Assessment of Dietary Sodium Intake Using the Scored Salt Questionnaire in Autosomal Dominant Polycystic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
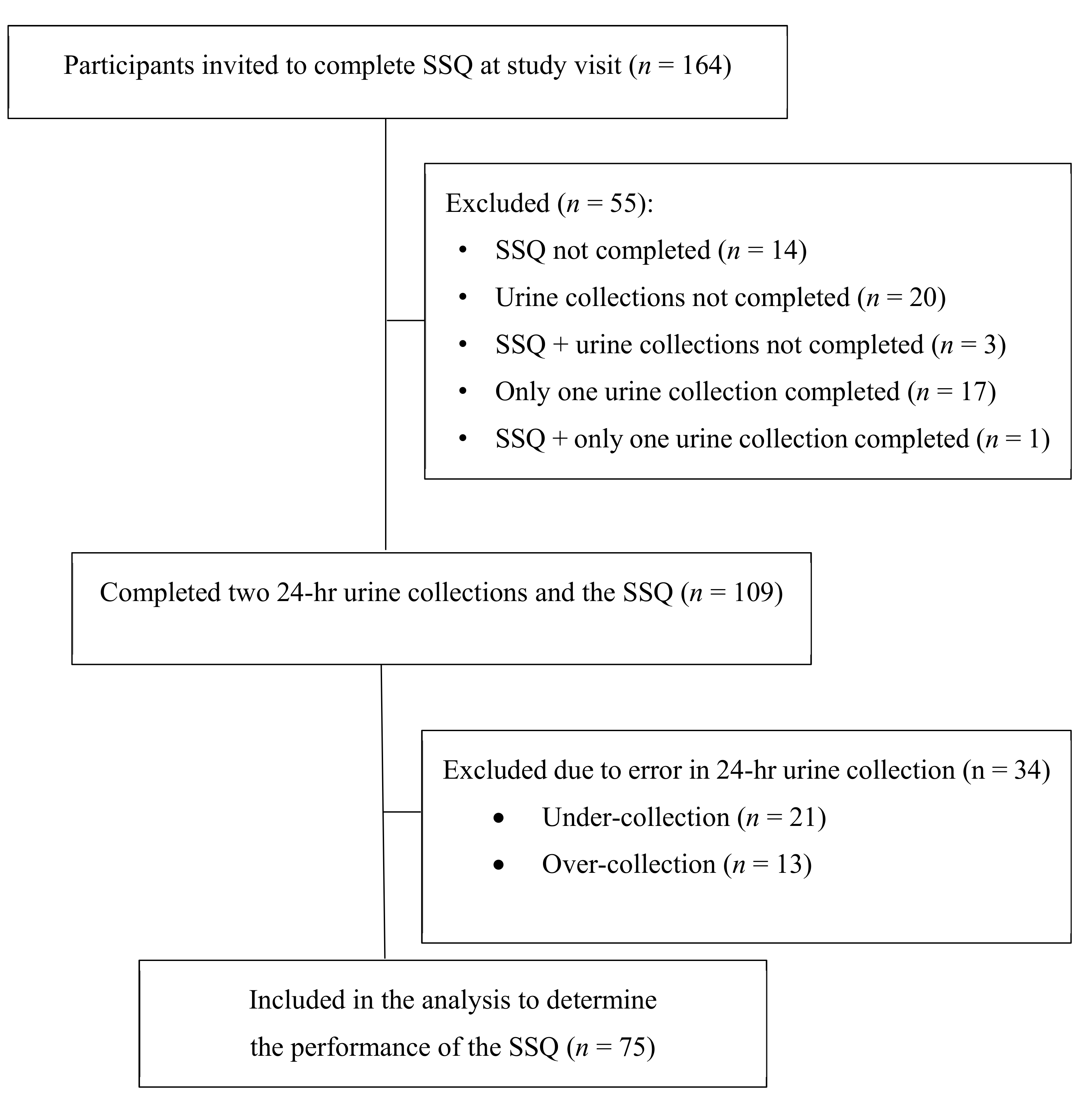
2.2. Study Design
2.3. Measurement of Urinary Sodium Excretion
2.4. Scored Salt Questionnaire
2.5. Statistical Analysis
3. Results
3.1. Characteristics of High and Low Sodium Intake Groups
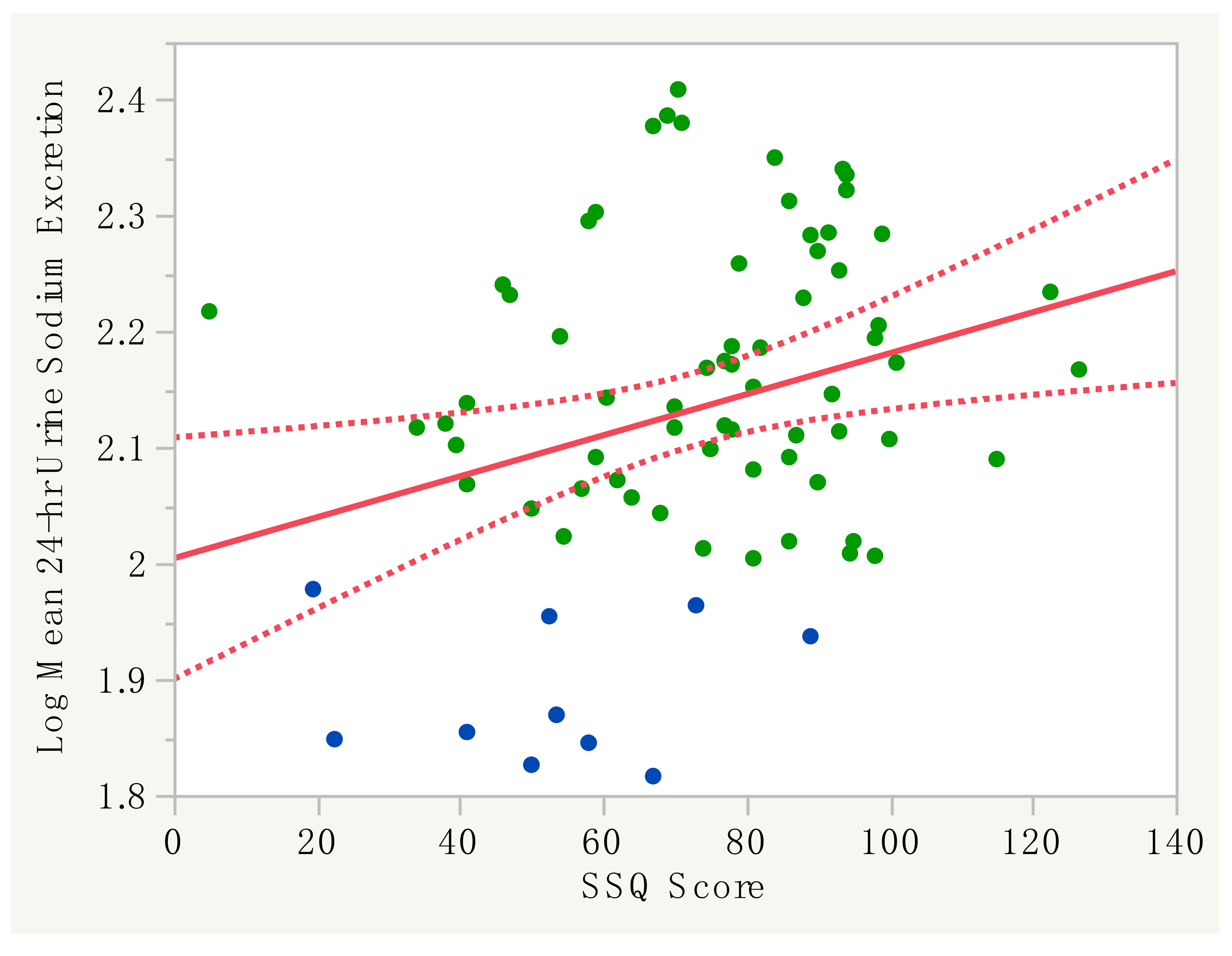
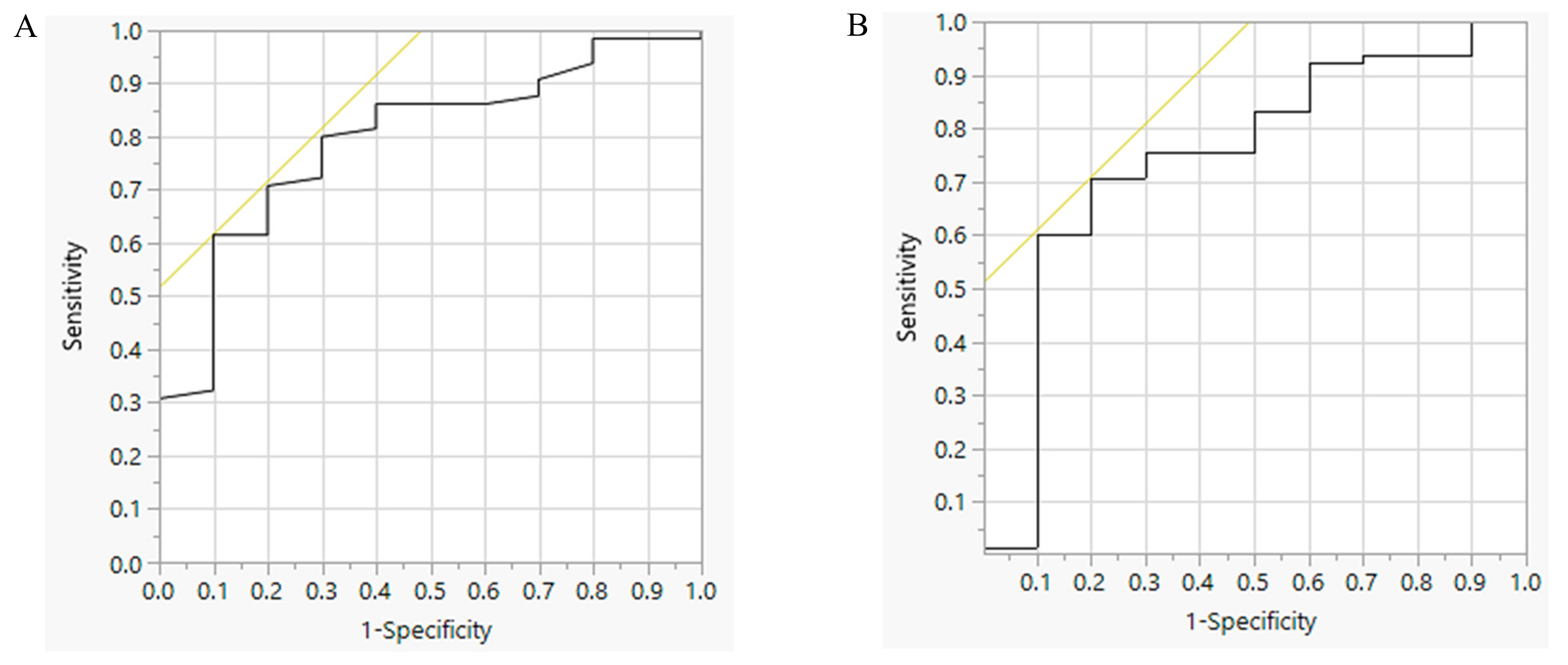
3.2. Performance of the SSQ with 24-H Urine Sodium Excretion
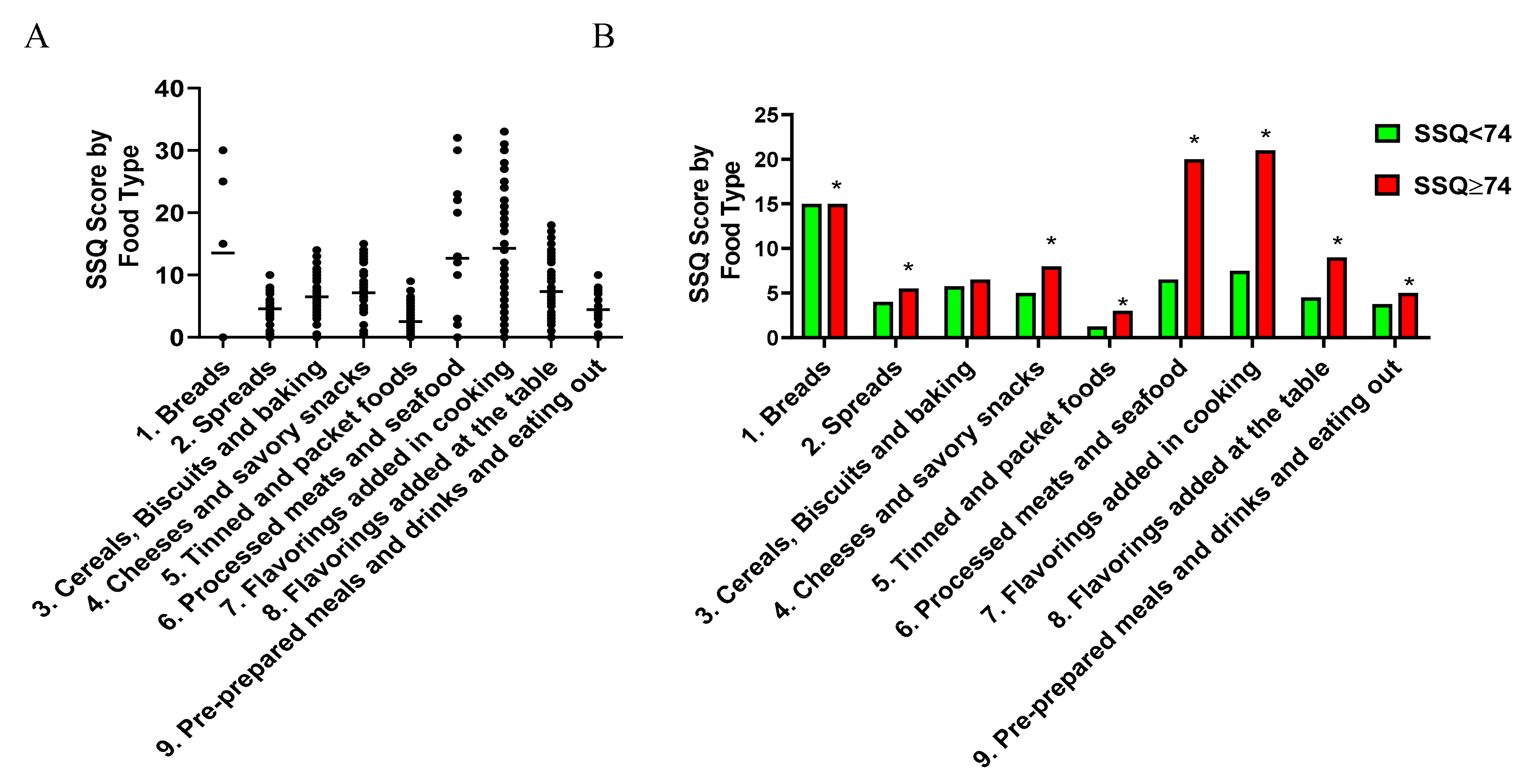
3.3. Characteristics of the Sodium-Rich Foods Types Identified by the SSQ
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gall, E.C.L.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef]
- Grantham, J.J. Rationale for early treatment of polycystic kidney disease. Pediatr. Nephrol. 2015, 30, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Ecder, T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr. Hypertens. Rev. 2013, 9, 2–11. [Google Scholar] [CrossRef]
- Carriazo, S.; Perez-Gomez, M.V.; Cordido, A.; Garcia-Gonzalez, M.A.; Sanz, A.B.; Ortiz, A.; Sanchez-Nino, M.D. Dietary care for ADPKD patients: Current status and future directions. Nutrients 2019, 11, 1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, V.E.; Abebe, K.Z.; Schrier, R.W.; Perrone, R.D.; Chapman, A.B.; Yu, A.S.; Braun, W.E.; Steinman, T.I.; Brosnahan, G.; Hogan, M.C.; et al. Dietary salt restriction is beneficial to the management of autosomal dominant polycystic kidney disease. Kidney Int. 2017, 91, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, V.E.; Grantham, J.J.; Chapman, A.B.; Mrug, M.; Bae, K.T.; King, B.F., Jr.; Wetzel, L.H.; Martin, D.; Lockhart, M.E.; Bennett, W.M.; et al. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., III; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; de Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; de Nicola, L.; Conte, G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.L.; Rangan, G.K.; Lopez-Vargas, P.; Tong, A. KHA-CARI autosomal dominant polycystic kidney disease guideline: Diet and lifestyle management. Semin. Nephrol. 2015, 6, 572. [Google Scholar] [CrossRef]
- Gimpel, C.; Bergmann, C.; Bockenhauer, D.; Breysem, L.; Cadnapaphornchai, M.A.; Cetiner, M.; Dudley, J.; Emma, F.; Konrad, M.; Harris, T.; et al. International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat. Rev. Nephrol. 2019, 15, 713–726. [Google Scholar] [CrossRef] [Green Version]
- Ars, E.; Bernis, C.; Fraga, G.; Martinez, V.; Martins, J.; Ortiz, A.; Rodriguez-Perez, J.C.; Sans, L.; Torra, R. Spanish working group on inherited kidney D. Spanish guidelines for the management of autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2014, 29, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soroka, S.; Alam, A.; Bevilacqua, M.; Girard, L.P.; Komenda, P.; Loertscher, R.; McFarlane, P.; Pandeya, S.; Tam, P.; Bichet, D.G. Updated Canadian Expert Consensus on Assessing Risk of Disease Progression and Pharmacological Management of Autosomal Dominant Polycystic Kidney Disease. Can. J. Kidney Health Dis. 2018, 5. [Google Scholar] [CrossRef]
- Tong, A.; Tunnicliffe, D.J.; Lopez-Vargas, P.; Mallett, A.; Patel, C.; Savige, J.; Campbell, K.; Patel, M.; Tchan, M.C.; Alexander, S.I.; et al. Identifying and integrating consumer perspectives in clinical practice guidelines on autosomal-dominant polycystic kidney disease. Nephrology 2016, 21, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.T.A.; Tran, D.; Pace, W.D.; Dickinson, M.; Vassalotti, J.; Carroll, J.; Withiam-Leitch, M.; Yang, M.; Satchidanand, N.; Staton, E.; et al. Extracting deep phenotypes for chronic kidney disease using electronic health records. EGEMS 2017, 5, 9. [Google Scholar] [CrossRef]
- Taylor, J.M.; Ptomey, L.; Hamilton-Reeves, J.M.; Sullivan, D.K.; Creed, C.; Carlson, S.E.; Wesson, D.E.; Grantham, J.J.; Gibson, C.A. Experiences and perspectives of polycystic kidney disease patients following a diet of reduced osmoles, protein, and acid precursors supplemented with water: A qualitative study. PLoS ONE 2016, 11, e0161043. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Sykes, L.; Paul, C.; Dary, O.; Legetic, B.; Campbell, N.R.; Cappuccio, F.P.; Sub-Group for R, Surveillance of the P-WHOREGfCDPTP-wDSR. Systematic review of studies comparing 24-h and spot urine collections for estimating population salt intake. Rev. Panam. Salud. Publica. 2012, 32, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Graffe, C.C.; Bech, J.N.; Lauridsen, T.G.; Pedersen, E.B. Urinary excretion of AQP2 and ENaC in autosomal dominant polycystic kidney disease during basal conditions and after a hypertonic saline infusion. Am. J. Physiol. Renal. Physiol. 2012, 302, 917–927. [Google Scholar] [CrossRef]
- Jensen, J.M.; Mose, F.H.; Kulik, A.E.; Bech, J.N.; Fenton, R.A.; Pedersen, E.B. Abnormal urinary excretion of NKCC2 and AQP2 in response to hypertonic saline in chronic kidney disease: An intervention study in patients with chronic kidney disease and healthy controls. BMC Nephrol. 2014, 15. [Google Scholar] [CrossRef] [Green Version]
- Campbell, N.R.C.; He, F.J.; Tan, M.; Cappuccio, F.P.; Neal, B.; Woodward, M.; Cogswell, M.E.; McLean, R.; Arcand, J.; MacGregor, G.; et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-h, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J. Clin. Hypertens. 2019, 21, 700–709. [Google Scholar] [CrossRef] [Green Version]
- Ross, L.; Chong, S.H.; Mason, B.; Healy, H. Development and evaluation of a scored sodium questionnaire-screening form for kidney disease patients. J. Ren. Nutr. 2016, 26, 159–167. [Google Scholar] [CrossRef]
- Tsuchihashi, T. Practical and personal education of dietary therapy in hypertensive patients. Hypertens. Res. 2020, 43, 6–12. [Google Scholar] [CrossRef]
- Mason, B.; Ross, L.; Gill, E.; Healy, H.; Juffs, P.; Kark, A. Development and validation of a dietary screening tool for high sodium consumption in Australian renal patients. J. Ren. Nutr. 2014, 24, 123–134. [Google Scholar] [CrossRef]
- Wong, A.T.Y.; Mannix, C.; Grantham, J.J.; Allman-Farinelli, M.; Badve, S.V.; Boudville, N.; Byth, K.; Chan, J.; Coulshed, S.; Edwards, M.E.; et al. Randomised controlled trial to determine the efficacy and safety of prescribed water intake to prevent kidney failure due to autosomal dominant polycystic kidney disease (PREVENT-ADPKD). BMJ Open 2018, 8, e018794. [Google Scholar] [CrossRef]
- Knuiman, J.T.; Hautvast, J.G.; van der Heyden, L.; Geboers, J.; Joossens, J.V.; Tornqvist, H.; Isaksson, B.; Pietinen, P.; Tuomilehto, J.; Poulsen, L.; et al. A multi-centre study on completeness of urine collection in 11 European centres. I. Some problems with the use of creatinine and 4-aminobenzoic acid as markers of the completeness of collection. Hum. Nutr. Clin. Nutr. 1986, 40, 229–237. [Google Scholar] [PubMed]
- John, K.A.; Cogswell, M.E.; Campbell, N.R.; Nowson, C.A.; Legetic, B.; Hennis, A.J.; Patel, S.M. Accuracy and usefulness of select methods for assessing complete collection of 24-h urine: A systematic review. J. Clin. Hypertens. 2016, 18, 456–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K.; Watanabe, T.; Kohri, T.; Yamasaki, M.; Watanabe, R.; Baba, K.; Shibata, K.; et al. Sensitivity and specificity of published strategies using urinary creatinine to identify incomplete 24-h urine collection. Nutrition 2008, 24, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jedrusik, P.; Symonides, B.; Gaciong, Z. Performance of 24-h urinary creatinine excretion-estimating equations in relation to measured 24-h urinary creatinine excretion in hospitalized hypertensive patients. Sci. Rep. 2019, 9, 3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbembo, A.L.; Walser, M. Nutritional assessment. In Nutritional Assessment, the John Hopkins Handbook; Imbembo, A.L., Walser, M., Margolis, S., Eds.; W.B. Saunders: Philadelphia, PA, USA, 1984; pp. 9–30. [Google Scholar]
- McLean, R.M.; Farmer, V.L.; Nettleton, A.; Cameron, C.M.; Cook, N.R.; Campbell, N.R.C.; Consortium, T. Assessment of dietary sodium intake using a food frequency questionnaire and 24-h urinary sodium excretion: A systematic literature review. J. Clin. Hypertens. 2017, 19, 1214–1230. [Google Scholar] [CrossRef] [Green Version]
- Grimes, C.A.; Kelley, S.J.; Stanley, S.; Bolam, B.; Webster, J.; Khokhar, D.; Nowson, C.A. Knowledge, attitudes and behaviours related to dietary salt among adults in the state of Victoria, Australia 2015. BMC Public Health 2017, 17, 532. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020. [Google Scholar] [CrossRef]
- McMahon, E.J.; Campbell, K.L.; Mudge, D.W.; Bauer, J.D. Achieving salt restriction in chronic kidney disease. Int. J. Nephrol. 2012, 720429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutlugun, A.A.; Arici, M.; Yildirim, T.; Turgut, D.; Yilmaz, R.; Altindal, M.; Altun, B.; Erdem, Y.; Yasavul, U.; Turgan, C. Daily sodium intake in chronic kidney disease patients during nephrology clinic follow-up: An observational study with 24-h urine sodium measurement. Nephron. Clin. Pract. 2011, 118, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Leonardis, D.; Mallamaci, F.; Enia, G.; Postorino, M.; Tripepi, G.; Zoccali, C.; Investigators, M.S. The MAURO study: Baseline characteristics and compliance with guidelines targets. J. Nephrol. 2012, 25, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Kimura, Y.; Kitaoka, C.; Sakata, T.; Abe, I.; Kawano, Y. Blood pressure control status and relationship between salt intake and lifestyle including diet in hypertensive outpatients treated at a general hospital. Clin. Exp. Hypertens. 2017, 39, 29–33. [Google Scholar] [CrossRef]
- Wright, H.H.; Kickbusch, H.; Swanepoel, E.; Gray, N. Factors influencing low sodium intake in people with non-dialysis dependent chronic kidney disease. J. Ren. Care 2020, 46, 95–105. [Google Scholar] [CrossRef]
- Gabow, P.A.; Ikle, D.W.; Holmes, J.H. Polycystic kidney disease: Prospective analysis of nonazotemic patients and family members. Ann. Intern. Med. 1984, 101, 238–247. [Google Scholar] [CrossRef]
- Christopher-Stine, L.; Petri, M.; Astor, B.C.; Fine, D. Urine protein-to-creatinine ratio is a reliable measure of proteinuria in lupus nephritis. J. Rheumatol. 2004, 31, 1557–1559. [Google Scholar]
- Jakobsen, J.; Ovesen, L.; Fagt, S.; Pedersen, A.N. Para-aminobenzoic acid used as a marker for completeness of 24 hour urine: Assessment of control limits for a specific HPLC method. Eur. J. Clin. Nutr. 1997, 51, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Amro, O.W.; Paulus, J.K.; Noubary, F.; Perrone, R.D. Low-osmolar diet and adjusted water intake for vasopressin reduction in autosomal dominant polycystic kidney disease: A pilot randomized controlled trial. Am. J. Kidney Dis. 2016, 68, 882–891. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.L.; Dixon, Z.; Himburg, S.; Huffman, F. Asian students change their eating patterns after living in the United States. J. Am. Diet. Assoc. 1999, 99, 54–57. [Google Scholar] [CrossRef]
| Parameter | Total n = 75 | 24-h Urine Na < 100 mmol/day n = 10 | 24-h Urine Na ≥ 100 mmol/day n = 65 | p |
|---|---|---|---|---|
| Age (years) | 44.6 ± 11.5 | 40.2 ± 12.5 | 45.3 ± 11.3 | 0.19 |
| Gender (F:M) (%) | 53:47 | 90:10 | 48:52 * | <0.01 |
| Height (m) | 1.71 ± 0.09 | 1.65 ± 0.08 | 1.72 ± 0.09 * | 0.04 |
| Weight (kg) | 76.1 ± 15.1 | 64.1 ± 11.9 | 78.0 ± 14.8 * | <0.01 |
| BMI (kg/m2) | 25.9 ± 3.8 | 23.5 ± 4.4 | 26.3 ± 3.6 * | 0.03 |
| Systolic BP (mm Hg) | 133 ± 14 | 125 ± 10 | 134 ± 14 * | 0.04 |
| Diastolic BP (mm Hg) | 85 ± 11 | 79 ± 8 | 86 ± 11 | 0.08 |
| Serum Cr (mmol/L) | 91 (70–123) | 77 (62–104) | 94 (70–126) | 0.12 |
| eGFR (mL/min/1.73m2) | 75 (53–105) | 85 (56–112) | 73 (52–104) | 0.39 |
| 24-h urine Na (mmol/day) | 132 (112–172) | 73 (69–91) | 142 (123–180) * | <0.01 |
| Parameter | TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | DOR | A | AUC | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSQ | 40 | 1 | 9 | 25 | 61.5% | 90.0% | 97.6% | 26.5% | 6.15 | 0.43 | 14.40 | 65.3 | 0.79 | <0.01 |
| BMI | 46 | 2 | 8 | 19 | 70.8% | 80.0% | 95.8% | 29.6% | 3.54 | 0.37 | 9.68 | 72.0 | 0.75 | 0.02 |
| SSQ in BMI < 25 Group (n = 29) | 12 | 1 | 7 | 9 | 57.0% | 87.5% | 92.3% | 43.8% | 2.87 | 0.20 | 14.52 | 65.5 | 0.72 | 0.13 |
| SSQ in BMI ≥ 25 Group (n = 46) | 44 | 0 | 2 | 0 | 100% | 100% | 100% | 100% | - | 0.00 | - | 100% | 1.00 | < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, A.T.Y.; Munt, A.; Allman-Farinelli, M.; Badve, S.V.; Boudville, N.; Coolican, H.; Chandra, A.N.; Coulshed, S.; Fernando, M.; Grantham, J.; et al. Assessment of Dietary Sodium Intake Using the Scored Salt Questionnaire in Autosomal Dominant Polycystic Kidney Disease. Nutrients 2020, 12, 3376. https://doi.org/10.3390/nu12113376
Wong ATY, Munt A, Allman-Farinelli M, Badve SV, Boudville N, Coolican H, Chandra AN, Coulshed S, Fernando M, Grantham J, et al. Assessment of Dietary Sodium Intake Using the Scored Salt Questionnaire in Autosomal Dominant Polycystic Kidney Disease. Nutrients. 2020; 12(11):3376. https://doi.org/10.3390/nu12113376
Chicago/Turabian StyleWong, Annette T. Y., Alexandra Munt, Margaret Allman-Farinelli, Sunil V. Badve, Neil Boudville, Helen Coolican, Ashley N. Chandra, Susan Coulshed, Mangalee Fernando, Jared Grantham, and et al. 2020. "Assessment of Dietary Sodium Intake Using the Scored Salt Questionnaire in Autosomal Dominant Polycystic Kidney Disease" Nutrients 12, no. 11: 3376. https://doi.org/10.3390/nu12113376
APA StyleWong, A. T. Y., Munt, A., Allman-Farinelli, M., Badve, S. V., Boudville, N., Coolican, H., Chandra, A. N., Coulshed, S., Fernando, M., Grantham, J., Haloob, I., Harris, D. C. H., Hawley, C. M., Holt, J., Johnson, D. W., Kumar, K., Lee, V. W., Lonergan, M., Mai, J., ... Rangan, G. K. (2020). Assessment of Dietary Sodium Intake Using the Scored Salt Questionnaire in Autosomal Dominant Polycystic Kidney Disease. Nutrients, 12(11), 3376. https://doi.org/10.3390/nu12113376







