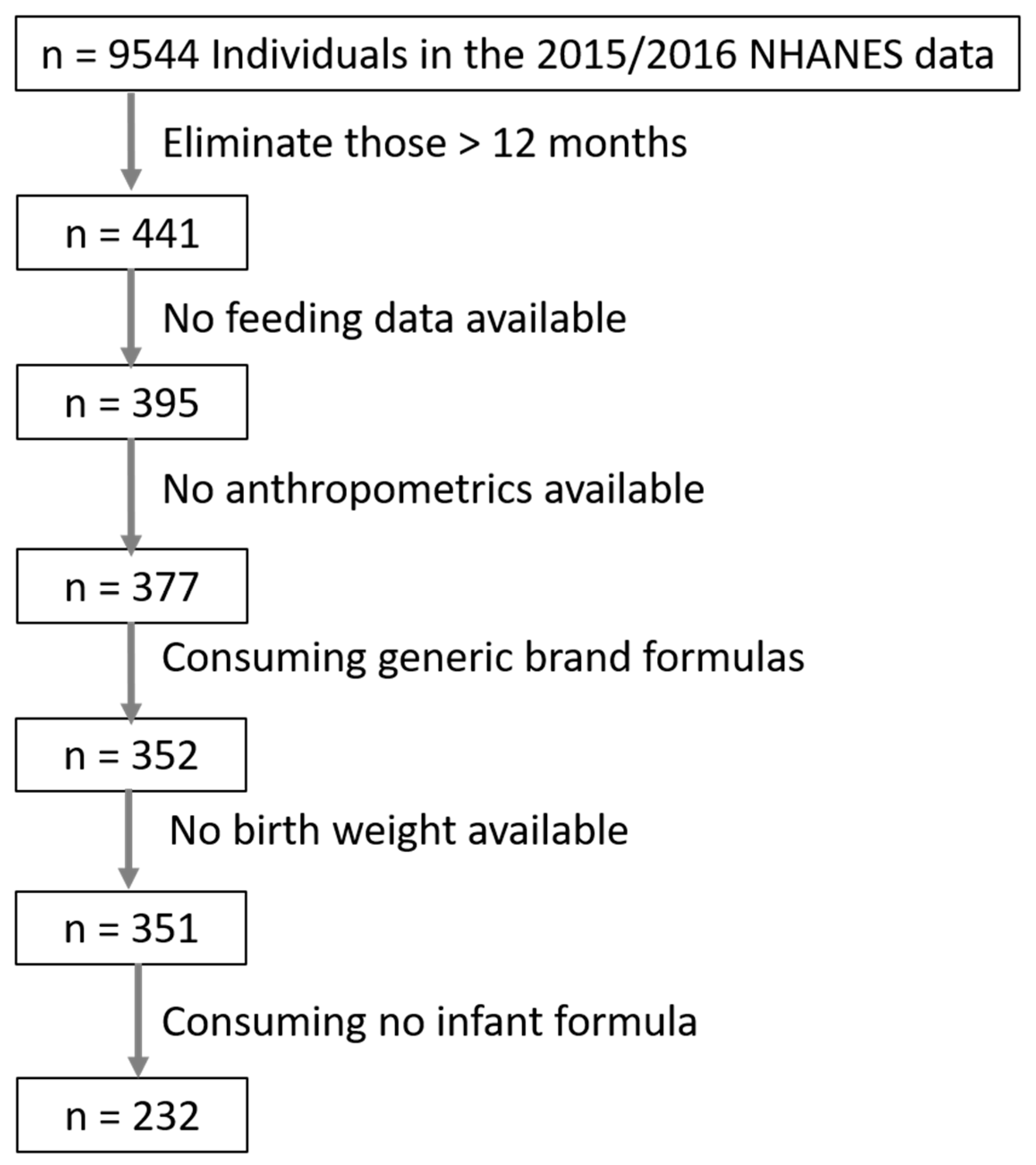
| Characteristic | Mean ± SD or % | n |
|---|---|---|
| Infant Sex (% male) | 47% | 350 |
| Birth Weight (g) | 3278 ± 533 | 350 |
| Birth Weight category | LBW = 7.7%; LGA = 6.6% | 350 |
| Breastfeeding Exclusivity 1 (all infants) | Exclusive Breastfeeding = 29% Mixed Feeding = 14% Exclusive Formula = 56% | 325 |
| Breastfeeding Exclusivity 1 (infants ≤ 6 months) | Exclusive Breastfeeding = 31% Mixed Feeding = 19% Exclusive Formula = 49% | 180 |
| Infant age at physical exam (months) | 6.4 ± 3.6 | 350 |
| Weight for age Z-score (WAZ) | 0.47 ±1.05 | 323 |
| Length for age Z-score (LAZ) | 0.21 ± 1.1 | 323 |
| Weight for Length Z-score (WLZ) | 0.51 ± 0.99 | 323 |
| Head Circumference for age Z-score (HCZ) | 1.00 ± 1.22 | 196 |
| Abdominal Circumference for age Z-score (ACZ) | 0.47 ± 0.99 | 255 |
| Macronutrient Source | Mean ± SD or % | n |
|---|---|---|
| Daily caloric intake from formula (kcal) | 531 ± 230 kcal | 230 |
| Protein size | Amino Acid Based = 0.4% Fully Hydrolyzed = 4.8% Partially Hydrolyzed = 22.4% Intact = 72.4% | 228 |
| Protein Source (if dairy formula) | Casein = 47.5 ± 25.0% Whey = 52.3 ± 25.2% | 228 |
| Protein source—% infants consuming soy | 7.4% | 228 |
| Carbohydrate—Source | Lactose = 64.7 ± 41.4% Glucose = 31.7 ± 36.3% Sucrose = 3.4 ± 8.6% | 228 |
| Carbohydrate source—% infants consuming sucrose | 15.4% | 228 |
| Fat Source | Palm Oil = 25.7 ± 22.4% Coconut/MCT Oil = 24.9 ± 5.9% Soy Oil = 25.4 ± 5.9% | 228 |
| Fat source—% infants by Palm Oil consumption categories | No palm oil = 41.2% 20–30% Palm oil = 3.1% ≥40% Palm oil = 55.6% | 228 |
| A. Infants < 6 Months | ||
| Independent Variable (Parameter) | Parameter p-Value | Parameter Estimate |
| WAZ (R2 = 0.10, n = 124) | ||
| Birth Weight category (reference = normal birth weight) | 0.001 | LBW: −1.04 ± 0.27, p = 0.0002 LGA: 0.923 ± 0.312, p = 0.003 |
| LAZ (R2 = 0.11, n = 124) | ||
| Birth Weight category (reference = normal birth weight) | 0.001 | LBW: −1.10 ± 0.29, p = 0.0003 LGA: 0.85 ± 0.33, p = 0.004 |
| WLZ (R2 = 0.08, n = 123) | ||
| Protein—Size (reference = intact protein) | 0.087 | Fully Hydrolyzed: 0.568 ± 0.267, p = 0.035 Partially Hydrolyzed: −0.245 ± 0.184, p = 0.185 |
| Protein—Soy | 0.143 | Non-soy based: 0.265 ± 0.180 |
| Fat—Palm Oil | 0.076 | Palm Oil-free: −0.185 ± 0.103, p = 0.076 |
| Formula—kcal consumed/day | 0.096 | 0.00062 ± 0.00037, p = 0.096 |
| HCZ (R2 = 0.03, n = 122) | ||
| Protein—Soy | 0.048 | Non-soy based: 0.424 ± 0.212 |
| ACZ (R2 = 0.10, n = 81) | ||
| Breastfeeding Category (reference = mixed fed) | 0.032 | Exclusively formula: 0.29 ± 0.13 |
| Protein—Soy | 0.059 | Non-soy based: 0.43 ± 0.22 |
| Fat—% Coconut oil | 0.054 | −1.108 ± 0.169 1 |
| B. Infants 6–12 Months | ||
| Independent Variable (Parameter) | Parameter p-Value | Parameter Estimate |
| WAZ (R2 = 0.15, n = 102) | ||
| Birth Weight category (reference = normal birth weight) | 0.002 | LBW: −1.00 ± 0.23, p < 0.0001 LGA: 0.96 ± 0.28, p = 0.0011 |
| Fat—Palm Oil | 0.264 | Palm Oil-free: 0.11 ± 0.10 |
| LAZ (R2 = 0.17, n = 103) | ||
| Birth Weight category (reference = normal birth weight) | <0.0001 | LBW: −1.03 ± 0.23, p < 0.0001 LGA: 1.12 ± 0.29, p = 0.0002 |
| WLZ (R2 = 0.06, n = 103) | ||
| Birth Weight category (reference = normal birth weight) | 0.033 | LBW: −0.634 ± 0.241, p = 0.0097 LGA: 0.534 ± 0.295, p = 0.073 |
| Fat—Palm Oil | 0.168 | Palm Oil-free: 0.142 ± 0.102 |
| ACZ (R2 = 0.09, n = 102) | ||
| Carbohydrate—Sucrose | 0.125 | Sucrose-free: −0.182 ± 0.118 |
| Birth Weight category (reference = normal birth weight) | 0.008 | LBW: −0.748 ± 0.235, p = 0.002 LGA: 0.752 ± 0.280, p = 0.008 |
| C. All Infants (0–12 Months) | ||
| Independent Variable (Parameter) | Parameter p-Value | Parameter Estimate |
| WAZ (R2 = 0.13, n = 227) | ||
| Birth Weight Category (reference = normal birth weight) | <0.0001 | LBW: −1.018 ± 0.175 (p < 0.0001) LGA: 0.925 ± 0.208 (p < 0.0001) |
| LAZ (R2 = 0.13, n = 227) | ||
| Birth Weight Category (reference = normal birth weight) | <0.0001 | LBW: −1.060 ± 0.184 (p < 0.0001); LGA: 0.986 ± 0.219 (p < 0.0001) |
| WLZ (R2 = 0.031, n = 225) | ||
| Birth Weight Category (reference = normal birth weight) | 0.029 | LBW: −0.486 ± 0.181 (p = 0.0008); LGA: 0.471 ± 0.216 (p = 0.030) |
| Carbohydrate—Sucrose | 0.073 | Sucrose-free: −0.185 ± 0.103 |
| Fat—% soy oil | 0.122 | 0.570 ± 0.366 1 |
| ACZ (R2 = 0.070, n = 183) | ||
| Birth Weight Category (reference = normal birth weight) | 0.005 | LBW: −0.655 ± 0.203 (p = 0.002) LGA: 0.685 ± 0.237 (p = 0.004) |
| Breastfeeding Category (reference = mixed fed) | 0.043 | Exclusively formula: 0.205 ± 0.100 |
| Protein—Soy | 0.205 | Non-soy based: 0.187 ± 0.147 |
| Carbohydrate—Sucrose | 0.080 | Sucrose-free: −0.175 ± 0.099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, B. Variation in Infant Formula Macronutrient Ingredients Is Associated with Infant Anthropometrics. Nutrients 2020, 12, 3465. https://doi.org/10.3390/nu12113465
Young B. Variation in Infant Formula Macronutrient Ingredients Is Associated with Infant Anthropometrics. Nutrients. 2020; 12(11):3465. https://doi.org/10.3390/nu12113465
Chicago/Turabian StyleYoung, Bridget. 2020. "Variation in Infant Formula Macronutrient Ingredients Is Associated with Infant Anthropometrics" Nutrients 12, no. 11: 3465. https://doi.org/10.3390/nu12113465
APA StyleYoung, B. (2020). Variation in Infant Formula Macronutrient Ingredients Is Associated with Infant Anthropometrics. Nutrients, 12(11), 3465. https://doi.org/10.3390/nu12113465





