Nutrition Can Help DNA Repair in the Case of Aging
Abstract
1. Introduction
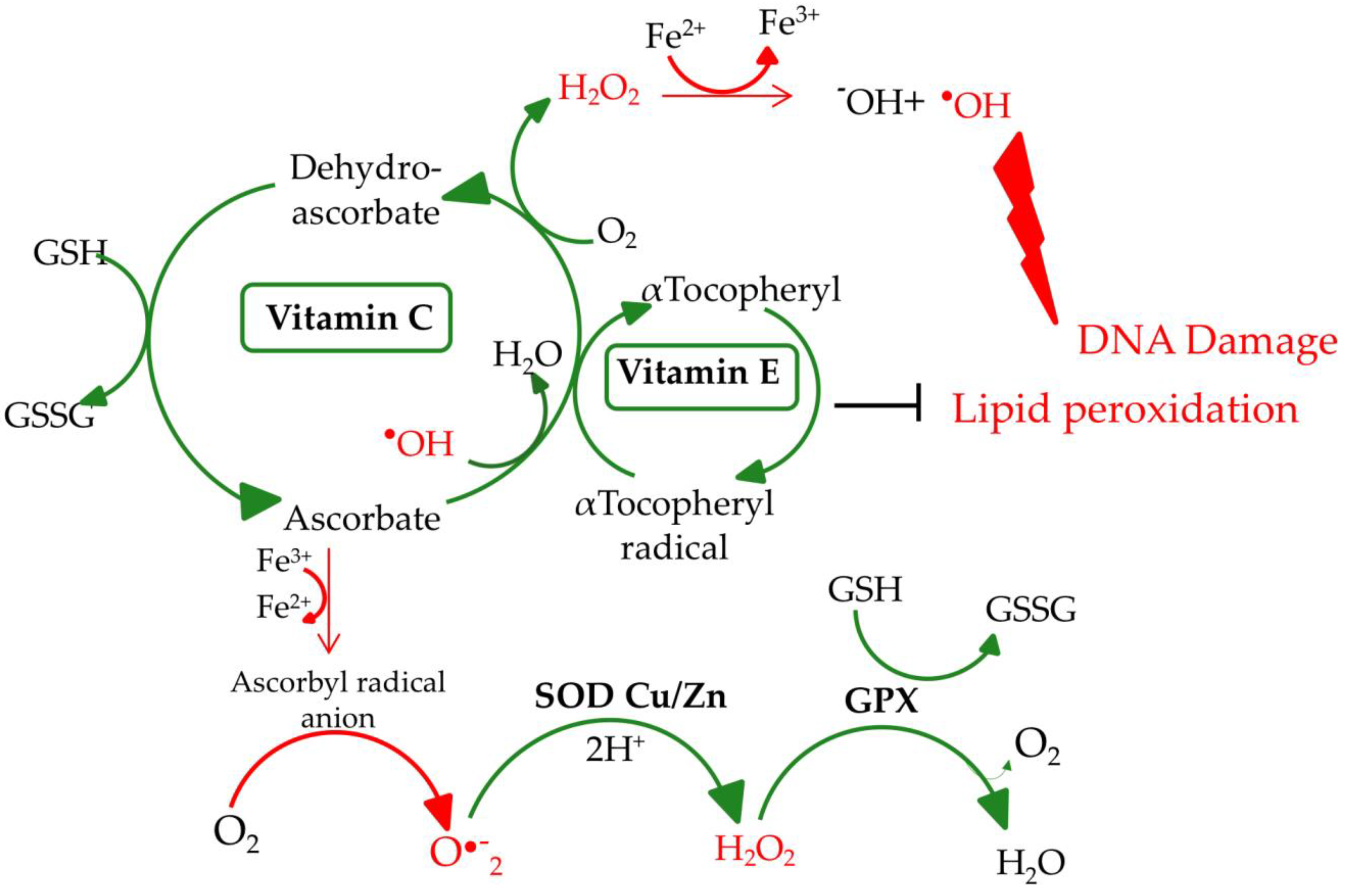
2. Biochemical Aspects of Selected Micronutrients
2.1. Selenium
2.2. Zinc
2.3. Vitamin E
2.4. Vitamin C
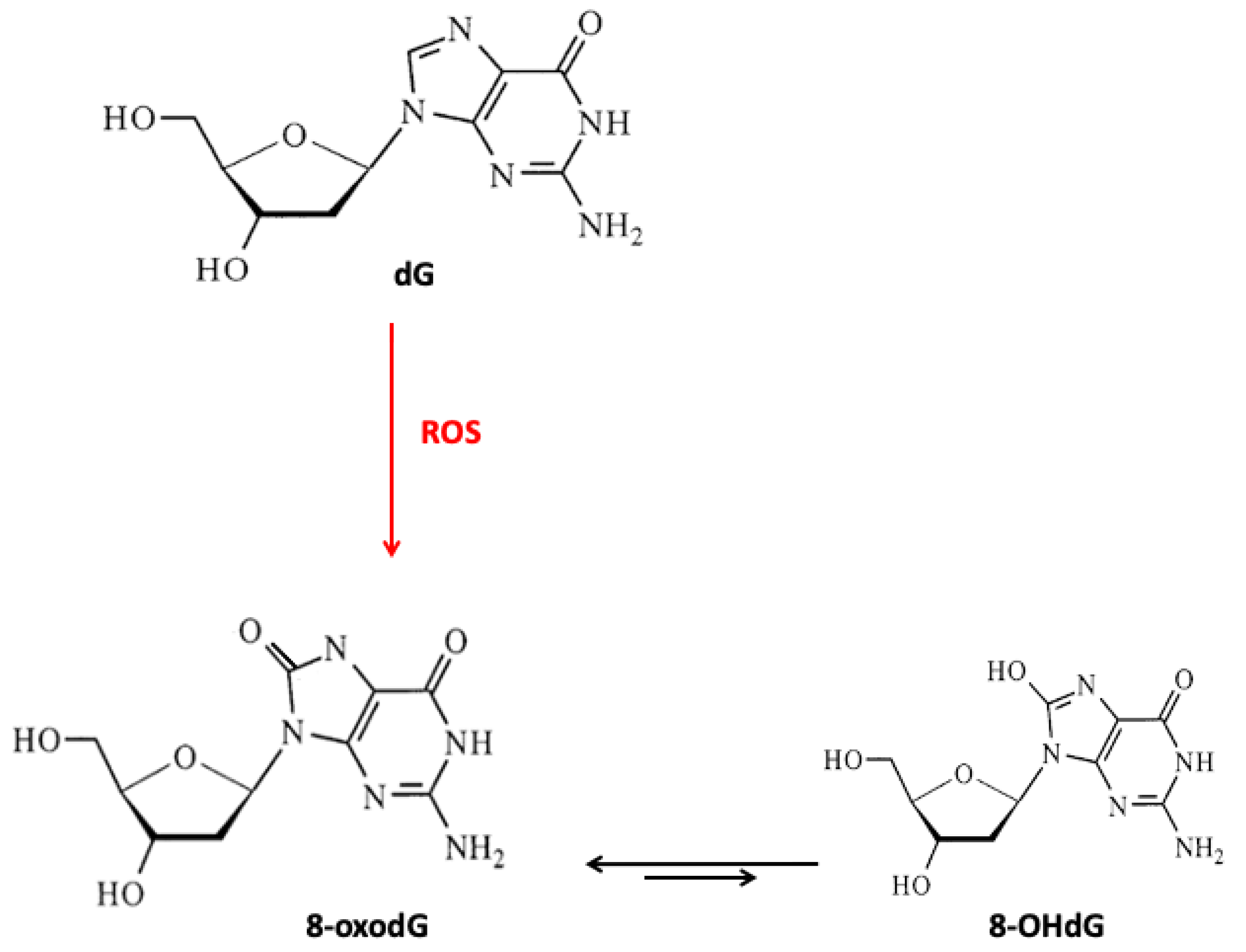
3. Micronutrients, DNA Damage, and Repair
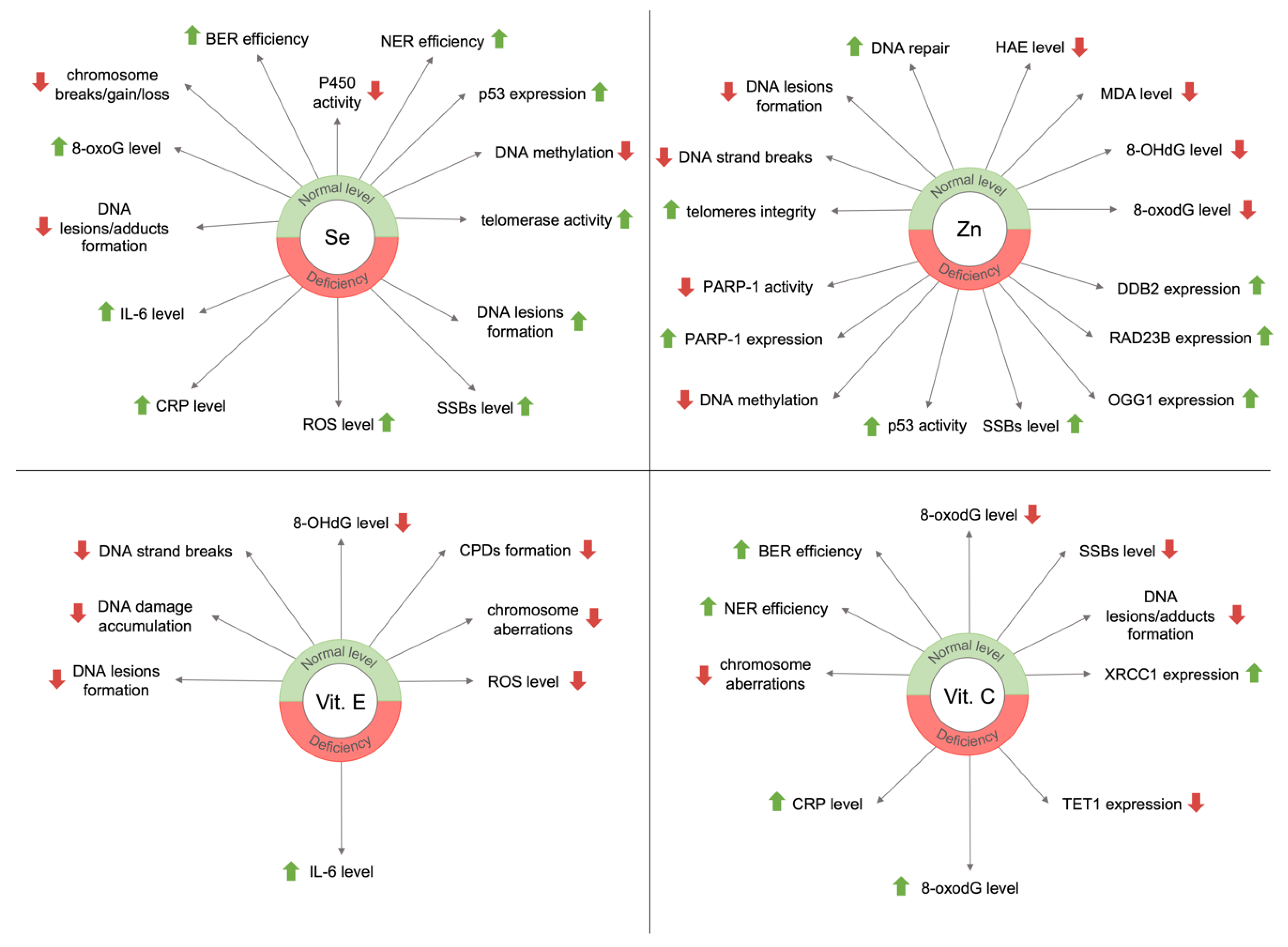
3.1. Selenium
3.2. Zinc
3.3. Vitamin E
3.4. Vitamin C
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutritio; World Health Organization, 2014; WHO Reference Number: WHO/NMH/NHD/14.1; Available online: https://apps.who.int/iris/bitstream/handle/10665/113048/WHO_NMH_NHD_14.1_eng.pdf?ua=1 (accessed on 30 October 2020).
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Tutusaus, M.P.; Martínez, S.F.; Roig, M.D.G.; García-Algar, O. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Poggiogalle, E.; Piredda, M.; Pinto, A.; Barbagallo, M.; Cucinotta, D.; Sergi, G. Anorexia and Eating Patterns in the Elderly. PLoS ONE 2013, 8, e63539. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Lattanzio, F.; Russo, A.; Tosato, M.; Barillaro, C.; Bernabei, R.; Onder, G. Effects of anorexia on mortality among older adults receiving home care: An observation study. J. Nutr. Health Aging 2012, 16, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wysokiński, A.; Sobów, T.; Kłoszewska, I.; Kostka, T. Mechanisms of the anorexia of aging—A review. AGE 2015, 37, 81. [Google Scholar] [CrossRef] [PubMed]
- Eshler, W.B. Interleukin 6: A cytokine for gerontologists. J. Am. Geriatr. Soc. 1993, 41, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Fagiolo, U.; Cossarizza, A.; Scala, E.; Fanales-Belasio, E.; Ortolani, C.; Cozzi, E. Increased cytokine production in mononuclear cells of healthy elderly people. Eur. J. Immunol. 1993, 23, 2375–2378. [Google Scholar] [CrossRef]
- Schuetz, P.; Bally, M.; Stanga, Z.; Keller, U. Loss of appetite in acutely ill medical inpatients: Physiological response or therapeutic target? An area of current uncertainty. Swiss Med. Wkly 2014, 144, w13957. [Google Scholar]
- Siepelmeyer, A.; Micka, A.; Simm, A.; Bernhardt, J. Nutritional Biomarkers of Aging. In Molecular Basis of Nutrition and Aging; Academic Press: Cambridge, MA, USA, 2016; pp. 109–120. [Google Scholar]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol 2018, 9, 477. [Google Scholar] [CrossRef]
- Karwowski, B.T. 5’8-cyklo-deoksyadenozyna. Podwójne uszkodzenie w obrębie pojedynczego nukleozydu/nukleotydu. Wiad Chem. 2010, 64, 1013–1048. [Google Scholar]
- Ba, X.; Boldogh, L. 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions. Redox Biol. 2018, 14, 669–678. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Philpott, M. Nutrition and Mutagenesis. Ann. Rev. Nutr. 2008, 28, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Winston, A.P. The clinical biochemistry of anorexia nervosa. Ann. Clin. Biochem. 2012, 49, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Arcelus, J. Mortality Rates in Patients with Anorexia Nervosa and Other Eating Disorders. Arch. Gen. Psychiatry 2011, 68, 724. [Google Scholar] [CrossRef]
- Klecha, B.; Bukowska, B. Selen w organizmie człowieka—charakterystyka pierwiastka i potencjalne zastosowanie terapeutyczne. Bromat. Chem. Toksykol. 2016, 4, 818–829. [Google Scholar]
- Carlson, B.A.; Yoo, M.H.; Shrimali, R.K.; Irons, R.; Gladyshev, V.N.; Hatfield, D.L.; Park, J.M. Role of selenium-containing proteins in T-cell and macrophage function. Proc. Nutr. Soc. 2010, 69, 300–310. [Google Scholar] [CrossRef]
- Wołonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postepy Hig. Med. Dosw. 2016, 70, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Hornick, J.; Istasse, L.; Dufrasne, I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Oliveras-López, M.-J.; Ruiz-Prieto, I.; Bolaños-Ríos, P.; De la Cerda, F.; Martín, F.; Jáuregui-Lobera, I. Antioxidant Activity and Nutritional Status in Anorexia Nervosa: Effects of Weight Recovery. Nutrients 2015, 7, 2193–2208. [Google Scholar]
- Abuja, P.M.; Albertini, R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin. Chim. Acta 2001, 306, 1–17. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Torres, S.; Guerra, M.P.; Lencastre, L.; Miller, K.; Vieira, F.M.; Roma-Torres, A.; Brandão, I.; Costa, P. Alexithymia in anorexia nervosa: The mediating role of depression. Psychiatry Res. 2015, 225, 99–107. [Google Scholar] [CrossRef]
- Hammad, G.; Legrain, Y.; Touat-Hamici, Z.; Duhieu, S.; Cornu, D.; Bulteau, A.-L.; Chavatte, L. Interplay between Selenium Levels and Replicative Senescence in WI-38 Human Fibroblasts: A Proteomic Approach. Antioxidants 2018, 7, 19. [Google Scholar] [CrossRef]
- Legrain, Y.; Touat-Hamici, Z.; Chavatte, L. Interplay between selenium levels, selenoprotein expression, and replicative senescence in wi-38 human fibroblasts. J. Biol. Chem. 2014, 289, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jing, H.; Zhang, J. Optimization of mycelia selenium polysaccharide extraction from Agrocybe cylindracea SL-02 and assessment of their antioxidant and anti-aging activities. PLoS ONE 2016, 11, e0160799. [Google Scholar]
- Yamashita, Y.; Yabu, T.; Yamashita, M. Discovery of the strong antioxidant selenoneine in tuna and selenium redox metabolism. World J. Biol. Chem. 2010, 1, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kłapcińska, B.; Poprzecki, S.; Danch, A.; Sobczak, A.; Kempa, K. Selenium Levels in Blood of Upper Silesian Population: Evidence of Suboptimal Selenium Status in a Significant Percentage of the Population. Biol. Trace Elem. Res. 2005, 108, 001–016. [Google Scholar] [CrossRef]
- Forte, G.; Deiana, M.; Pasella, S.; Baralla, A.; Occhineri, P.; Mura, I. Metals in plasma of nonagenarians and centenarians living in a key area of longevity. Exp. Gerontol. 2014, 60, 197–206. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; Lattanzio, F.; Bustacchini, S.; di Stefano, G.; Moresi, R. Selenium Concentrations and Mortality Among Community-Dwelling Older Adults: Results from ilSIRENTE Study. J. Nutr. Health Aging 2018, 22, 608–612. [Google Scholar] [CrossRef]
- Combs, G.F.; Scott, M.L. Nutritional Interrelationships of Vitamin E and Selenium. BioScience 1977, 27, 467–473. [Google Scholar] [CrossRef]
- MacFarquhar, J.K. Acute Selenium Toxicity Associated With a Dietary Supplement. Arch. Intern. Med. 2010, 170, 256. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical, endocrinological and biochemical effects of zinc deficiency. Clin. Endocrinol. Metab. 1985, 14, 567–589. [Google Scholar] [CrossRef]
- Prasad, A.S. Effects of Zinc Deficiency on Th1 and Th2 Cytokine Shifts. J. Infect. Dis. 2000, 182, S62–S68. [Google Scholar] [CrossRef]
- DePasquale-Jardieu, P.; Fraker, P.J. Interference in the Development of a Secondary Immune Response in Mice by Zinc Deprivation: Persistence of Effects. J. Nutr. 1984, 114, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Cassandri, M.; Smirnov, A.; Novelli, F. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; Kelley, A.M.; McClung, J.P. Metallothionein and Zinc Transporter Expression in Circulating Human Blood Cells as Biomarkers of Zinc Status: A Systematic Review. Adv Nutr. 2016, 7, 735–746. [Google Scholar] [CrossRef]
- Inoue, K.; Takano, H.; Shimada, A.; Satoh, M. Metallothionein as an Anti-Inflammatory Mediator. Mediat. Inflamm. 2009, 2009, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Metallothionein/disulfide interactions, oxidative stress, and the mobilization of cellular zinc. Neurochem. Int. 1995, 27, 111–117. [Google Scholar] [CrossRef]
- Yang, X.; Doser, T.A.; Fang, C.X.; Nunn, J.M.; Janardhanan, R.; Zhu, M. Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: Role of oxidative stress. FASEB J. 2006, 20, 1024–1026. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef]
- Izquierdoa, M.; Domíngueza, D.; Ignacio, J.; Salehab, J.R.; Hernández-Cruza, C.M.; Zamoranoa, M.J.; Hamre, K. Interaction between taurine, vitamin E and vitamin C in microdiets for gilthead seabream (Sparus aurata) larvae. Aquaculture 2019, 498, 246–253. [Google Scholar] [CrossRef]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Tokoferole i tokotrienole jako witamina E. Chemik 2014, 68, 585–591. [Google Scholar]
- Elkamil, A.; Johansen, K.K.; Aasly, J. Ataxia with Vitamin E Deficiency in Norway. J. Mov. Disord. 2015, 8, 33–36. [Google Scholar] [CrossRef]
- Moyano, D.; Sierra, C.; Brandi, N.; Artuch, R.; Mira, A.; García-Tornel, S.; Vilaseca, M. Antioxidant status in anorexia nervosa. Int. J. Eat. Disord. 1997, 25, 99–103. [Google Scholar] [CrossRef]
- Marcus, J.B. Nutritional and Physical Concerns in Aging. In Aging, Nutrition and Taste; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128135280. [Google Scholar]
- Skowrońska, A.; Sójta, K.; Strzelecki, D. Refeeding syndrome as treatment complication of anorexia nervosa. Psychiatr. Pol. 2019, 53, 1113–1123. [Google Scholar] [CrossRef]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Briançon, S. The SU.VI.MAX Study. Arch. Intern. Med. 2004, 164, 2335. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant Supplements for Preventing Gastrointestinal Cancers. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; Li, J.-Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.-Q. Nutrition Intervention Trials in Linxian, China: Supplementation with Specific Vitamin/Mineral Combinations, Cancer Incidence, and Disease-Specific Mortality in the General Population. JNCI 1993, 85, 1483–1491. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, 157–165. [Google Scholar]
- Bei, R. Effects of Vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Druga Twarz Tlenu. Wolne Rodniki w Przyrodzie; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006. [Google Scholar]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Lenton, K.J.; Therriault, H.; Cantin, A.M.; Fülöp, T.; Payette, H.; Wagner, J.R. Direct correlation of glutathione and ascorbate and their dependence on age and season in human lymphocytes. Am. J. Clin. Nutr. 2000, 71, 1194–1200. [Google Scholar] [CrossRef]
- Mezzetti, A.; Lapenna, D.; Romano, F.; Costantini, F.; Pierdomenico, S.D.; De Cesare, D. Systemic Oxidative Stress and Its Relationship with Age and Illness. J. Am. Geriatr. Soc. 1996, 44, 823–827. [Google Scholar] [CrossRef]
- Langlois, M.; Duprez, D.; Delanghe, J.; De Buyzere, M.; Clement, D.L. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation 2001, 103, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Date, C.; Kokubo, Y.; Yoshiike, N.; Matsumura, Y.; Tanaka, H. Serum Vitamin C Concentration Was Inversely Associated with Subsequent 20-Year Incidence of Stroke in a Japanese Rural Community: The Shibata Study. Stroke 2000, 31, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A. Vitamin C and cardiovascular disease: A review. J. Am. Coll. Nutr. 1992, 11, 107–125. [Google Scholar]
- Sesso, H.D.; Buring, J.E.; Christen, W.G. Vitamins E and C in the prevention of cardiovascular disease in men. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Boeing, H.; Stelmach-Mardas, M.; Gottschald, M.; Dietrich, S.; Hoffmann, G.; Chaimani, A. Dietary supplements and risk of cause specific death, cardiovascular disease, and cancer: A systematic review and meta-analysis of primary prevention trials. Adv. Nutr. 2017, 8, 27–39. [Google Scholar] [CrossRef]
- Ashor, A.W.; Siervo, M.; Mathers, J.C.; Vitamin, C. Antioxidant Status, and Cardiovascular Aging. In Molecular Basis of Nutrition and Aging; Academic Press: Cambridge, MA, USA, 2016; pp. 609–619. [Google Scholar]
- DiTroia, S.P.; Percharde, M.; Guerquin, M.-J.; Wall, E.; Collignon, E.; Ebata, K.T.; Mesh, K.; Mahesula, S.; Agathocleous, M.; Laird, D.J.; et al. Maternal vitamin C regulates reprogramming of DNA methylation and germline development. Nature 2015, 573, 271–275. [Google Scholar] [CrossRef]
- Ciesielski, P.; Jóźwiak, P.; Krześlak, A. Białka TET a modyfikacje epigenetyczne w nowotworach. Postepy Hig. Med. Dosw. 2015, 69, 1371–1383. [Google Scholar] [CrossRef]
- Fu, H.L.; Ma, Y.; Lu, L.G.; Hou, P.; Li, B.J.; Jin, W.L.; Cui, D.X. TET1 exerts its tumor suppressor function by interacting with p53-EZH2 pathway in gastric cancer. J. Biomed. Nanotechnol. 2014, 10, 1217–1230. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Podmore, I.D.; Herbert, K.E.; Mistry, N.; Mistry, P.; Lunec, J. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett. 1998, 439, 363–367. [Google Scholar] [CrossRef]
- Bevan, R.J.; Mistry, N.; Patel, P.R.; Halligan, E.P.; Dove, R.; Lunec, J. Can vitamin C induce nucleotide excision repair? Support from in vitro evidence. Br. J. Nutr. 2009, 103, 686–695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lalonde, M. A new perspective on the health of Canadians. AARN News Lett. 1976, 32, 1–5. [Google Scholar]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Geront. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.-M. ROS and Brain Diseases: The Good, the Bad, and the Ugly. Oxidative Med. Cell. Longev. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.; Ferreira, G.; Trevenzoli, I.; Oliveira, K.; de Melo Reis, R. Fatty Acids, Antioxidants and Physical Activity in Brain Aging. Nutrients 2017, 9, 1263. [Google Scholar] [CrossRef]
- Terman, A.; Gustafsson, B.; Brunk, U. Autophagy, organelles and aging. J. Pathol. 2007, 211, 134–143. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Dhillon, V.; Bull, C.; Fenech, M. Telomeres, Aging, and Nutrition. In Molecular Basis of Nutrition and Aging; Malavolta, M., Nocchegiani, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 129–140. [Google Scholar]
- Hewitt, G.; Jurk, D.; Marques, F.D.M.; Correia-Melo, C.; Hardy, T.; Gackowska, A. Telomeres are favored targets of a persistent DNA damage response in aging and stress-induced senescence. Nat. Comm. 2012, 3, 708. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.P.; Cortinhas, A.; Bento, T.; Leitão, J.C.; Collins, A.R.; Gaivã, I.; Mota, M.P. Aging and DNA damage in humans: A meta-analysis study. Aging 2014, 6, 432–439. [Google Scholar] [CrossRef]
- Wolf, F.; Fasanella, S.; Tedesco, B.; Cavallini, G.; Donati, A.; Bergamini, E.; Cittadini, A. Peripheral lymphocyte 8-OHdG levels correlate with age-associated increase of tissue oxidative DNA damage in Sprague- Dawley rats. Protective effects of caloric restriction. Exp. Geront. 2005, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Olinski, R.; Siomek, A.; Rozalski, R.; Gackowski, D.; Foksinski, M.; Guz, J.; Dziaman, T.; Szpila, A.; Tudek, B. Oxidative damage to DNA and antioxidant status in aging and age-related diseases. ABP 2007, 54, 11–26. [Google Scholar] [CrossRef]
- Zenger, F.; Russmann, S.; Junker, E.; Wuthrich, C.; Bui, M.H.; Lauterburg, B.H. Decreased glutathione in patients with anorexia nervosa. Risk factor for toxic liver injury? Eur. J. Clin. Nutr. 2004, 58, 238–243. [Google Scholar] [CrossRef]
- Ames, B.N. Micronutrient Deficiencies: A Major Cause of DNA Damage. Ann. N. Y. Acad. Sci. 1999, 889, 87–106. [Google Scholar] [CrossRef]
- Fenech, M. Nutritional treatment of genome instability: A paradigm shift in disease prevention and in the setting of recommended dietary allowances. Nutr. Res. Rev. 2003, 16, 109–122. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Karunasinghe, N.; Zhu, S.; Wang, A.H. Selenium and its’ role in the maintenance of genomic stability. Mutat. Res. Fund. Mol. M 2012, 733, 100–110. [Google Scholar] [CrossRef]
- Rao, L.; Puschner, B.; Prolla, T.A. Gene Expression Profiling of Low Selenium Status in the Mouse Intestine: Transcriptional Activation of Genes Linked to DNA Damage, Cell Cycle Control and Oxidative Stress. J. Nutr. 2001, 131, 3175–3181. [Google Scholar] [CrossRef]
- Karunasinghe, N.; Ryan, J.; Tuckey, J.; Masters, J. DNA Stability and Serum Selenium Levels in a High-Risk Group for Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 391–397. [Google Scholar]
- Dziaman, T.; Huzarski, T.; Gackowski, D.; Rozalski, R.; Siomek, A.; Szpila, A.; Olinski, R. Selenium Supplementation Reduced Oxidative DNA Damage in Adnexectomized BRCA1 Mutations Carriers. Cancer Epidem Biomar. 2009, 18, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Zhao, R.; Song, G.; Zhong, W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogen 2008, 29, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Lancia, J.; Mathur, A.; Smith, M. Selenium Protection from DNA Damage Involves a Ref1/p53/Brca1 Protein Complex. Anticanc. Res. 2006, 26, 899–904. [Google Scholar]
- De Rosa, V.; Lu, P.E.; Forestier, A.; Favier, A.; Hincal, F.; Diamond, A.M.; Douki, T.; Rachidi, W. Low doses of selenium specifically stimulate the repair of oxidative DNA damage in LNCaP prostate cancer cells. Free Radic. Res. 2016, 46, 105–116. [Google Scholar] [CrossRef]
- Zachara, B.A.; Gromadzinska, J.; Palus, J.; Zbrog, Z.; Swiech, R.; Twardowska, E.; Wasowicz, W. The Effect of Selenium Supplementation in the Prevention of DNA Damage in White Blood Cells of Hemodialyzed Patients: A Pilot Study. Biol. Trace Elem. Res. 2010, 142, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C.; Beal, D.; Blouin, E.; Leccia, M.T.; Roussel, A.M.; Rachidi, W. Age-Dependent Protective Effect of Selenium against UVA Irradiation in Primary Human Keratinocytes and the Associated DNA Repair Signature. Oxid. Med. Cell Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Shimada, T.; El-Bayoumy, K.; Upadhyaya, P.; Sutter, T.R.; Guengerich, F.P.; Yamazaki, H. Inhibition of human cytochrome P450-catalyzed oxidations of xenobiotics and procarcinogens) by synthetic organoselenium compounds. Cancer Res. 1997, 57, 4757–4764. [Google Scholar]
- Cong, Y.; Chi, Q.; Teng, X.; Li, S. The Protection of Selenium Against Cadmium-Induced Mitochondrial Damage via the Cytochrome P450 in the Livers of Chicken. Biol. Trace Elem. Res. 2019, 190, 484–492. [Google Scholar] [CrossRef]
- Sun, L.-H.; Zhang, N.-Y.; Zhu, M.-K.; Zhao, L.; Zhou, J.-C.; Qi, D.-S. Prevention of Aflatoxin B1 Hepatoxicity by Dietary Selenium Is Associated with Inhibition of Cytochrome P450 Isozymes and Up-Regulation of 6 Selenoprotein Genes in Chick Liver. J. Nutr. 2015, 146, 655–661. [Google Scholar] [CrossRef]
- Willett, W.C.; Polk, B.F.; Morris, J.S.; Stampfer, M.J.; Pressel, S.; Rosner, B. Prediagnostic serum selenium and risk of cancer. Lancet 1983, 2, 130–134. [Google Scholar] [CrossRef]
- Virtamo, J.; Valkeila, E.; Alfthan, G.; Punsar, S.; Huttunen, J.K.; Karvonen, M.J. Serum selenium and risk of cancer. A prospective follow-up of nine years. Cancer 1987, 60, 145–148. [Google Scholar] [CrossRef]
- Pagmantidis, V.; Méplan, C.; van Schothorst, E.M.; Keijer, J.; Hesketh, J.E. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am. J. Clin. Nutr. 2008, 87, 181–189. [Google Scholar] [CrossRef]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the Interaction Between Selenium and Zinc on DNA Repair in Association with Cancer Prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.; Dedoussis, G.; Jajte, J.; Malavolta, M.; Mocchegiani, E.; Bürkle, A. Effect of zinc on cellular poly(ADP-ribosyl)ation capacity. Exp. Gerontol. 2008, 43, 409–414. [Google Scholar] [CrossRef][Green Version]
- Ronson, G.E.; Piberger, A.L.; Higgs, M.R.; Olsen, A.L.; Stewart, G.S.; McHugh, P.J.; Lakin, N.D. PARP1 and PARP2 stabilize replication forks at base excision repair intermediates through Fbh1-dependent Rad51 regulation. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pines, A.; Vrouwe, M.G.; Marteijn, J.A.; Typas, D.; Luijsterburg, M.S.; Cansoy, M.; Mullenders, L. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol. 2012, 199, 235–249. [Google Scholar] [CrossRef]
- Mangerich, A.; Burkle, A. Pleiotropic cellular functions of PARP1 in longevity and aging: Genome maintenance meets inflammation. Oxidative Med. Cell Longev. 2012, 2012, 321653. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Thomas, P.; Zalewski, P.; Graham, R.D.; Fenech, M. The effect of zinc sulphate and zinc carnosine on genome stability and cytotoxicity in the WIL2-NS human lymphoblastoid cell line. Mut. Res. Gen. Toxicol. Environm. Mutagen. 2011, 720, 22–33. [Google Scholar] [CrossRef]
- Ho, E.; Courtemanche, C.; Ames, B.N. Zinc Deficiency Induces Oxidative DNA Damage and Increases P53 Expression in Human Lung Fibroblasts. J. Nutr. 2003, 133, 2543–2548. [Google Scholar] [CrossRef]
- Song, Y.; Chung, C.S.; Bruno, R.S.; Traber, M.G.; Brown, K.H.; King, K.J. Dietary zinc restriction and repletion affects DNA integrity in healthy men. Am. J. Clin. Nutrit. 2009, 90, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Thomas, P.; Zalewski, P.; Fenech, M. Zinc supplementation influences genomic stability biomarkers, antioxidant activity, and zinc transporter genes in an elderly Australian population with low zinc status. Mol. Nutr. Food Res. 2015, 59, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Thomas, P.; Zalewski, P.; Fenech, M. The role of zinc in genomic stability. Mutat. Res. Fund. Mol. M 2012, 733, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Letavayová, L.; Vlčková, V.; Brozmanová, J. Selenium: From cancer prevention to DNA damage. Toxicology 2006, 227, 1–14. [Google Scholar] [CrossRef]
- Maret, W. The redox biology of redox-inert zinc ions. Free Rad. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef]
- el-Nahas, S.M.; Mattar, F.E.; Mohamed, A.A. Radioprotective effect of Vitamins C and E. Mutat. Res. 1993, 301, 143–147. [Google Scholar] [CrossRef]
- Konopacka, M.; Widel, M.; Rzeszowska-Wolny, J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. Mut. Res. Gen. Toxicol. Environm. Mutagen. 1998, 417, 85–94. [Google Scholar] [CrossRef]
- Delinasios, G.J.; Karbaschi, M.; Cooke, M.S.; Young, A.R. Vitamin E inhibits the UVAI induction of “light” and “dark” cyclobutane pyrimidine dimers, and oxidatively generated DNA damage, in keratinocytes. Sci. Rep. 2018, 8, 1–12. [Google Scholar]
- Fantappiè, O.; Lodovici, M.; Fabrizio, P.; Marchetti, S.; Fabbroni, V.; Solazzo, M. Vitamin E Protects DNA from Oxidative Damage in Human Hepatocellular Carcinoma Cell Lines. Free Rad. Res. 2004, 38, 751–759. [Google Scholar] [CrossRef]
- La Fata, G.; van Vliet, N.; Barnhoorn, S.; Brandt, R.M.C. Vitamin E supplementation reduces cellular loss in the brain of a premature aging mouse model. J. Prev. Alz. Dis. 2017, 4, 226–235. [Google Scholar]
- Jenkinson, A.M.; Collins, A.R.; Duthie, S.J.; Wahle, K.W.J.; Duthie, G.G. The effect of increased intakes of polyunsaturated fatty acids and vitamin E on DNA damage in human lymphocytes. FASEB J. 1999, 13, 2138–2142. [Google Scholar] [CrossRef] [PubMed]
- Goon, J.A.; Nor Azman, N.H.E.; Abdul Ghani, S.M.; Hamid, Z.; Wan Ngah, W.Z. Comparing palm oil tocotrienol rich fraction with α-tocopherol supplementation on oxidative stress in healthy older adults. Clin. Nutr. ESPEN 2017, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhori, M.; Singh, K.; Marar, T.; Chilakapati, M.K. Exploring the effect of vitamin E in cancer chemotherapy-A biochemical and biophysical insight. J. Biophoton. 2018, 11, e201800104. [Google Scholar] [CrossRef]
- Fenech, M.; Dreosti, I.; Aitken, C. Vitamin-E supplements and their effect on vitamin-E status in blood and genetic damage rate in peripheral blood lymphocytes. Carcinogenesis 1997, 18, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Astley, S.; Langrish-Smith, A.; Southon, S.; Sampson, M. Vitamin E supplementation and oxidative damage to DNA and plasma LDL in type 1 diabetes. Diabetes Care 1999, 22, 1626–1631. [Google Scholar] [CrossRef]
- Bianchini, F. Oxidative DNA damage in human lymphocytes: Correlations with plasma levels of alpha-tocopherol and carotenoids. Carcinogenesis 2000, 21, 321–324. [Google Scholar] [CrossRef][Green Version]
- Capuron, L.; Moranis, A.; Combe, N.; Cousson-Gélie, F.; Fuchs, D.; De Smedt-Peyrusse, V. Vitamin E status and quality of life in the elderly: Influence of inflammatory processes. Br. J. Nutr. 2009, 102, 1390. [Google Scholar] [CrossRef]
- Sram, R.J.; Binkova, B.; Rossner, P. Vitamin C for DNA damage prevention. Mut. Res. Gen. Toxicol. Environm. Mutagen. 2012, 733, 39–49. [Google Scholar] [CrossRef]
- Fraga, C.G.; Motchnik, P.A.; Shigenaga, M.K.; Helbock, H.J.; Jacob, R.A.; Ames, B.N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. PNAS 1991, 88, 11003–11006. [Google Scholar] [CrossRef]
- Simon, A.S.; Chithra, V.; Vijayan, A.; Dinesh, R.D.; Vijayakumar, T. Altered DNA repair, oxidative stress and antioxidant status in coronary artery disease. J. Biosci. 2013, 38, 385–389. [Google Scholar] [CrossRef]
- Rossner, P.; Uhlirova, K.; Beskid, O.; Rossnerova, A.; Svecova, V.; Sram, R.J. Expression of XRCC5 in peripheral blood lymphocytes is upregulated in subjects from a heavily polluted region in the Czech Republic. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2011, 713, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Slyskova, J.; Lorenzo, Y.; Karlsen, A.; Carlsen, M.H.; Novosadova, V.; Blomhoff, R.; Collins, A.R. Both genetic and dietary factors underlie individual differences in DNA damage levels and DNA repair capacity. DNA Repair 2014, 16, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, S.; Loft, S.; Riso, P.; Porrini, M.; Risom, L.; Poulsen, H.E.; Møller, P. DNA repair phenotype and dietary antioxidant supplementation. Br. J. Nutr. 2008, 99, 1018–1024. [Google Scholar] [CrossRef]
- Walston, J.; Xue, Q.; Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M. Serum Antioxidants, Inflammation, and Total Mortality in Older Women. Am. J. Epidem. 2005, 163, 18–26. [Google Scholar] [CrossRef]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef]
- Von Zglinicki, T.; Pilger, R.; Sitte, N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Rad. Biol. Med. 2000, 28, 64–74. [Google Scholar] [CrossRef]
- Crogan, N.L. Nutritional Problems Affecting Older Adults. Nurs. Clin. N Am. 2017, 52, 433–445. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak-Barańska, J.; Boguszewska, K.; Karwowski, B.T. Nutrition Can Help DNA Repair in the Case of Aging. Nutrients 2020, 12, 3364. https://doi.org/10.3390/nu12113364
Kaźmierczak-Barańska J, Boguszewska K, Karwowski BT. Nutrition Can Help DNA Repair in the Case of Aging. Nutrients. 2020; 12(11):3364. https://doi.org/10.3390/nu12113364
Chicago/Turabian StyleKaźmierczak-Barańska, Julia, Karolina Boguszewska, and Boleslaw T. Karwowski. 2020. "Nutrition Can Help DNA Repair in the Case of Aging" Nutrients 12, no. 11: 3364. https://doi.org/10.3390/nu12113364
APA StyleKaźmierczak-Barańska, J., Boguszewska, K., & Karwowski, B. T. (2020). Nutrition Can Help DNA Repair in the Case of Aging. Nutrients, 12(11), 3364. https://doi.org/10.3390/nu12113364





