Fennel for Reducing Pain in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Data Sources
2.2. Study Selection
2.2.1. Types of Studies
2.2.2. Types of Participants
2.2.3. Types of Interventions
2.2.4. Types of Comparisons
2.2.5. Outcome Measures
- −
- The primary outcome was pain, especially the reduction in menstrual pain occurring during trial intervention or as an intervention result, which was measured using validated scales such as the visual analog scale (VAS). The pain outcome presented in the form of dichotomous outcomes was also included.
- −
- Adverse events (AEs) were studied as secondary outcome.
2.3. Data Extraction and Risk-of-Bias Assessment
2.4. Data Synthesis
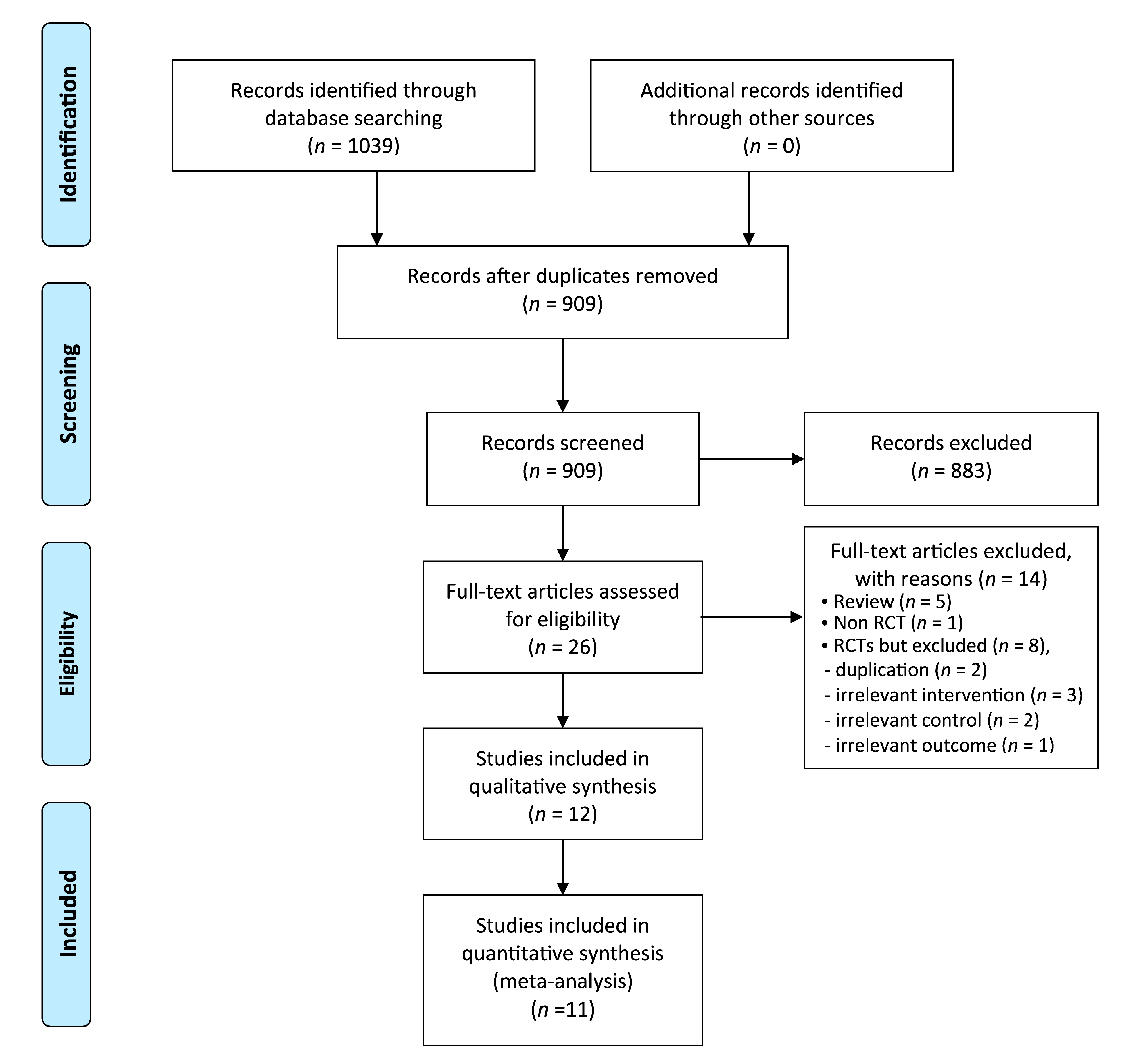
3. Results
3.1. Description of the Included Trials
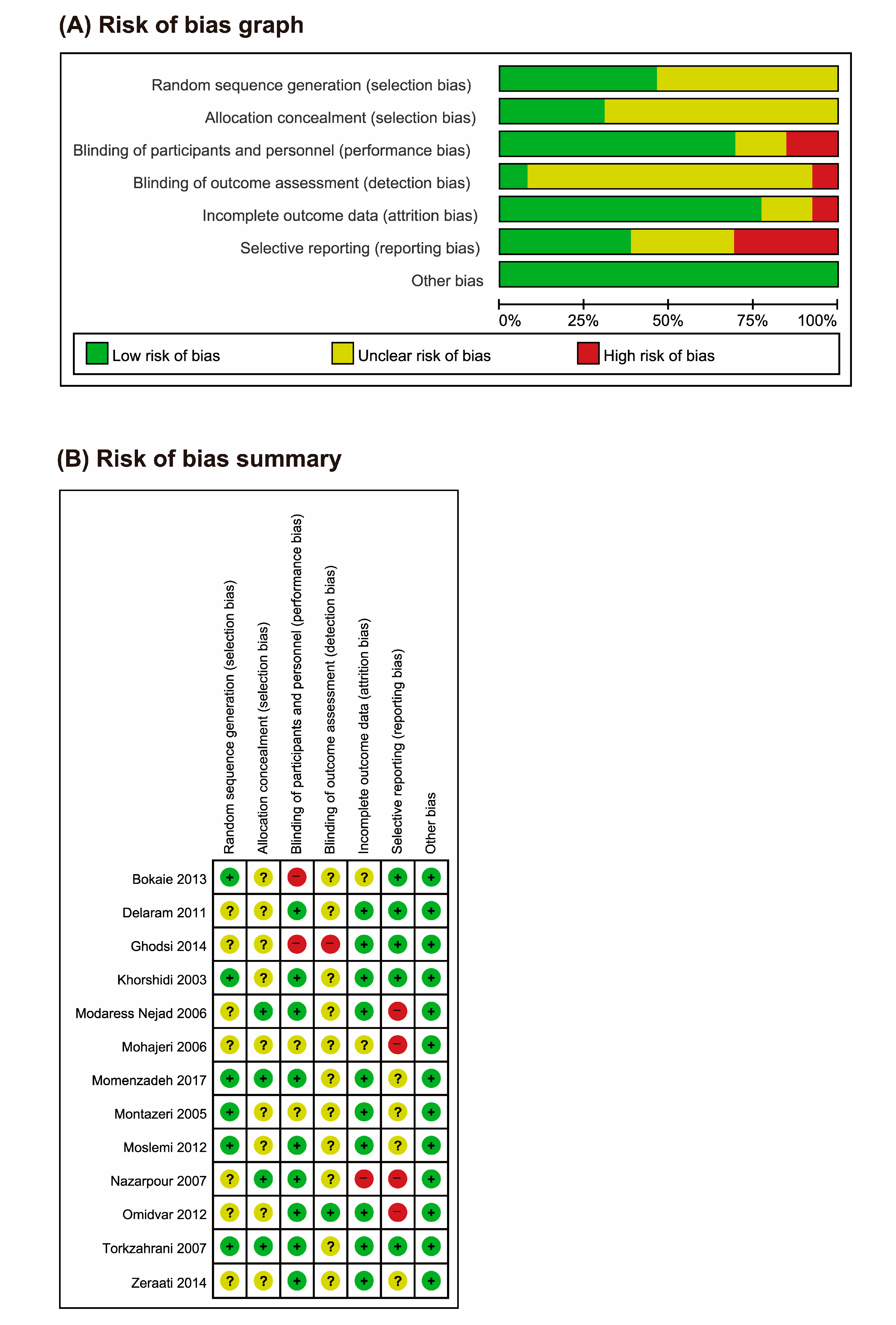
3.2. Risk of Bias (ROB)
3.3. Outcome Measurements
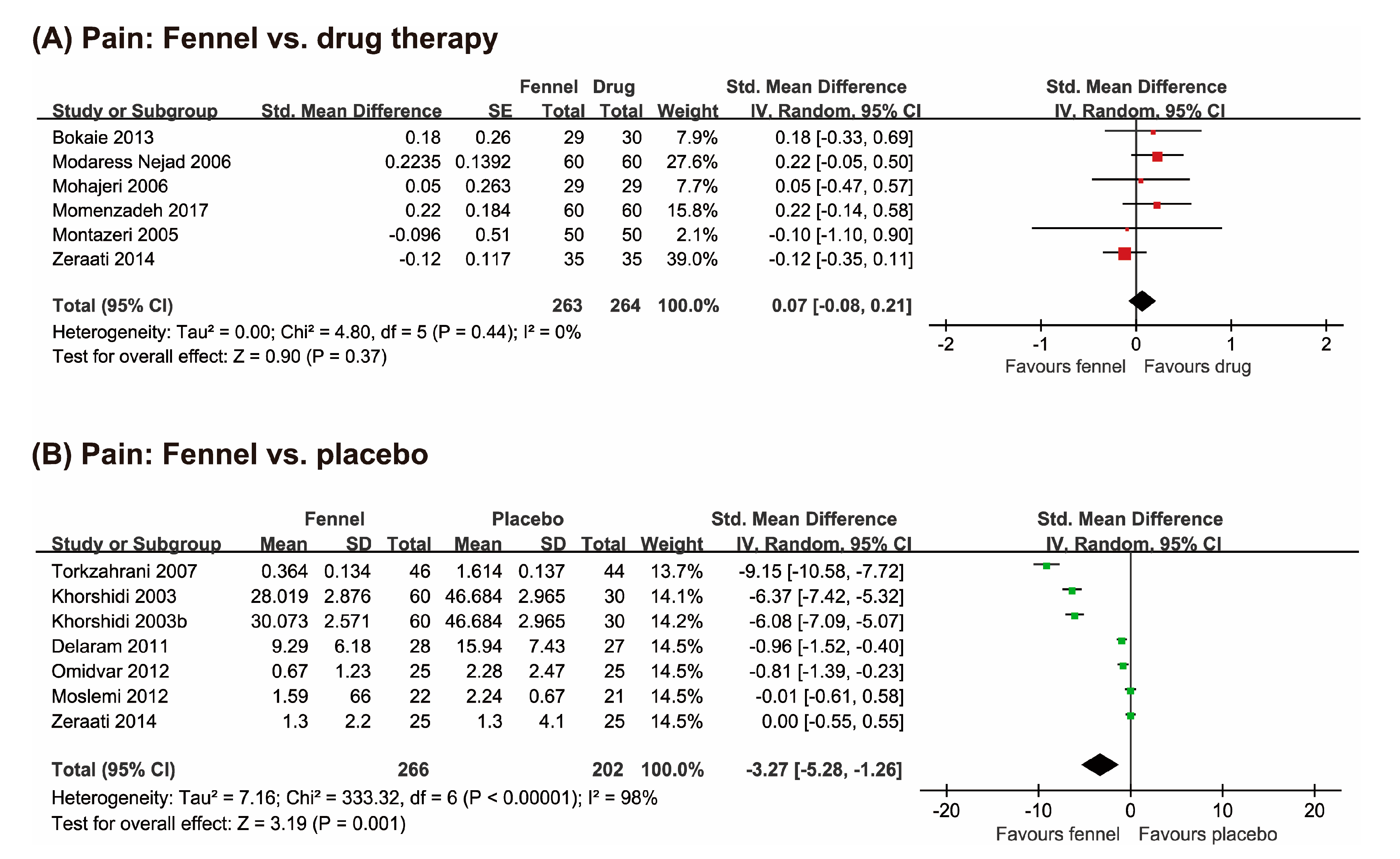
3.3.1. Pain
Fennel vs. Drug Therapies
Fennel vs. Placebo
3.3.2. Adverse Events (AEs)
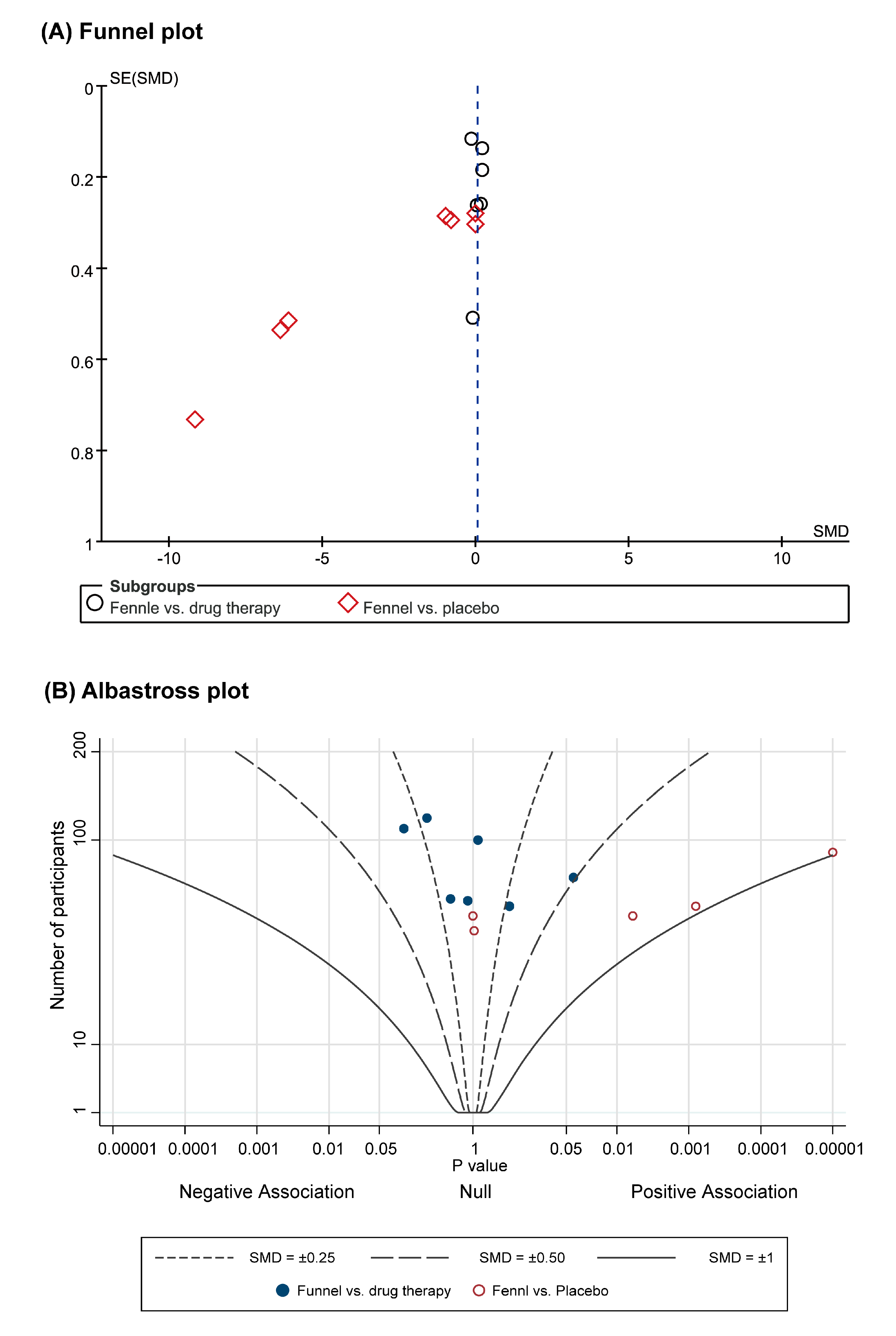
3.3.3. Publication Bias and Albatross Plot
4. Discussion
4.1. Summary of the Main Results
4.2. Overall Completeness and Applicability of the Evidence
4.3. Certainty of the Evidence
4.4. Potential Biases in the Review Process
4.5. Agreements and Disagreements with Other Studies or Reviews
4.6. Implication for Clinical Practice
4.7. Implications for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Meniratta, V. Primary and secondary dysmenorrhea, premenstrual syndrome, and premenstrual dysphoric disorder. In Comprehensive Gynecology, 7th ed.; Lobo, R.A., Gershenson, D.M., Lentz, G.M., Valea, F.A., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 815–828. [Google Scholar]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Al-Jefout, M.; Seham, A.F.; Jameel, H.; Randa, A.Q.; Oday, A.M.; Luscombe, G. Dysmenorrhea: Prevalence and impact on quality of life among young adult Jordanian females. J. Pediatr. Adolesc. Gynecol. 2015, 28, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, G.; Özdemir, F.; Pasinlioglu, T. Dysmenorrhea prevalence among adolescents in eastern Turkey: Its effects on school performance and relationships with family and friends. J. Pediatr. Adolesc. Gynecol. 2010, 23, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; Rangel-Flores, E.; Carrillo-Alarcón, L.C.; Veras-Godoy, H.A. Prevalence and impact of primary dysmenorrhea among Mexican high school students. Int. J. Gynecol. Obstet. 2009, 107, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Pitangui, A.C.R.; Gomes, M.R.D.A.; Lima, A.S.; Schwingel, P.A.; Albuquerque, A.P.D.S.; De Araújo, R.C. Menstruation disturbances: Prevalence, characteristics, and effects on the activities of daily living among adolescent girls from Brazil. J. Pediatr. Adolesc. Gynecol. 2013, 26, 148–152. [Google Scholar] [CrossRef]
- Wong, L.P.; Khoo, E.M. Dysmenorrhea in a multiethnic population of adolescent Asian girls. Int. J. Gynecol. Obstet. 2009, 108, 139–142. [Google Scholar] [CrossRef]
- Ju, H.; Jones, M.; Mishra, G.D. The prevalence and risk factors of dysmenorrhea. Epidemiol. Rev. 2013, 36, 104–113. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Proctor, M.; Farquhar, C.; Derks, R.S. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst. Rev. 2010, CD001751. [Google Scholar] [CrossRef]
- Proctor, M.; Farquhar, C. Diagnosis and management of dysmenorrhoea. Br. Med. J. 2006, 332. [Google Scholar] [CrossRef]
- Pattanittum, P.; Kunyanone, N.; Brown, J.; Sangkomkamhang, U.S.; Barnes, J.; Seyfoddin, V.; Marjoribanks, J. Dietary supplements for dysmenorrhoea. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Burnett, M.A.; Antao, V.; Black, A.; Feldman, K.; Grenville, A.; Lea, R.; Lefebvre, G.; Pinsonneault, O.; Robert, M. Prevalence of primary dysmenorrhea in Canada. J. Obstet. Gynaecol. Can. 2005, 27, 765–770. [Google Scholar] [CrossRef]
- Ghodsi, Z.; Asltoghiri, M. The effect of fennel on pain quality, symptoms, and menstrual duration in primary dysmenorrhea. J. Pediatr. Adolesc. Gynecol. 2014, 27, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Research Registry. Available online: https://www.researchregistry.com/ (accessed on 7 October 2020).
- Higgins, J.; Altman, D.; Sterne, J. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1: The Cochrane Collaboration; Higgins, J., Green, S., Eds.; Wiley-Blackwell: West Sussex, UK, 2011; pp. 187–241. [Google Scholar]
- Harrison, S. ALBATROSS: Stata Module to Create Albatross Plots; Statistical Software Components S458296; Boston College Department of Economics: Boston, MA, USA, 2017. [Google Scholar]
- Harrison, S.; Jones, H.E.; Martin, R.M.; Lewis, S.J.; Higgins, J.P. The albatross plot: A novel graphical tool for presenting results of diversely reported studies in a systematic review. Res. Synth. Methods 2017, 8, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bokaie, M.; Farajkhoda, T.; Enjezab, B.; Khoshbin, A.; Mojgan, K.Z. Oral fennel (Foeniculum vulgare) drop effect on primary dysmenorrhea: Effectiveness of herbal drug. Iran. J. Nurs. Midwifery Res. 2013, 18, 128–132. [Google Scholar] [PubMed]
- Modarressnejad, V.; Asadipour, M. Comparison of the effectiveness of fennel and mefenamic acid on pain intensity in dysmenorrhoea. East. Mediterr. Health J. 2006, 12, 423–427. [Google Scholar]
- Mohajeri, M.; Bakhshi, S.; Behzadian, N.; Tanha, A.; Nikzadeh, M. Comparison the effects of herb foeniculum vulgare (Fennel) with mefenamic acid in treatment of primary dysmenorrhea. Med. Sci. J. Islamic Azad Univ. 2006, 2, 122–126. [Google Scholar]
- Momenzadeh, F.; Toghiri, M.A.; Taghizadeh, M.; Mahlioji, M.; Rafiee, F. Comparison the effect of fennel and mefenamic acid on severity of primary dysmenorrhea. Iran. J. Obstet. Gynecol. Infertil. 2017, 20, 44–49. [Google Scholar] [CrossRef]
- Montazeri, S.; Manoochehri, E.; Abedi, P. Comparison the effect of Fennel and Ibuprofen on primary dysmenorrhea in highschool students. Med. Sci. J. Islamic Azad Univ. 2005, 1, 17–24. [Google Scholar]
- Nazarpour, S.; Azimi, H. Comparison of therapeutic effects of Fennelin and Mefenamic Acid on primary dysmenorrhea. Majallahi Dan. Ulumi Pizishkii Maz. 2007, 17, 54–61. [Google Scholar]
- Zeraati, F.; Shobeiri, F.; Nazari, M.; Araghchian, M.; Bekhradi, R. Comparative evaluation of the efficacy of herbal drugs (fennelin and vitagnus) and mefenamic acid in the treatment of primary dysmenorrhea. Iran. J. Nurs. Midwifery Res. 2014, 19, 581–584. [Google Scholar]
- Moslemi, L.; Bekhradi, R.; Moghaddam, G. Comparative effect of fennel extract on the intensity of primary dysmenorrhea. Afr. J. Microbiol. Res. 2012, 6, 1770–1773. [Google Scholar] [CrossRef]
- Omidvar, S.; Esmailzadeh, S.; Baradaran, M.; Basirat, Z. Effect of fennel on pain intensity in dysmenorrhoea: A placebo-controlled trial. Ayu 2012, 33, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Torkzahrani, S.; Akhavan-Amjadi, M.; Mojab, F.; Alavimajd, H. Clinical effects of foeniculum vulgare extract on primary dysmenorrhea. J. Reprod. Infertil. 2007, 8, 45–51. [Google Scholar]
- Khorshidi, N.; Ostad, S.; Mosaddegh, M.; Soodi, M. Clinical effects of fennel essential oil on primary dysmenorrhea. Iran. J. Pharm. Res. 2003, 2, 89–93. [Google Scholar]
- Delaram, M.F.N. The effect of fennel on the primary dysmenorrhea in students of Shahrekord University of Medical Sciences. Jundishapur J. Health Sci. 2011, 10, 81–88. [Google Scholar]
- Mahboubi, M. Foeniculum vulgareas valuable plant in management of women’s health. J. Menopausal Med. 2019, 25, 1–14. [Google Scholar] [CrossRef]
- Salehi, A.; Marzban, M.; Amini, F. Effect of foeniculum vulgare on primary dysmenorrhea: A systematic review and meta-analysis. Womens Health Bull. 2019, 6, e74240. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Q.; Wang, X. Efficacy of herbal medicine (cinnamon/fennel/ginger) for primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Sachse, C.; Uehleke, B. Systematischer review zur wirksamkeit von fenchel bei primärer dysmenorrhoe. Z. Phytother. 2015, 36, 150–156. [Google Scholar] [CrossRef]
- Sharghi, M.; Mansurkhani, S.M.; Ashtary-Larky, D.; Kooti, W.; Niksefat, M.; Firoozbakht, M.; Behzadifar, M.; Azami, M.; Servatyari, K.; Jouybari, L. An update and systematic review on the treatment of primary dysmenorrhea. JBRA Assist. Reprod. 2019, 23, 51–57. [Google Scholar] [CrossRef]
- Abdollahi, N.G.; Mirghafourvand, M.; Mollazadeh, S. The effects of fennel on menstrual bleeding: A systematic review and meta-analysis. J. Complement. Integr. Med. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, H.W.; Khalil, M.K.M.; Lim, H.S. Aromatherapy for Managing Pain in Primary Dysmenorrhea: A systematic review of randomized placebo-controlled trials. J. Clin. Med. 2018, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Lee, M.K.; Min, E.; Kim, M.E.; Fike, G.C.; Hur, M.H. Effects of aromatherapy on dysmenorrhea: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2018, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
| First Author (Year) [Ref] | Sample Size/Analyzed Age (Year) Severity | Intervention | Control | Treatment Duration | Main Outcome | Result | Design AEs Registration No. |
|---|---|---|---|---|---|---|---|
| Bokaie (2013) [18] | 60/59 8–25 years moderate to severe | (A) Fennel (oil, oral, 2%, 25 drops/6 h, from beginning of pain or 1st menstrual day, n = 29), plus (B) | (B) Mefenamic acid (250 mg, n = 30) | 1 month | Pain (VAS) | MD 0.40 [−0.74, 1.54], NS | Parallel Not assessed IRCT 201107096826N2 |
| Modaressnejad (2006) [19] | 120/110 13–18 n.r. | (A) Fennel (oil, oral, 30 drops/6 h, n = 55) | (B) Mefenamic acid (250 mg/6 h, n = 55) | 2 consecutive months (first three days of menstruation) | Pain (AMVMS) | RR 1.10 [0.89, 1.36], NS | Parallel Not assessed NA |
| Mohajeri (2006) [20] | 58/58 18–28 n.r. | (A) Fennel (oil, oral, 25 drops, 3 times day, n = 29) | (B) Mefenamic acid (500 mg loading dose then 250 mg, 3 times daily, n = 29) | 6 consecutive months | Pain (VAS) | RR 0.96 [0.79, 1.16], NS | Parallel Not assessed NA |
| Momenzadeh (2017) [21] | 120/120 18–23 moderate to severe | (A) Fennel (oil, oral, capsule, 30 mg, n = 60) | (B) Mefenamic acid (250 mg capsule, n = 60) | 2 consecutive months (3 days before onset of menstruation up to first three days) | Pain (AMVMS) | MD 0.13 [−0.08, 0.34], NS | Parallel No AEs reported NA |
| Montazeri (2005) [22] | 120/100 15–19 moderate to severe pain | (A) Fennel (oil, oral, 2%, 20–30 drop/4–6 h, n = 50) | (B) Ibuprofen (400 mg, per 4–6 h, n = 50) | 3 consecutive months (first month with no intervention followed by 2 months of intervention) | Pain (AMVMS) | RR 0.94 [0.71, 1.25], NS | Parallel Not assessed NA |
| Nazarpour (2007) [23] | 120/104 17–25 moderate to severe | (A) Fennel (oil, oral, 2%, 20–30 drops/4 to 8 h, n = 36) | (B) Mefenamic acid (250 mg/6 h, n = 36) (C) Placebo (same shape and same order, n = 32) | 2 consecutive months | Pain (VAS) | A vs. B: MD −0.58 [NA], p < 0.05 A vs. C: MD −0.09 [NA], NS | Parallel Not assessed NA |
| Zeraati (2014) [24] | 105/105 18–25 mild to moderate | (A) Fennel (oil, oral, 30 drops/4 h, n = 25) | (B) Mefenamic acid (capsules 250 mg/4 h, n = 30) (C) Placebo(30 drops/4 h, n = 25) * (D) Vitagnus (40 drops daily in the morning, n = 25) | 3 consecutive months (1 day before the start of the cycle until the third day for three cycles: one without any drugs and then two cycles with them) | Pain (VAS) | A. vs. B: MD −0.38 [−1.93, 1.17], NS A. vs. C: MD 0.00 [−1.82, 1.82], NS | Parallel Not assessed NA |
| Moslemi (2012) [25] | 65/63 n.r.Moderate to severe | (A) Fennel (extract, capsule, 46 mg, n = 22) | (B) Placebo (n = 21) * (C) Vt E (n = 20) | 2 consecutive months (every 6 h for 3 days for two consecutive cycles) | Pain (AMVMS) | MD −0.65 [−1.02, −0.28], p < 0.001 | Parallel Not assessed IRCT201106046705N1 |
| Omidvar (2012) [26] | 50/50 15–24 Moderate to severe | (A) Fennel (extract, capsules, 30 mg, n = 25) | (B) Placebo (capsules, wheat flour, n = 25) | 2 consecutive months (four times daily for 3 days for two consecutive cycles) | Pain (VAS) | MD −1.61 [−2.69, −0.53], p < 0.01 | Parallel Not assessed NA |
| Torkzahrani (2007) [27] | 130/90 17–30 moderate to severe | (A) Fennel (46 mg/capsule, 5 capsules daily, n = 46) | (B) Placebo (same shape and same order, n = 44) | 2 consecutive months (from the 1st day of menstrual bleeding, capsules were administered only for first three days) | Pain (AMVMS) | MD −1.25 [−1.31,−1.19], p < 0.001 | Parallel Not assessed NA |
| Khorshidi (2003) [28] | 60/55 17–25 mild to moderate | (A) Fennel (oil, oral, 1%, n = 18) (B) Fennel (oil, oral 2%, n = 19) | (C) Placebo (n.r., n = 16) | 1 time, administrated as soon as pain felt | Pain (Likert scale) | A vs. C: MD −18.66 [−20.48, −16.85], p < 0.001 B vs. C: MD −16.61 [−18.42, −14.80], p < 0.001 | Cross-over AE reported (no details) NA |
| Delaram (2011) [29] | 60/55 8–25 Severe | (A) Fennel (oil, oral, 30 drops/8 h, n = 28) | (B) Placebo (same shape and same order, n = 27) | 2 consecutive months (3 days before onset of menstrual bleeding to three first days of bleeding) | Pain (VAS) | MD −6.65 [−10.27, −3.03], p < 0.001 | Parallel AE reported Nausea and vomiting (A:1; B:1) NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.W.; Ang, L.; Lee, M.S.; Alimoradi, Z.; Kim, E. Fennel for Reducing Pain in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 3438. https://doi.org/10.3390/nu12113438
Lee HW, Ang L, Lee MS, Alimoradi Z, Kim E. Fennel for Reducing Pain in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2020; 12(11):3438. https://doi.org/10.3390/nu12113438
Chicago/Turabian StyleLee, Hye Won, Lin Ang, Myeong Soo Lee, Zainab Alimoradi, and Eunseop Kim. 2020. "Fennel for Reducing Pain in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 12, no. 11: 3438. https://doi.org/10.3390/nu12113438
APA StyleLee, H. W., Ang, L., Lee, M. S., Alimoradi, Z., & Kim, E. (2020). Fennel for Reducing Pain in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 12(11), 3438. https://doi.org/10.3390/nu12113438







