Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications
Abstract
1. Introduction
1.1. Historical Context
1.2. Composition
| Compound | Molecular Group | % in Dry Venom | Biological Activity | Type of Study | Reference |
|---|---|---|---|---|---|
| Melittin and isoforms | Peptide | 50–60 | -Antibacterial -Anti-inflammatory -Anti-arrhythmic -Anti-secretory -Anti-cancer -Anti-arthritis -Anti-atherosclerotic -Antiviral -Pro-apoptotic -Anti-apoptotic -Analgesic -Anti-fibrotic -Anti-diabetic -Haemolysis -Antiangiogenic -Wound healing -Antifungal -Anti-nociceptive | -In vitro -In vivo -In vivo -In vivo -In vitro -In vitro -In vivo -In vitro -In vitro -In vitro -In vivo -In vitro -In vivo -In vitro -In vitro -In vitro -In vitro -In vivo | [18] [19] [20] [19] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [27] |
| Apamin | Peptide | 1–3 | -Antifungal -Anti-fibrotic -Anti-cancer -Anti-inflammatory -Anti-atherosclerotic -Antibacterial -Neuroprotection | -In vitro -In vivo -In vitro -In vivo -In vivo -In vitro -In vivo | [34] [35] [36] [37] [38] [39] [40] |
| MCD | Peptide | 1–3 | -Anti-inflammatory -Anti-allergic | -In vivo -In vitro | [14] [41] |
| Secapin | Peptide | 1–2 | -Antifungal -Antibacterial -Anti-elastolytic -Anti-fibrinolytic | -In vitro -In vitro -In vitro -In vitro | [15] [15] [15] [15] |
| Adolapin | Peptide | 0.1–0.8 | -Anti-inflammatory -Anti-nociceptive -Antipyretic | -In vitro -In vitro | [16] [16] [42] |
| PLA2 (Api m 1) | Enzyme | 10–12 | -Antibacterial -Anti-arthritis -Antiparasitic -Neuroprotective -Anti-cancer -Antiviral -Inflammatory -Antigenicity -Allergenicity -Nociceptive -Neuronal activation -Nerve regeneration | -In vitro -In vivo -In vitro -In vivo -In vitro -In vitro -In vivo -In vivo -In vivo -In vivo -In vivo -In vivo | [39] [43] [44,45] [46] [47] [48] [49] [50] [51] [50] [52] [53] |
| Hyaluronidase (Api m 2) | Enzyme | 1.5–2 | -Spreading factor by hyaluronic acid activation -Allergenicity | - | [54] [55] |
| Compound | Molecular Group | % in Dry |
|---|---|---|
| Aminobutyric acid | Biologic amine | 1 |
| Dopamine | Biologic amine | 0.1–1 |
| Histamine | Biologic amine | 0.5–2 |
| Noradrealine | Biologic amine | 0.1–0.5 |
| Acid phosphatase | Enzyme | 1 |
| Phosphatase | Enzyme | 1 |
| PLA B | Enzyme | 1 |
| α-Glucosidase | Enzyme | 0.6 |
| Acetylcholine | Ester | – |
| Icarapin | Glycoprotein | – |
| P, Ca and Mg | Minerals | 3–4 |
| Apamin | Peptide | 1–3 |
| Cardiopep | Peptide | 0.7 |
| Cecropin A | Peptide | – |
| Melittin-F | Peptide | 0.01 |
| Melittin-S | Peptide | 1–2 |
| Minimine | Peptide | 2–3 |
| Pamine | Peptide | 2 |
| Procamine A,B | Peptide | 1–2 |
| Secapin | Peptide | 1–2 |
| Tertiapin | Peptide | 0.1 |
| Phospholipids | Phospholipids | 1–3 |
| α-D-Glucosidase | Protein | <1 |
| Dipeptidyl peptidase IV | Protein | <1 |
| Lysiphosppholipase | Protein | <1 |
| MRJP8 | Protein | – |
| MRJP9 | Protein | – |
| Phospholipase B | Protein | <1 |
| Vitellogenin | Protein | – |
| Glucose, fructose | Sugars | 2–4 |
| Complex ethers | Volatile compounds | 4–8 |
1.3. Biological Activities of Bee Venom
2. Bee Venom Composition
2.1. Peptides
2.1.1. Melittin
2.1.2. Apamin
2.1.3. Mast Cell-Degranulating Peptide
2.1.4. Minor Peptides
2.2. Enzymes
2.2.1. Phospholipase A2
2.2.2. Hyaluronidase
2.2.3. Other Enzymes
3. Biological Activities of Bee Venom
3.1. Antioxidant Activity
3.2. Antimicrobial Activity
3.3. Anti-Inflammatory Activity
3.4. Neuroprotective Effects
3.5. Antitumor Effects
4. Clinical Applications
5. Future Perspectives and New Approaches
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Generic | |
| BV | Bee Venom |
| DW | Dry Weight |
| RA | Rheumatoid Arthritis |
| PD | Parkinson’s Disease |
| MS | Multiple Sclerosis |
| LF | Liver Fibrosis |
| CNS | Central Nervous System |
| AD | Alzheimer’s Disease |
| Components | |
| Aa | Amino acid |
| PLA2 | Phospholipase A2 |
| MCD | Mast Cell-Degranulating Peptide |
| NO | Nitric Oxide |
| bPLA2 | PLA2 Derived From Bee |
| MDA | Malondialdehyde |
| ASA | Acetylsalicylic Acid |
| LPS | Lipopolysaccharides |
| DPIV | Dipeptidyl Peptidase IV |
| GST | Glutathione S-Transferase |
| GSH | Glutathione Content |
| GPx | Glutathione Peroxidase |
| SOD | Superoxide Dismutase |
| Kir | Inward Rectifier Potassium |
| Cellular components | |
| TLR | Toll-Like Receptors |
| DR | Death Receptor |
| CD | Cluster of Differentiation |
| TNF | Tumor Necrosis Factors |
| TAM | Tumor-Associated Macrophages |
| IL | Interleukin |
| NF-kB | Inhibitory effect of the nuclear factor kappa-B |
| Antioxidant activity | |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TAS | Plasma Total Antioxidant Status |
| TOS | Total Oxidant Status |
| OSI | Oxidative Stress Index |
| FRAP | Ferric Reducing/Antioxidant Power |
| ABTS | 2, 20-Azinobis 3-Ethylbenzothiazoline-6-Sulfonic Acid |
| TAC | Total Antioxidant Capacity |
References
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee Venom Composition: From Chemistry to Biological Activity. Stud. Nat. Prod. Chem. 2018, 60, 459–484. [Google Scholar] [CrossRef]
- Hellner, M.; Winter, D.; Von Georgi, R.; Münstedt, K. Apitherapy: Usage and experience in German beekeepers. Evidence-Based Complement. Altern. Med. 2008, 5, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.R.; Lin, L.T.; Xiao, L.Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, M.; Sherman, R.A.; Gileva, O.S.; Kim, C.M.H. Biotherapy—History, Principles and Practice; Springer: Dordrecht, The Netherlands, 2013; ISBN 9789400765849. [Google Scholar]
- Benton, A.W.; Morse, R.A.; Stewart, J.D. Venom collection from honey bees. Science 1963, 142, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Bicudo de Almeida-Muradian, L.; Monika Barth, O.; Dietemann, V.; Eyer, M.; Freitas, A.d.S.d.; Martel, A.C.; Marcazzan, G.L.; Marchese, C.M.; Mucignat-Caretta, C.; Pascual-Maté, A.; et al. Standard methods for Apis mellifera honey research. J. Apic. Res. 2020, 59, 1–62. [Google Scholar] [CrossRef]
- Krell, R. Value-added products from beekeeping. In FAO Agriculture Server Bulletin; No. 124; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996; ISBN 92-5-103819-8. [Google Scholar]
- Ali, M. Studies on Bee Venom and Its Medical Uses. Int. J. Adv. Res. 2012, 1, 1–15. [Google Scholar]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pacáková, V.; Štulík, K.; Thi Hau, P.; Jelínek, I.; Vinš, I.; Sýkora, D. Comparison of high-performance liquid chromatography and capillary electrophoresis for the determination of some bee venom components. J. Chromatogr. A 1995, 700, 187–193. [Google Scholar] [CrossRef]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Jakob, T.; Rafei-Shamsabadi, D.; Spillner, E.; Müller, S. Diagnostik der Hymenopteren-giftallergie: Aktuelle Konzepte und Entwicklungen mit besonderem Fokus auf die molekulare Allergiediagnostik. Allergo J. 2017, 26, 33–48. [Google Scholar] [CrossRef]
- Mourre, C.; Fournier, C.; Soumireu-Mourat, B. Apamin, a blocker of the calcium-activated potassium channel, induces neurodegeneration of Purkinje cells exclusively. Brain Res. 1997, 778, 405–408. [Google Scholar] [CrossRef]
- Banks, B.E.C.; Dempsey, C.E.; Vernon, C.A.; Warner, J.A.; Yamey, J. Anti-inflammatory activity of bee venom peptide 401 (mast cell degranulating peptide) and compound 48/80 results from mast cell degranulation in vivo. Br. J. Pharmacol. 1990, 99, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, B.Y.; Yoon, H.J.; Choi, Y.S.; Jin, B.R. Secapin, a bee venom peptide, exhibits anti-fibrinolytic, anti-elastolytic, and anti-microbial activities. Dev. Comp. Immunol. 2016, 63, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Shkenderov, S.; Koburova, K. Adolapin—A newly isolated analgetic and anti-inflammatory polypeptide from bee venom. Toxicon 1982, 20, 317–321. [Google Scholar] [CrossRef]
- Schumacher, M.J. Lethality of ‘killer’ bee stings. Nature 1989, 337, 413. [Google Scholar]
- Marques Pereira, A.F.; Albano, M.; Bérgamo Alves, F.C.; Murbach Teles Andrade, B.F.; Furlanetto, A.; Mores Rall, V.L.; Delazari dos Santos, L.; de Oliveira Orsi, R.; Fernandes, A., Jr. Influence of apitoxin and melittin from Apis mellifera bee on Staphylococcus aureus strains. Microb. Pathog. 2020, 141, 104011. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Abd-Elhakim, Y.M.; Ismail, S.A.A. Involvement of the anti-inflammatory, anti-apoptotic, and anti-secretory activity of bee venom in its therapeutic effects on acetylsalicylic acid-induced gastric ulceration in rats. Toxicology 2019, 419, 11–23. [Google Scholar] [CrossRef]
- Yalcin, M.; Aydin, C.; Savci, V. Cardiovascular effect of peripheral injected melittin in normotensive conscious rats: Mediation of the central cholinergic system. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 341–347. [Google Scholar] [CrossRef]
- Lim, H.N.; Baek, S.B.; Jung, H.J. Bee venom and its peptide component melittin suppress growth and migration of melanoma cells via inhibition of PI3K/Akt/mTOR and MAPK pathways. Molecules 2019, 24, 929. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Shin, J.M.; Bae, Y.S.; Cho, H.J.; Park, K.K.; Choe, J.Y.; Han, S.M.; Moon, S.K.; Kim, W.J.; Choi, Y.H.; et al. Melittin has a chondroprotective effect by inhibiting MMP-1 and MMP-8 expressions via blocking NF-κB and AP-1 signaling pathway in chondrocytes. Int. Immunopharmacol. 2015, 25, 400–405. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.H.; Kim, K.H.; Lee, W.R.; Kim, K.S.; Park, K.K. Melittin inhibits atherosclerosis in LPS/high-fat treated mice through atheroprotective actions. J. Atheroscler. Thromb. 2011, 18, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.M.; Tao, W.H.; Diao, Y.L.; Fang, P.H.; Wang, J.J.; Bo, P.; Qian, F. Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J. Gastroenterol. 2016, 22, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Park, J.H.; Kim, K.H.; Park, Y.Y.; Han, S.M.; Park, K.K. Protective effects of melittin on transforming growth factor-β1 injury to hepatocytes via anti-apoptotic mechanism. Toxicol. Appl. Pharmacol. 2011, 256, 209–215. [Google Scholar] [CrossRef]
- Choi, S.; Chae, H.K.; Heo, H.; Hahm, D.H.; Kim, W.; Kim, S.K. Analgesic effect of melittin on oxaliplatin-induced peripheral neuropathy in rats. Toxins 2019, 11, 396. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Wei, L.; Wang, Z.; Ma, W.; Liu, F.; Shen, Y.; Zhang, S.; Zhang, X.; Hang, Y.; et al. Anti-fibrotic effect of melittin on TRIM47 expression in human embryonic lung fibroblast through regulating TRIM47 pathway. Life Sci. 2020, 256. [Google Scholar] [CrossRef]
- Khulan, T.S.; Ambaga, M. Effect of Honey Bee Venom (Apis mellifera) on Hyperglycemia and Hyperlipidemia in Alloxan Induced Diabetic Rabbits. J. Diabetes Metab. 2016, 6, 3–6. [Google Scholar] [CrossRef]
- Hincha, D.K.; Crowe, J.H. The lytic activity of the bee venom peptide melittin is strongly reduced by the presence of negatively charged phospholipids or chloroplast galactolipids in the membranes of phosphatidylcholine large unilamellar vesicles. Biochim. Biophys. Acta Biomembr. 1996, 1284, 162–170. [Google Scholar] [CrossRef]
- Shin, J.M.; Jeong, Y.J.; Cho, H.J.; Park, K.K.; Chung, I.K.; Lee, I.K.; Kwak, J.Y.; Chang, H.W.; Kim, C.H.; Moon, S.K.; et al. Melittin Suppresses HIF-1α/VEGF Expression through Inhibition of ERK and mTOR/p70S6K Pathway in Human Cervical Carcinoma Cells. PLoS ONE 2013, 8, e69380. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, Y.J.; Park, K.K.; Cho, H.J.; Chung, I.K.; Min, K.S.; Kim, M.; Lee, K.G.; Yeo, J.H.; Park, K.K.; et al. Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-κB and AP-1-dependent MMP-9 expression. Mol. Cells 2010, 29, 209–215. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M. Anti-fungal properties and mechanisms of melittin. Appl. Microbiol. Biotechnol. 2020, 104, 6513–6526. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ye, M.K.; Choi, S.Y.; Park, K.K. The effects of melittin and apamin on airborne fungi-induced chemical mediator and extracellular matrix production from nasal polyp fibroblasts. Toxins 2017, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; An, H.J.; Kim, W.H.; Park, Y.Y.; Park, K.D.; Park, K.K. Apamin suppresses biliary fibrosis and activation of hepatic stellate cells. Int. J. Mol. Med. 2017, 39, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Lee, Y.M.; Cho, S.N.; Son, E.; Song, C.H.; Kim, D.S. Apamin from bee venom suppresses inflammation in a murine model of gouty arthritis. J. Ethnopharmacol. 2020, 257, 112860. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.H.; Kim, K.H.; Lee, W.R.; Pak, S.C.; Han, S.M.; Park, K.K. The protective effect of apamin on LPS/fat-induced atherosclerotic mice. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Leandro, L.F.; Mendes, C.A.; Casemiro, L.A.; Vinholis, A.H.C.; Cunha, W.R.; De Almeida, R.; Martins, C.H.G. Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. An. Acad. Bras. Cienc. 2015, 87, 147–155. [Google Scholar] [CrossRef]
- Mohammadi-Rad, M.; Ghasemi, N.; Aliomrani, M. Evaluation of apamin effects on myelination process in C57BL/6 mice model of multiple sclerosis. Res. Pharm. Sci. 2019, 14, 424–431. [Google Scholar] [CrossRef]
- Buku, A.; Price, J.A.; Mendlowitz, M.; Masur, S. Mast cell degranulating peptide binds to RBL-2H3 mast cell receptors and inhibits IgE binding. Peptides 2001, 22, 1993–1998. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; Obeid, D.E.; Sabatier, J.M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Duchez, A.C.; Boudreau, L.H.; Naika, G.S.; Rousseau, M.; Cloutier, N.; Levesque, T.; Gelb, M.H.; Boilard, E. Respective contribution of cytosolic phospholipase A2α and secreted phospholipase A2 IIA to inflammation and eicosanoid production in arthritis. Prostaglandins Other Lipid Mediat. 2019, 143, 106340. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, M.; Sinou, V.; Payré, C.; Jeammet, L.; Parzy, D.; Grellier, P.; Deregnaucourt, C.; Lambeau, G. Antimalarial activity of human group IIA secreted phospholipase A2 in relation to enzymatic hydrolysis of oxidized lipoproteins. Infect. Immun. 2019, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Boutrin, M.C.F.; Foster, H.A.; Pentreath, V.W. The effects of bee (Apis mellifera) venom phospholipase A2 on Trypanosoma brucei brucei and enterobacteria. Exp. Parasitol. 2008, 119, 246–251. [Google Scholar] [CrossRef]
- Ham, H.J.; Han, J.H.; Lee, Y.S.; Kim, K.C.; Yun, J.; Kang, S.K.; Park, Y.S.; Kim, S.H.; Hong, J.T. Bee Venom Soluble Phospholipase A2 Exerts Neuroprotective Effects in a Lipopolysaccharide-Induced Mouse Model of Alzheimer’s Disease via Inhibition of Nuclear Factor-Kappa B. Front. Aging Neurosci. 2019, 11, 1–14. [Google Scholar] [CrossRef]
- Ho, J.N.; Lee, S.B.; Lee, S.S.; Yoon, S.H.; Kang, G.Y.; Hwang, S.G.; Um, H.D. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol. Cancer Ther. 2010, 9, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Fenard, D.; Lambeau, G.; Maurin, T.; Lefebvre, J.C.; Doglio, A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001, 60, 341–347. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.Y.; Shin, J.; Hwang, J.T.; Jeon, H.N.; Bae, H. Dose-dependent neuroprotective effect of standardized bee venom phospholipase A2 against MPTP-induced Parkinson’s disease in mice. Front. Aging Neurosci. 2019, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.C.T.; Toyama, M.; Marangoni, S.; Oliveira, B.; Cirino, G.; Antunes, E.; De Nucci, G. Effect of crotapotin and heparin on the rat paw oedema induced by different secretory phospholipases A2. Toxicon 2000, 38, 199–208. [Google Scholar] [CrossRef]
- Corthésy, B.; Lassus, A.; Terrettaz, J.; Tranquart, F.; Bioley, G. Efficacy of a therapeutic treatment using gas-filled microbubble-associated phospholipase A2 in a mouse model of honeybee venom allergy. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 957–966. [Google Scholar] [CrossRef]
- Baek, H.; Jang, H.I.; Jeon, H.N.; Bae, H. Comparison of administration routes on the protective effects of Bee Venom Phospholipase A2 in a mouse model of Parkinson’s disease. Front. Aging Neurosci. 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Ye, M.; Chung, H.S.; Lee, C.; Hyun Song, J.; Shim, I.; Kim, Y.S.; Bae, H. Bee venom phospholipase A2 ameliorates motor dysfunction and modulates microglia activation in Parkinson’s disease alpha-synuclein transgenic mice. Exp. Mol. Med. 2016, 48, e244. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Frost, G.I.; Stern, R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Marković-Housley, Z.; Miglierini, G.; Soldatova, L.; Rizkallah, P.J.; Müller, U.; Schirmer, T. Crystal structure of hyaluronidase, a major allergen of bee venom. Structure 2000, 8, 1025–1035. [Google Scholar] [CrossRef]
- Van Vaerenbergh, M.; Debyser, G.; Devreese, B.; de Graaf, D.C. Exploring the hidden honeybee (Apis mellifera) venom proteome by integrating a combinatorial peptide ligand library approach with FTMS. J. Proteom. 2014, 99, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.; Estevinho, M.M.; Choupina, A.B.; Sousa-Pimenta, M.; Estevinho, L.M. An overview of the bioactive compounds, therapeutic properties and toxic effects of apitoxin. Food Chem. Toxicol. 2019, 134, 110864. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lariviere, W.R. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: A double-edged sword. Prog. Neurobiol. 2010, 92, 151–183. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.R.P.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef]
- Lee, J.E.; Shah, V.K.; Lee, E.J.; Oh, M.S.; Choi, J.J. Melittin—A bee venom component—Enhances muscle regeneration factors expression in a mouse model of skeletal muscle contusion. J. Pharmacol. Sci. 2019, 140, 26–32. [Google Scholar] [CrossRef]
- PubChem Compound Summary: Melittin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/16133648#section=UN-GHS-Classification (accessed on 16 September 2020).
- Sun, X.; Chen, S.; Li, S.; Yan, H.; Fan, Y.; Mi, H. Deletion of two C-terminal Gln residues of 12–26-residue fragment of melittin improves its antimicrobial activity. Peptides 2005, 26, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef] [PubMed]
- Klocek, G.; Schulthess, T.; Shai, Y.; Seelig, J. Thermodynamics of melittin binding to lipid bilayers. Aggregation and pore formation. Biochemistry 2009, 48, 2586–2596. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, S.; Li, J.; Yuan, B.; Yang, K.; Ma, Y. Molecular details on the intermediate states of melittin action on a cell membrane. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2234–2241. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Lee, M.T.; Sun, T.L.; Hung, W.C.; Huang, H.W. Process of inducing pores in membranes by melittin. Proc. Natl. Acad. Sci. USA 2013, 110, 14243–14248. [Google Scholar] [CrossRef]
- Hood, J.L.; Jallouk, A.P.; Campbell, N.; Ratner, L.; Wickline, S.A. Cytolytic nanoparticles attenuate HIV-1 infectivity. Antivir. Ther. 2013, 18, 95–103. [Google Scholar] [CrossRef]
- Uddin, M.B.; Lee, B.H.; Nikapitiya, C.; Kim, J.H.; Kim, T.H.; Lee, H.C.; Kim, C.G.; Lee, J.S.; Kim, C.J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, B.; Huang, C.; Meng, X.M.; Bian, E.B.; Li, J. Melittin restores PTEN expression by down-regulating HDAC2 in human hepatocelluar carcinoma HepG2 cells. PLoS ONE 2014, 9, e95520. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Liu, J.; Dai, B.; Xu, G.; Shen, G.; Luo, Q.; Zhang, Z. Immune modulation of liver sinusoidal endothelial cells by melittin nanoparticles suppresses liver metastasis. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- PubChem Apamin, N-acetyl-4-(N6-acetyl-L-lysine)-78114-13-3. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/91808948 (accessed on 17 September 2020).
- Palma, M.S. Hymenoptera Insect Peptides. In Handbook of Biologically Active Peptides: Venom Peptides; Elsevier Inc.: Los Angeles, CA, USA, 2013; pp. 416–422. ISBN 9780123850959. [Google Scholar]
- Lamy, C.; Goodchild, S.J.; Weatherall, K.L.; Jane, D.E.; Liégeois, J.F.; Seutin, V.; Marrion, N.V. Allosteric block of KCa2 channels by apamin. J. Biol. Chem. 2010, 285, 27067–27077. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.C.d.M.; Ramos, E.R.d.P.; Ambiel, C.R.; Correia-de-Sá, P.; Alves-Do-Prado, W. Apamin reduces neuromuscular transmission by activating inhibitory muscarinic M2 receptors on motor nerve terminals. Eur. J. Pharmacol. 2010, 626, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Han, S.M.; Park, K.K. Therapeutic effects of apamin as a bee venom component for non-neoplastic disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ye, M.K.; Choi, S.Y.; Park, K.K. Anti-inflammatory effect of bee venom in an allergic chronic rhinosinusitis mouse model. Mol. Med. Rep. 2018, 17, 6632–6638. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Lee, S.J.; Park, J.Y.; Park, K.D.; Han, S.M.; Kim, M.K.; et al. Apamin inhibits TNF-α-and IFN-γ-induced inflammatory cytokines and chemokines via suppressions of NF-κB signaling pathway and STAT in human keratinocytes. Pharmacol. Rep. 2017, 69, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, J.H.; Kim, K.H.; Lee, W.R.; An, H.J.; Min, B.K.; Han, S.M.; Kim, K.S.; Park, K.K. Apamin inhibits THP-1-derived macrophage apoptosis via mitochondria-related apoptotic pathway. Exp. Mol. Pathol. 2012, 93, 129–134. [Google Scholar] [CrossRef]
- Ziai, M.R.; Russek, S.; Wang, H.-C.; Beer, B.; Blume, A.J. Mast Cell Degranulating Peptide: A Multi-functional Neurotoxin. J. Pharm. Pharmacol. 1990, 42, 457–461. [Google Scholar] [CrossRef]
- PubChem Mast Cell Degranulating Peptide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/16132290 (accessed on 17 September 2020).
- Buku, A.; Price, J.A. Further studies on the structural requirements for mast cell degranulating (MCD) peptide-mediated histamine release. Peptides 2001, 22, 1987–1991. [Google Scholar] [CrossRef]
- Buku, A. Mast cell degranulating (MCD) peptide: A prototypic peptide in allergy and inflammation. Peptides 1999, 20, 415–420. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Zwiener, R.D. Immunology of Bee Venom. Clin. Rev. Allergy Immunol. 2018, 54, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Buku, A.; Mendlowitz, M.; Condie, B.A.; Price, J.A. Partial alanine scan of mast cell degranulating peptide (MCD): Importance of the histidine-and arinine residues. J. Pept. Sci. 2004, 10, 313–317. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, X.X.; Sheng, Y.X.; Zhang, J.L.; Yu, D.Q. A novel peptide from Apis mellifera and solid-phase synthesis of its analogue. Chin. Chem. Lett. 2012, 23, 1161–1164. [Google Scholar] [CrossRef]
- PubChem Secapin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Secapin (accessed on 18 September 2020).
- Vlasak, R.; Kreil, G. Nucleotide sequence of cloned cDNAs coding for preprosecapin, a major product of queen-bee venom glands. Eur. J. Biochem. 1984, 145, 279–282. [Google Scholar] [CrossRef]
- Hou, C.; Guo, L.; Lin, J.; You, L.; Wu, W. Production of antibacterial peptide from bee venom via a new strategy for heterologous expression. Mol. Biol. Rep. 2014, 41, 8081–8091. [Google Scholar] [CrossRef]
- Mourelle, D.; Brigatte, P.; Bringanti, L.D.B.; De Souza, B.M.; Arcuri, H.A.; Gomes, P.C.; Baptista-Saidemberg, N.B.; Ruggiero Neto, J.; Palma, M.S. Hyperalgesic and edematogenic effects of Secapin-2, a peptide isolated from Africanized honeybee (Apis mellifera) venom. Peptides 2014, 59, 42–52. [Google Scholar] [CrossRef]
- Jung, S.Y.; Lee, K.W.; Choi, S.M.; Yang, E.J. Bee venom protects against rotenone-induced cell death in NSC34 motor neuron cells. Toxins 2015, 7, 3715–3726. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvǎtescu, C.A.; Ifteni, P.; Pleş, L. Anticancer activity of toxins from bee and snake venom-an overview on ovarian cancer. Molecules 2018, 23, 692. [Google Scholar] [CrossRef]
- Kitamura, H.; Yokoyama, M.; Akita, H.; Matsushita, K.; Kurachi, Y.; Physiology, C.; Cardiovascular, N.; Ya, M. Tertiapin Potently and Selectively Blocks Muscarinic K+ Channels in Rabbit Cardiac Myocytes 1. J. Pharmacol. Exp. Ther. 2000, 293, 196–205. [Google Scholar] [PubMed]
- PubChem. Tertiapin LQ. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/90488935 (accessed on 18 September 2020).
- Bidaud, I.; Chong, A.C.Y.; Carcouet, A.; Waard, S.D.; Charpentier, F.; Ronjat, M.; Waard, M.D.; Isbrandt, D.; Wickman, K.; Vincent, A.; et al. Inhibition of G protein-gated K + channels by tertiapin-Q rescues sinus node dysfunction and atrioventricular conduction in mouse models of primary bradycardia. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Klem, A.M.; Lewis, J.H.; Lu, Z. Mechanisms of inward-rectifier K+ channel inhibition by tertiapin-Q. Biochemistry 1999, 38, 14294–14301. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.L.; O’Connor, R. Procamine and Other Basic Peptides in the Venom of the Honeybee (Apis mellifera). J. Agric. Food Chem. 1974, 22, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Lowy, P.H.; Sarmiento, L.; Mitchell, H.K. Polypeptides minimine and melittin from bee venom: Effects on Drosophila. Arch. Biochem. Biophys. 1971, 145, 338–343. [Google Scholar] [CrossRef]
- Vick, J.A.; Shipman, W.H.; Brooks, R. Beta adrenergic and anti-arrhythmic effects of cardiopep, a newly isolated substance from whole bee venom. Toxicon 1974, 12, 139–142. [Google Scholar] [CrossRef]
- Van Vaerenbergh, M.; Cardoen, D.; Formesyn, E.M.; Brunain, M.; Van Driessche, G.; Blank, S.; Spillner, E.; Verleyen, P.; Wenseleers, T.; Schoofs, L.; et al. Extending the honey bee venome with the antimicrobial peptide apidaecin and a protein resembling wasp antigen 5. Insect Mol. Biol. 2013, 22, 199–210. [Google Scholar] [CrossRef]
- Welker, S.; Markert, Y.; Köditz, J.; Mansfeld, J.; Ulbrich-Hofmann, R. Disulfide bonds of phospholipase A2 from bee venom yield discrete contributions to its conformational stability. Biochimie 2011, 93, 195–201. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Putz, T.; Ramoner, R.; Gander, H.; Rahm, A.; Bartsch, G.; Bernardo, K.; Ramsay, S.; Thurnher, M. Bee venom secretory phospholipase A2 and phosphatidylinositol-homologues cooperatively disrupt membrane integrity, abrogate signal transduction and inhibit proliferation of renal cancer cells. Cancer Immunol. Immunother. 2007, 56, 627–640. [Google Scholar] [CrossRef]
- Kudo, I.; Murakami, M. Phospholipase A2 Enzymes. Prostaglandins Other Lipids Mediat. 2020, 68–69, 3–58. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Thwin, M.M.; Chow, T.K.V.; Bow, H.; Yap, E.H.; Thong, T.W.J. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J. Appl. Microbiol. 2007, 102, 650–659. [Google Scholar] [CrossRef]
- Chen, M.; Aoki-Utsubo, C.; Kameoka, M.; Deng, L.; Terada, Y.; Kamitani, W.; Sato, K.; Koyanagi, Y.; Hijikata, M.; Shindo, K.; et al. Broad-spectrum antiviral agents: Secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Bee venom phospholipase A2: Yesterday’s enemy becomes today’s friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef]
- Lambeau, G.; Schmid-Alliana, A.; Lazdunski, M.; Barhanin, J. Identification and purification of a very high affinity binding protein for toxic phospholipases A2 in skeletal muscle. J. Biol. Chem. 1990, 265, 9526–9532. [Google Scholar]
- Lambeau, G.; Barhanin, J.; Schweitz, H.; Qar, J.; Lazdunski, M. Identification and properties of very high affinity brain membrane-binding sites for a neurotoxic phospholipase from the taipan venom. J. Biol. Chem. 1989, 264, 11503–11510. [Google Scholar] [PubMed]
- Nicolas, J.P.; Lin, Y.; Lambeau, G.; Ghomashchi, F.; Lazdunski, M.; Gelb, M.H. Localization of structural elements of bee venom phospholipase A2 involved in N-type receptor binding and neurotoxicity. J. Biol. Chem. 1997, 272, 7173–7181. [Google Scholar] [CrossRef]
- Chung, E.S.; Lee, G.; Lee, C.; Ye, M.; Chung, H.; Kim, H.; Bae, S.S.; Hwang, D.-S.; Bae, H. Bee Venom Phospholipase A 2, a Novel Foxp3 + Regulatory T Cell Inducer, Protects Dopaminergic Neurons by Modulating Neuroinflammatory Responses in a Mouse Model of Parkinson’s Disease. J. Immunol. 2015, 195, 4853–4860. [Google Scholar] [CrossRef]
- Baek, H.; Park, S.Y.; Ku, S.J.; Ryu, K.; Kim, Y.; Bae, H.; Lee, Y.S. Bee venom phospholipase A2 induces regulatory T cell populations by suppressing apoptotic signaling pathway. Toxins 2020, 12, 198. [Google Scholar] [CrossRef]
- Palm, N.W.; Rosenstein, R.K.; Yu, S.; Schenten, D.D.; Florsheim, E.; Medzhitov, R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 2013, 39, 976–985. [Google Scholar] [CrossRef]
- Taketomi, Y.; Ueno, N.; Kojima, T.; Sato, H.; Murase, R.; Yamamoto, K.; Tanaka, S.; Sakanaka, M.; Nakamura, M.; Nishito, Y.; et al. Mast cell maturation is driven via a group III phospholipase A 2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 2013, 14, 554–563. [Google Scholar] [CrossRef]
- Dos Santos-Pinto, J.R.A.; Perez-Riverol, A.; Lasa, A.M.; Palma, M.S. Diversity of peptidic and proteinaceous toxins from social Hymenoptera venoms. Toxicon 2018, 148, 172–196. [Google Scholar] [CrossRef]
- Hossen, M.S.; Shapla, U.M.; Gan, S.H.; Khalil, M.I. Impact of bee venom enzymes on diseases and immune responses. Molecules 2017, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, T.; Bockisch, B.; Spillner, E.; Ring, J.; Bredehorst, R.; Ollert, M.W. Molecular cloning and expression in insect cells of honeybee venom allergen acid phosphatase (Api m 3). J. Allergy Clin. Immunol. 2006, 117, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Seismann, H.; Bockisch, B.; Braren, I.; Cifuentes, L.; McIntyre, M.; Rühl, D.; Ring, J.; Bredehorst, R.; Ollert, M.W.; et al. Identification, Recombinant Expression, and Characterization of the 100 kDa High Molecular Weight Hymenoptera Venom Allergens Api m 5 and Ves v 3. J. Immunol. 2010, 184, 5403–5413. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Lee, K.S.; Kim, B.Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.Y.; Wan, H.; Li, J.; Jin, B.R. Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev. Comp. Immunol. 2018, 85, 51–60. [Google Scholar] [CrossRef]
- Somwongin, S.; Chantawannakul, P.; Chaiyana, W. Antioxidant activity and irritation property of venoms from Apis species. Toxicon 2018, 145, 32–39. [Google Scholar] [CrossRef]
- El-Hanoun, A.; El-Komy, A.; El-Sabrout, K.; Abdella, M. Effect of bee venom on reproductive performance and immune response of male rabbits. Physiol. Behav. 2020, 223, 112987. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Kaleli, S. Anti-inflammatory and antioxidative properties of honey bee venom on Freund’s Complete Adjuvant-induced arthritis model in rats. Toxicon 2019, 161, 4–11. [Google Scholar] [CrossRef]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef]
- Marcos, J.F.; Beachy, R.N.; Houghten, R.A.; Blondelle, S.E.; Pérez-Payá, E. Inhibition of a plant virus infection by analogs of melittin. Proc. Natl. Acad. Sci. USA 1995, 92, 12466–12469. [Google Scholar] [CrossRef]
- Wachinger, M.; Saermark, T.; Erfle, V. Influence of amphipathic peptides on the HIV-1 production in persistently infected T lymphoma cells. FEBS Lett. 1992, 309, 235–241. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The protective effects of melittin on propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J. Invest. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.; Jiang, J.; Chen, Z.J. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 1999, 13, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-κB and MAPK pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef]
- Jin, Y.; Myung, L.; Oh, J.; Hun, D.; Yong, L.; Lee, S.; Lee, J.; Hyun, D.; Cheol, K.; Choi, H. Anti—Inflammatory effect of bee venom in phthalic anhydride—Induced atopic dermatitis animal model. Inflammopharmacology 2019, 56, 106–112. [Google Scholar] [CrossRef]
- Ozturk, A.B.; Bayraktar, R.; Gogebakan, B.; Mumbuc, S.; Bayram, H. Comparison of inflammatory cytokine release from nasal epithelial cells of non-atopic non-rhinitic, allergic rhinitic and polyp subjects and effects of diesel exhaust particles in vitro. Allergol. Immunopathol. 2017, 45, 473–481. [Google Scholar] [CrossRef]
- Otón, T.; Carmona, L. The epidemiology of established rheumatoid arthritis. Best Pr. Res. Clin. Rheumatol. 2019, 33, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, S.S.; Kim, J.H.; Bae, C.S.; Choi, S.H. Inhibitory effect of whole bee venom in adjuvant-induced arthritis. In Vivo 2005, 19, 801–806. [Google Scholar]
- Kwon, Y.B.; Lee, J.D.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Beitz, A.J.; Lee, J.H. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain 2001, 90, 271–280. [Google Scholar] [CrossRef]
- Davies, J.; Riede, P.; van Langevelde, K.; Teh, J. Recent developments in advanced imaging in gout. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X1984442. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-κB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef]
- Chung, E.S.; Kim, H.; Lee, G.; Park, S.; Kim, H.; Bae, H. Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of Parkinson’s disease: Role of regulatory T cells. Brain. Behav. Immun. 2012, 26, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.; Lee, G.; Jang, H.; Kim, S.S.; Yoon, H.; Kang, G.H.; Hwang, D.S.; Kim, S.K.; Chung, H.S.; et al. Phospholipase A2 inhibits cisplatin-induced acute kidney injury by modulating regulatory T cells by the CD206 mannose receptor. Kidney Int. 2015, 88, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chung, H.S.; Lee, C.; Yoon, M.S.; Yu, A.R.; Kim, J.S.; Hwang, D.S.; Shim, I.; Bae, H. Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J. Neuroinflammation 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Badshah, H.; Ali, T.; Kim, M.O. Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFκB signaling pathway. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.L.; Giesbergen, P.C.L.M.; De Rijk, M.C.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Incidence of parkinsonism and Parkinson disease in a general population: The Rotterdam Study. Neurology 2004, 63, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Jung, K.; Baek, H.; Kang, M.; Kim, N.; Lee, S.Y.; Bae, H. Dust Mite Extract Induced Atopic Dermatitis Like Skin Lesions in Mice. Toxins 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Choi, M.S.; Kwak, D.H.; Oh, K.W.; Yoon, D.Y.; Han, S.B.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venomin prostate cancer cells through activation of caspase pathway via inactivation of NF-κB. Prostate 2011, 71, 801–812. [Google Scholar] [CrossRef]
- Qin, G.; Chen, Y.; Li, H.; Xu, S.; Li, Y.; Sun, J.; Rao, W.; Chen, C.; Du, M.; He, K.; et al. Melittin inhibits tumor angiogenesis modulated by endothelial progenitor cells associated with the SDF-1α/CXCR4 signaling pathway in a UMR-106 osteosarcoma xenograft mouse model. Mol. Med. Rep. 2016, 14, 57–68. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, H.; Ge, Y.; Liu, J.; Cai, J.; Qin, Q.; Zhan, L.; Zhang, C.; Xu, L.; Liu, Z.; et al. Melittin enhances radiosensitivity of hypoxic head and neck squamous cell carcinoma by suppressing HIF-1α. Tumor Biol. 2014, 35, 10443–10448. [Google Scholar] [CrossRef]
- Hu, H.; Chen, D.; Li, Y.; Zhang, X. Effect of polypeptides in bee venom on growth inhibition and apoptosis induction of the human hepatoma cell line SMMC-7721 in-vitro and Balb/c nude mice in-vivo. J. Pharm. Pharmacol. 2006, 58, 83–89. [Google Scholar] [CrossRef]
- Jang, M.H.; Shin, M.C.; Lim, S.; Han, S.M.; Park, H.J.; Shin, I.; Lee, J.S.; Kim, K.A.; Kim, E.H.; Kim, C.J. Bee venom induces apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human lung cancer cell line NCI-H1299. J. Pharmacol. Sci. 2003, 91, 95–104. [Google Scholar] [CrossRef]
- Hait, W.N.; Grais, L.; Benz, C.; Cadman, E.C. Inhibition of growth of leukemic cells by inhibitors of calmodulin: Phenothiazines and melittin. Cancer Chemother. Pharmacol. 1985, 14, 202–205. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Lee, C.; Bae, S.J.S.; Joo, H.; Bae, H. Melittin suppresses tumor progression by regulating tumorassociated macrophages in a Lewis lung carcinoma mouse model. Oncotarget 2017, 8, 54951–54965. [Google Scholar] [CrossRef]
- Soliman, C.; Eastwood, S.; Truong, V.K.; Ramsland, P.A.; Elbourne, A. The membrane effects of melittin on gastric and colorectal cancer. PLoS ONE 2019, 14, 1–16. [Google Scholar] [CrossRef]
- Mcf, C.; Breast, M.-H.; Daniluk, K.; Kutwin, M.; Grodzik, M.; Wierzbicki, M.; Strojny, B.; Szczepaniak, J.; Sosnowska, M. Use of Selected Carbon Nanoparticles as Melittin. Materials 1870, 13, 90. [Google Scholar]
- Cho, S.Y.; Shim, S.R.; Rhee, H.Y.; Park, H.J.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Park, S.U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Park. Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Park, S.U.; Park, H.J.; Cho, S.Y.; Park, J.M.; Ko, C.N. Efficacy of combined treatment with acupuncture and Bee venom acupuncture for Parkinson’s disease: Double blind randomized controlled trial. J. Neurol. Sci. 2017, 381, 724. [Google Scholar] [CrossRef]
- Khalil, W.K.B.; Assaf, N.; Elshebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi, D.Y.; Park, D.S. Antinociceptive effect and the mechanism of bee venom acupuncture (Apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by α2-Adrenoceptors. Brain Res. 2006, 1073–1074, 305–310. [Google Scholar] [CrossRef]
- Cai, M.D.; Choi, S.M.; Yang, E.J. The effects of bee venom acupuncture on the central nervous system and muscle in an animal hsOD1G93A mutant. Toxins 2015, 7, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, B.P.; Cai, L. Therapeutic effect of melittin on a rat model of chronic prostatitis induced by Complete Freund’s Adjuvant. Biomed. Pharmacother. 2017, 90, 921–927. [Google Scholar] [CrossRef]
- Yin, D.; Chen, M.; Yang, N.; Wu, A.Z.; Xu, D.; Tsai, W.C.; Yuan, Y.; Tian, Z.; Chan, Y.H.; Shen, C.; et al. Role of apamin-sensitive small conductance calcium-activated potassium currents in long-term cardiac memory in rabbits. Hear Rhythm 2018, 15, 761–769. [Google Scholar] [CrossRef]
- Tighilet, B.; Leonard, J.; Mourre, C.; Chabbert, C. Apamin treatment accelerates equilibrium recovery and gaze stabilization in unilateral vestibular neurectomized cats: Cellular and behavioral aspects. Neuropharmacology 2019, 144, 133–142. [Google Scholar] [CrossRef]
- Hartmann, A.; Müllner, J.; Meier, N.; Hesekamp, H.; Van Meerbeeck, P.; Habert, M.O.; Kas, A.; Tanguy, M.L.; Mazmanian, M.; Oya, H.; et al. Bee venom for the treatment of Parkinson disease—A randomized controlled clinical trial. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef]
- Cherniack, E.P.; Govorushko, S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon 2018, 154, 74–78. [Google Scholar] [CrossRef]
- Park, J.H.; Yim, B.K.; Lee, J.H.; Lee, S.; Kim, T.H. Risk associated with bee venom therapy: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126971. [Google Scholar] [CrossRef]
- Vazquez-Revuelta, P.; Madrigal-Burgaleta, R. Death due to live bee acupuncture apitherapy. J. Investig. Allergol. Clin. Immunol. 2018, 28, 45–46. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Shin, J.S.; Lee, J.; Lee, Y.J.; Kim, M.R.; Shin, Y.S.; Park, K.B.; Kim, E.J.; Kim, M.J.; Lee, J.W.; et al. Safety of essential bee venom pharmacopuncture as assessed in a randomized controlled double-blind trial. J. Ethnopharmacol. 2016, 194, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Schmelzer, C.E.H.; Neubert, R.H.H.; Kokot, Z.J. Characterization of honeybee venom by MALDI-TOF and nanoESI-QqTOF mass spectrometry. J. Pharm. Biomed. Anal. 2011, 54, 273–278. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv. Drug Deliv. Rev. 2020, (in press). [CrossRef] [PubMed]
- Xing, L.; Dawei, C.; Liping, X.; Rongqing, Z. Oral colon-specific drug delivery for bee venom peptide: Development of a coated calcium alginate gel beads-entrapped liposome. J. Control. Release 2003, 93, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Chen, D.; Hao, T.; Zhao, X.; Hu, H.; Ma, X. Effect of bee venom peptide-copolymer interactions on thermosensitive hydrogel delivery systems. Int. J. Pharm. 2007, 345, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Peeler, D.J.; Thai, S.N.; Cheng, Y.; Horner, P.J.; Sellers, D.L.; Pun, S.H. pH-sensitive polymer micelles provide selective and potentiated lytic capacity to venom peptides for effective intracellular delivery. Biomaterials 2019, 192, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.M.; Kim, J.H.; Cho, C.W.; Jeon, J.W.; Park, J.K.; Lee, S.H.; Jung, B.G.; Lee, B.J. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and respiratory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunopathol. 2018, 200, 40–51. [Google Scholar] [CrossRef]
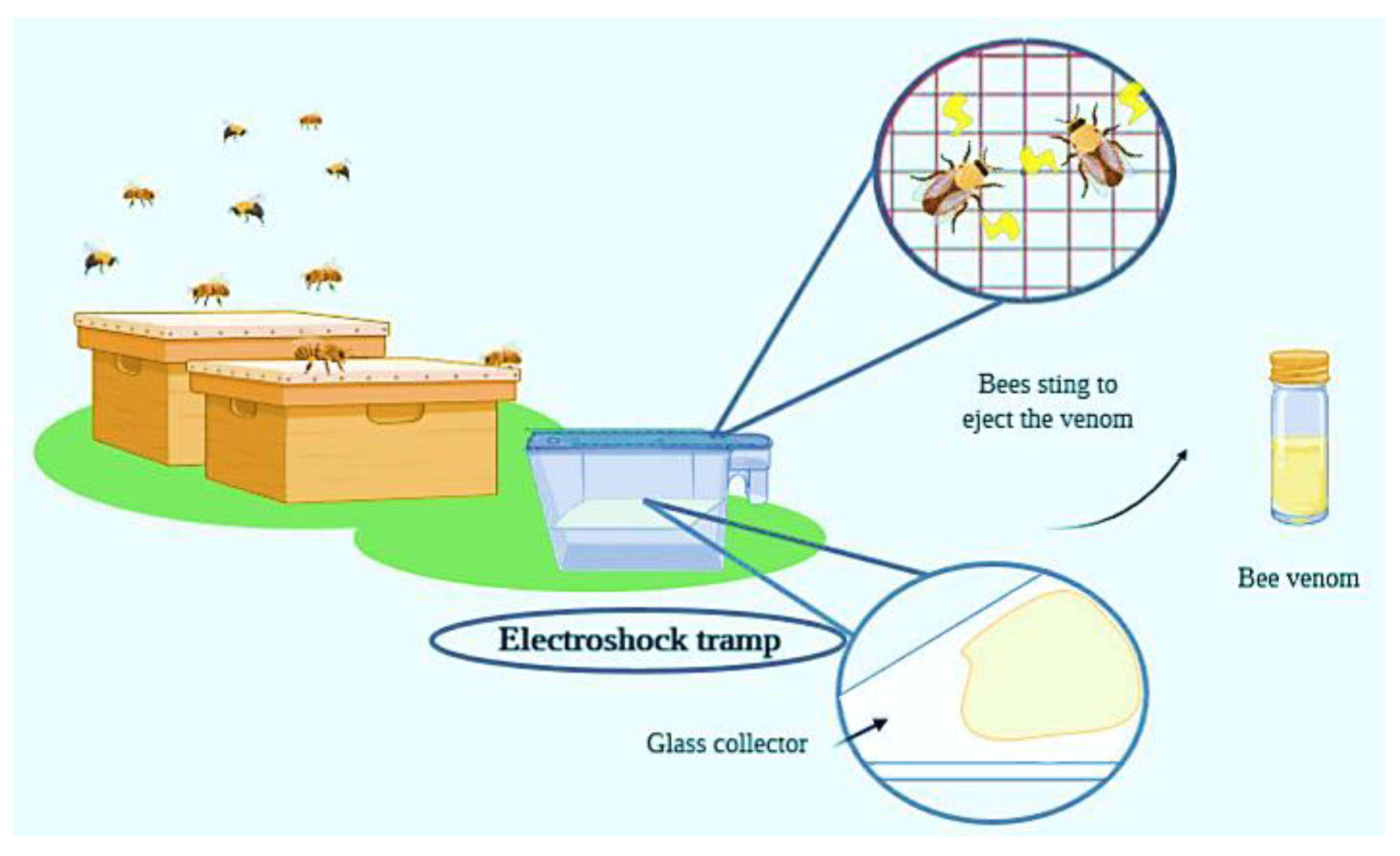
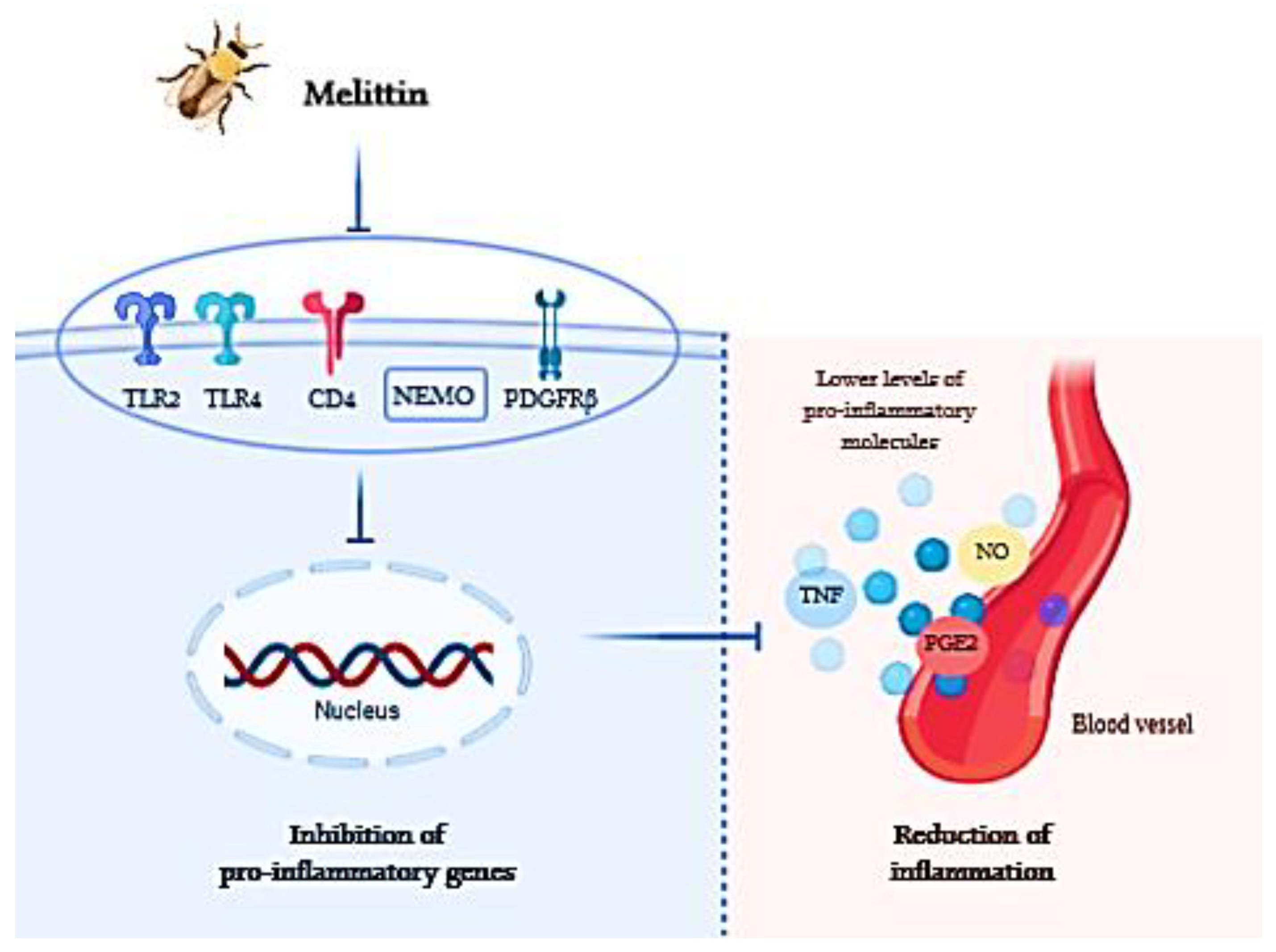
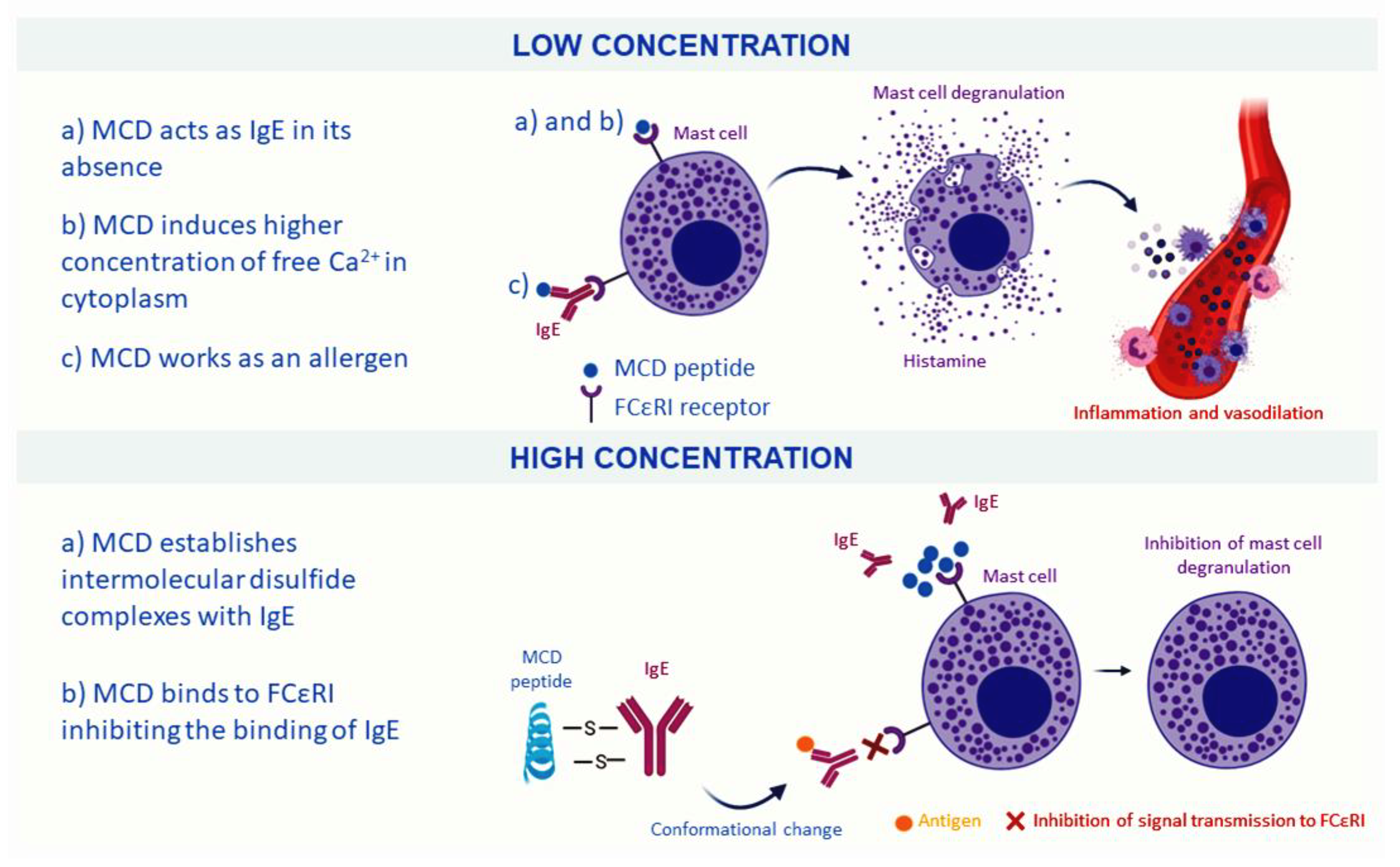
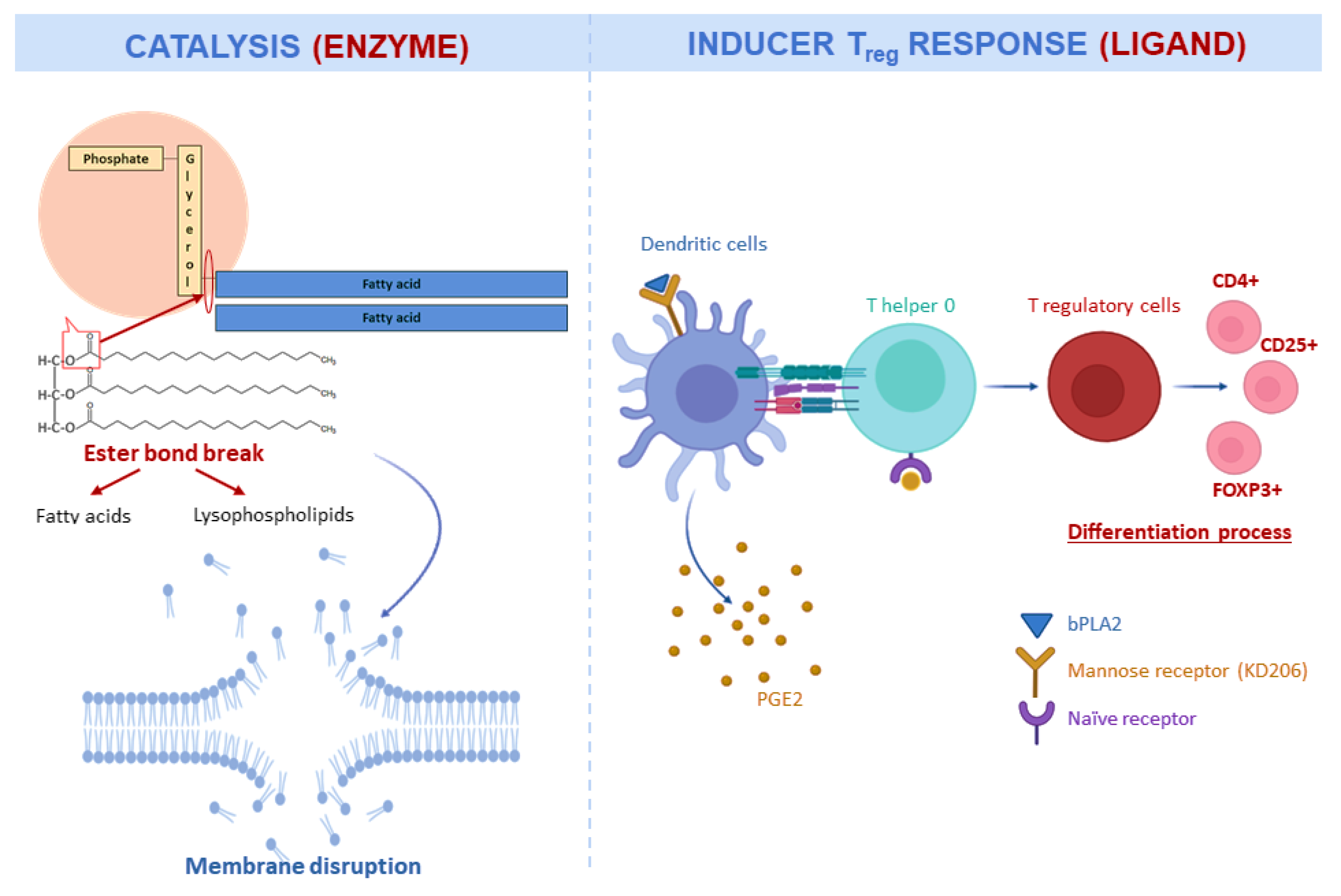
| Component | Organism | Effective Dose (µg/mL) | Component | Organism | Effective Dose (µg/mL) |
|---|---|---|---|---|---|
| BV | Acinetobacter baumannii BAA | MIC 30 | Melittin | Acinetobacter baumannii BAA | MIC 30 |
| BV | Bacillus subtilis | MIC 8 | Melittin | Candida krusei | MIC 30 |
| BV | Candida albicans | MIC 60 | Melittin | Candida krusei | MIC 30 |
| BV | Candida krusei | MIC 60 | Melittin | Escherichia coli | MIC 30 |
| BV | Candida parapsilosis | MIC 60 | Melittin | Streptococcus pyogenes | MIC 10 |
| BV | Clindamycin-resistant P. acnes | MIC 0.067 | Melittin | Staphylococcus aureus Amme | MIC 6 |
| BV | Enterococcus casseliflavus | MIC 10 | Melittin | Streptococcus agalactiae | MIC 30 |
| BV | Escherichia coli | MIC 60 | Melittin | MRSA | MIC 10 |
| BV | Klebsiella pneumoniae | MIC 30 | Melittin | Bacillus subtilis | MIC 6 |
| BV | MRSA | MIC 60 | Melittin | Klebsiella oxytoca | MIC 60 |
| BV | Propionibacterium acnes | MIC 0.086 | Melittin | Staphylococcus aureus BAA | MIC 8 |
| BV | Shigella flexneri | MIC 60 | Melittin | Staphylococcus aureus | MIC 10 |
| BV | Staphylococcus aureus | MIC 10 | Melittin | Staphylococcus saprophyticus | MIC 10 |
| BV | Staphylococcus aureus Amme | MIC 60 | Melittin | Staphylococcus aureus Amme | MIC 6 |
| BV | Staphylococcus aureus BAA | MIC 30 | Melittin | Candida candida | MIC 9.961 |
| BV | Staphylococcus epidermidis | MIC 0.104 | Melittin | Staphylococcus epidermidis | MIC 10 |
| BV | Staphylococcus epidermidis | MIC 60 | Melittin | Lactobacillus casei | MIC 4 |
| BV | Staphylococcus saprophyticus | MIC 10 | Melittin | Enterococcus faecalis | MIC 6 |
| BV | Streptococcus agalactiae | MIC 40 | Melittin | Candida krusei | MIC 30 |
| BV | Streptococcus pyogenes | MIC 0.121 | Melittin | Listeria monocytogenes | MIC 12.5 |
| BV | Streptococcus thermophilus | MIC 30 | Melittin | Escherichia coli | MIC 56.92 |
| PLA2 | Citrobacter freundii | MBC 1000 | Melittin | Staphylococcus aureus | MIC 8.5 |
| PLA2 | Enterobacter clocae | MBC 10000 | Melittin | Staphylococcus aureus amme | MIC 6 |
| PLA2 | Escherichia coli | MBC 10000 | Melittin | Enterococcus casseliflavus | MIC 8 |
| PLA2 | Lactobacillus caser | MBC 400 | Melittin | Enterococcus faecalis VanB | MIC 50 |
| PLA2 | Trypanosoma brucei | MBC 1 | Melittin | Enterococcus faecalis | MIC 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. https://doi.org/10.3390/nu12113360
Carpena M, Nuñez-Estevez B, Soria-Lopez A, Simal-Gandara J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients. 2020; 12(11):3360. https://doi.org/10.3390/nu12113360
Chicago/Turabian StyleCarpena, Maria, Bernabe Nuñez-Estevez, Anton Soria-Lopez, and Jesus Simal-Gandara. 2020. "Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications" Nutrients 12, no. 11: 3360. https://doi.org/10.3390/nu12113360
APA StyleCarpena, M., Nuñez-Estevez, B., Soria-Lopez, A., & Simal-Gandara, J. (2020). Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients, 12(11), 3360. https://doi.org/10.3390/nu12113360







