Chronological Age Interacts with the Circadian Melatonin Receptor 1B Gene Variation, Determining Fasting Glucose Concentrations in Mediterranean Populations. Additional Analyses on Type-2 Diabetes Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Demographic, Anthropometric, Biochemical, Clinical, and Lifestyle Variables
2.3. DNA Isolation and Genotyping
2.4. Statistical Analysis
3. Results
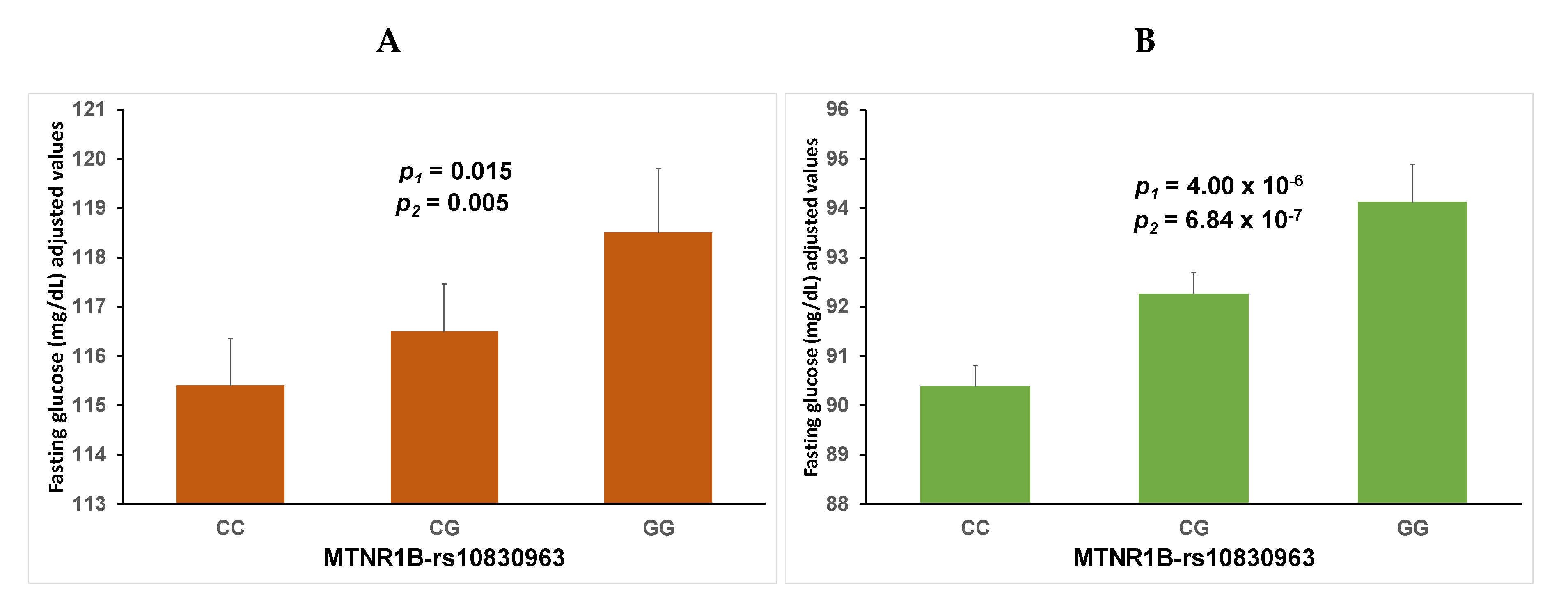
3.1. Association between the MTNR1B Polymorphism and Fasting Plasma Glucose in Mediterranean Subjects Aged 18 to 80 Years (Discovery Cohort)
3.1.1. Associations in the Whole Population
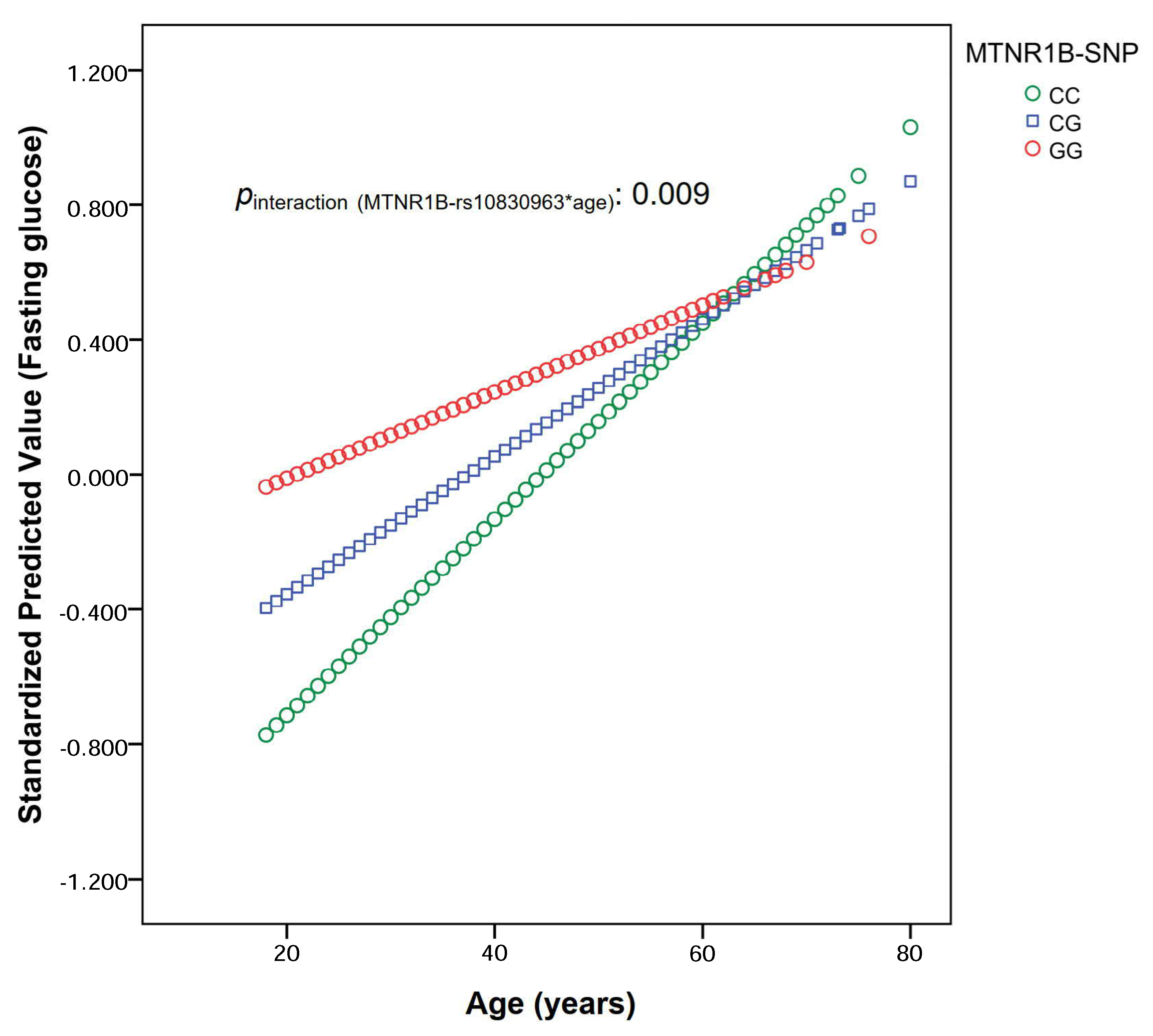
3.1.2. Modulations by Age
3.2. Association between the MTNR1B Polymorphism and Fasting Plasma Glucose and Type-2 Diabetes in an Elderly Population (Replication Cohort 1)
3.3. Association between the MTNR1B Polymorphism, Fasting Plasma Glucose, and Type-2 Diabetes in Another Elderly Population (Replication Cohort 2). Exploratory Analysis of the Influence of Parity in the Effects of the Polymorphism on Type-2 Diabetes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bush, C.L.; Blumberg, J.B.; El-Sohemy, A.; Minich, D.M.; Ordovás, J.M.; Reed, D.G.; Behm, V.A.Y. Toward the Definition of Personalized Nutrition: A Proposal by The American Nutrition Association. J. Am. Coll. Nutr. 2020, 39, 5–15. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef]
- Rodgers, G.P.; Collins, F.S. Precision Nutrition-the Answer to “What to Eat to Stay Healthy”. JAMA 2020. [Google Scholar] [CrossRef]
- Hawley, J.A.; Sassone-Corsi, P.; Zierath, J.R. Chrono-nutrition for the prevention and treatment of obesity and type 2 diabetes: From mice to men. Diabetologia 2020, 63, 2253–2259. [Google Scholar] [CrossRef]
- Lai, C.-Q.; Corella, D.; Demissie, S.; Cupples, L.A.; Adiconis, X.; Zhu, Y.; Parnell, L.D.; Tucker, K.L.; Ordovas, J.M. Dietary intake of n-6 fatty acids modulates effect of apolipoprotein A5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size: The Framingham Heart Study. Circulation 2006, 113, 2062–2070. [Google Scholar] [CrossRef]
- Lai, C.-Q.; Smith, C.E.; Parnell, L.D.; Lee, Y.-C.; Corella, D.; Hopkins, P.; Hidalgo, B.A.; Aslibekyan, S.; Province, M.A.; Absher, D.; et al. Epigenomics and metabolomics reveal the mechanism of the APOA2-saturated fat intake interaction affecting obesity. Am. J. Clin. Nutr. 2018, 108, 188–200. [Google Scholar] [CrossRef]
- Khodarahmi, M.; Jafarabadi, M.A.; Farhangi, M.A. Melanocortin-4 receptor (MC4R) rs17782313 polymorphism interacts with Dietary Approach to Stop Hypertension (DASH) and Mediterranean Dietary Score (MDS) to affect hypothalamic hormones and cardio-metabolic risk factors among obese individuals. Genes Nutr. 2020, 15, 13. [Google Scholar] [CrossRef]
- Wang, D.D.; Hu, F.B. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018, 6, 416–426. [Google Scholar] [CrossRef]
- Simino, J.; Shi, G.; Bis, J.C.; Chasman, D.I.; Ehret, G.B.; Gu, X.; Guo, X.; Hwang, S.-J.; Sijbrands, E.; Smith, A.V.; et al. Gene-age interactions in blood pressure regulation: A large-scale investigation with the CHARGE, Global BPgen, and ICBP Consortia. Am. J. Hum. Genet. 2014, 95, 24–38. [Google Scholar] [CrossRef]
- Simino, J.; Kume, R.; Kraja, A.T.; Turner, S.T.; Hanis, C.L.; Sheu, W.; Chen, I.; Jaquish, C.; Cooper, R.S.; Chakravarti, A.; et al. Linkage analysis incorporating gene-age interactions identifies seven novel lipid loci: The Family Blood Pressure Program. Atherosclerosis 2014, 235, 84–93. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Brown-Gentry, K.; Goodloe, R.; Glenn, K.; Yang, W.; Kornegay, N.; Pui, C.-H.; Relling, M.V.; Crawford, D.C. Evidence for age as a modifier of genetic associations for lipid levels. Ann. Hum. Genet. 2011, 75, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Shirts, B.H.; Hasstedt, S.J.; Hopkins, P.N.; Hunt, S.C. Evaluation of the gene-age interactions in HDL cholesterol, LDL cholesterol, and triglyceride levels: The impact of the SORT1 polymorphism on LDL cholesterol levels is age dependent. Atherosclerosis 2011, 217, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kinoshita, T.; Asada, T.; Ohashi, Y. Log-linear models for assessing gene-age interaction and their application to case-control studies of the apolipoprotein E (apoE) gene in Alzheimer’s disease. J. Hum. Genet. 2003, 48, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.W.; Brandl, C.; Grassmann, F.; Gorski, M.; Stark, K.; Loss, J.; Weber, B.H.F.; Heid, I.M. International Age-related Macular Degeneration Genomics Consortium (IAMDGC) Investigating the modulation of genetic effects on late AMD by age and sex: Lessons learned and two additional loci. PLoS ONE 2018, 13, e0194321. [Google Scholar] [CrossRef]
- Denker, E.; Ebbesson, L.O.E.; Hazlerigg, D.G.; Macqueen, D.J. Phylogenetic Reclassification of Vertebrate Melatonin Receptors to Include Mel1d. Genetics 2019, 9, 3225–3238. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Liu, N.; Cao, X.; Yang, Z.; Lu, B.; Hu, R.; Wang, X.; Wen, J. Melatonin exerts an inhibitory effect on insulin gene transcription via MTNR1B and the downstream Raf-1/ERK signaling pathway. Int. J. Mol. Med. 2018, 41, 955–961. [Google Scholar] [CrossRef]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef]
- Hu, C.; Jia, W. Linking MTNR1B Variants to Diabetes: The Role of Circadian Rhythms. Diabetes 2016, 65, 1490–1492. [Google Scholar] [CrossRef]
- Sparsø, T.; Bonnefond, A.; Andersson, E.; Bouatia-Naji, N.; Holmkvist, J.; Wegner, L.; Grarup, N.; Gjesing, A.P.; Banasik, K.; Cavalcanti-Proença, C.; et al. G-allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release: Studies involving 19,605 Europeans. Diabetes 2009, 58, 1450–1456. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Li, H.; Qi, Q.; Langenberg, C.; Loos, R.J.F.; Lin, X. MTNR1B rs10830963 is associated with fasting plasma glucose, HbA1C and impaired beta-cell function in Chinese Hans from Shanghai. BMC Med. Genet. 2010, 11, 59. [Google Scholar] [CrossRef]
- Staiger, H.; Machicao, F.; Schäfer, S.A.; Kirchhoff, K.; Kantartzis, K.; Guthoff, M.; Silbernagel, G.; Stefan, N.; Häring, H.-U.; Fritsche, A. Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine beta-cell function. PLoS ONE 2008, 3, e3962. [Google Scholar] [CrossRef] [PubMed]
- Rosta, K.; Al-Aissa, Z.; Hadarits, O.; Harreiter, J.; Nádasdi, Á.; Kelemen, F.; Bancher-Todesca, D.; Komlósi, Z.; Németh, L.; Rigó, J.; et al. Association Study with 77 SNPs Confirms the Robust Role for the rs10830963/G of MTNR1B Variant and Identifies Two Novel Associations in Gestational Diabetes Mellitus Development. PLoS ONE 2017, 12, e0169781. [Google Scholar] [CrossRef]
- Kelliny, C.; Ekelund, U.; Andersen, L.B.; Brage, S.; Loos, R.J.F.; Wareham, N.J.; Langenberg, C. Common genetic determinants of glucose homeostasis in healthy children: The European Youth Heart Study. Diabetes 2009, 58, 2939–2945. [Google Scholar] [CrossRef]
- Tan, X.; Ciuculete, D.-M.; Schiöth, H.B.; Benedict, C. Associations between chronotype, MTNR1B genotype and risk of type 2 diabetes in UK Biobank. J. Intern. Med. 2020, 287, 189–196. [Google Scholar] [CrossRef]
- Langlois, C.; Abadi, A.; Peralta-Romero, J.; Alyass, A.; Suarez, F.; Gomez-Zamudio, J.; Burguete-Garcia, A.I.; Yazdi, F.T.; Cruz, M.; Meyre, D. Evaluating the transferability of 15 European-derived fasting plasma glucose SNPs in Mexican children and adolescents. Sci. Rep. 2016, 6, 36202. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.; Sharp, S.J.; Timpson, N.J.; Bouatia-Naji, N.; Warrington, N.M.; Kanoni, S.; Beilin, L.J.; Brage, S.; Deloukas, P.; Evans, D.M.; et al. Association of genetic Loci with glucose levels in childhood and adolescence: A meta-analysis of over 6000 children. Diabetes 2011, 60, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Chen, G.; Shriner, D.; Doumatey, A.; Gerry, N.P.; Herbert, A.; Huang, H.; Zhou, J.; Christman, M.F.; Adeyemo, A.; et al. Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia 2011, 54, 783–788. [Google Scholar] [CrossRef]
- de Luis Román, D.A.; Primo, D.; Aller, R.; Izaola, O. Association of the rs10830963 polymorphism in MTNR1B with fasting glucose, serum adipokine levels and components of metabolic syndrome in adult obese subjects. Nutr. Hosp. 2019, 36, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C.; Zhang, W.; Zabaneh, D.; Sehmi, J.; Jain, P.; McCarthy, M.I.; Froguel, P.; Ruokonen, A.; Balding, D.; Jarvelin, M.-R.; et al. Common genetic variation near melatonin receptor MTNR1B contributes to raised plasma glucose and increased risk of type 2 diabetes among Indian Asians and European Caucasians. Diabetes 2009, 58, 2703–2708. [Google Scholar] [CrossRef]
- Langenberg, C.; Pascoe, L.; Mari, A.; Tura, A.; Laakso, M.; Frayling, T.M.; Barroso, I.; Loos, R.J.F.; Wareham, N.J.; Walker, M.; et al. Common genetic variation in the melatonin receptor 1B gene (MTNR1B) is associated with decreased early-phase insulin response. Diabetologia 2009, 52, 1537–1542. [Google Scholar] [CrossRef]
- Rönn, T.; Wen, J.; Yang, Z.; Lu, B.; Du, Y.; Groop, L.; Hu, R.; Ling, C. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia 2009, 52, 830–833. [Google Scholar] [CrossRef]
- Bouatia-Naji, N.; Bonnefond, A.; Cavalcanti-Proença, C.; Sparsø, T.; Holmkvist, J.; Marchand, M.; Delplanque, J.; Lobbens, S.; Rocheleau, G.; Durand, E.; et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 2009, 41, 89–94. [Google Scholar] [CrossRef]
- Prokopenko, I.; Langenberg, C.; Florez, J.C.; Saxena, R.; Soranzo, N.; Thorleifsson, G.; Loos, R.J.F.; Manning, A.K.; Jackson, A.U.; Aulchenko, Y.; et al. Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 2009, 41, 77–81. [Google Scholar] [CrossRef]
- Wu, L.; Cui, L.; Tam, W.H.; Ma, R.C.W.; Wang, C.C. Genetic variants associated with gestational diabetes mellitus: A meta-analysis and subgroup analysis. Sci. Rep. 2016, 6, 30539. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Z.; Li, H.; Bai, J.; Liu, X.; Ye, H. Relationship between melatonin receptor 1B (rs10830963 and rs1387153) with gestational diabetes mellitus: A case-control study and meta-analysis. Arch. Gynecol. Obstet. 2016, 294, 55–61. [Google Scholar] [CrossRef]
- Huerta-Chagoya, A.; Vázquez-Cárdenas, P.; Moreno-Macías, H.; Tapia-Maruri, L.; Rodríguez-Guillén, R.; López-Vite, E.; García-Escalante, G.; Escobedo-Aguirre, F.; Parra-Covarrubias, A.; Cordero-Brieño, R.; et al. Genetic determinants for gestational diabetes mellitus and related metabolic traits in Mexican women. PLoS ONE 2015, 10, e0126408. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.D.; Goodarzi, M.O.; Langefeld, C.D.; Wang, N.; Guo, X.; Taylor, K.D.; Fingerlin, T.E.; Norris, J.M.; Buchanan, T.A.; Xiang, A.H.; et al. Genetic Variants Associated with Quantitative Glucose Homeostasis Traits Translate to Type 2 Diabetes in Mexican Americans: The GUARDIAN (Genetics Underlying Diabetes in Hispanics) Consortium. Diabetes 2015, 64, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xiang, A.H.; Trigo, E.; Takayanagi, M.; Beale, E.; Lawrence, J.M.; Hartiala, J.; Richey, J.M.; Allayee, H.; Buchanan, T.A.; et al. Genetic variation in MTNR1B is associated with gestational diabetes mellitus and contributes only to the absolute level of beta cell compensation in Mexican Americans. Diabetologia 2014, 57, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Benedict, C. Increased Risk of Myocardial Infarction Among Patients with Type 2 Diabetes Who Carry the Common rs10830963 Variant in the MTNR1B Gene. Diabetes Care 2020, 43, 2289–2292. [Google Scholar] [CrossRef] [PubMed]
- Huopio, H.; Cederberg, H.; Vangipurapu, J.; Hakkarainen, H.; Pääkkönen, M.; Kuulasmaa, T.; Heinonen, S.; Laakso, M. Association of risk variants for type 2 diabetes and hyperglycemia with gestational diabetes. Eur. J. Endocrinol. 2013, 169, 291–297. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, Z.-X.; Wang, Y.-C.; Ma, Y.-S.; Zhang, F.; Che, W.; Fu, D.; Wang, X.-F. Association between the melatonin receptor 1B gene polymorphism on the risk of type 2 diabetes, impaired glucose regulation: A meta-analysis. PLoS ONE 2012, 7, e50107. [Google Scholar] [CrossRef]
- Lyssenko, V.; Nagorny, C.L.F.; Erdos, M.R.; Wierup, N.; Jonsson, A.; Spégel, P.; Bugliani, M.; Saxena, R.; Fex, M.; Pulizzi, N.; et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 2009, 41, 82–88. [Google Scholar] [CrossRef]
- Tuomi, T.; Nagorny, C.L.F.; Singh, P.; Bennet, H.; Yu, Q.; Alenkvist, I.; Isomaa, B.; Östman, B.; Söderström, J.; Pesonen, A.-K.; et al. Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell Metab. 2016, 23, 1067–1077. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.-X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [CrossRef]
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation: Melatonin in human sleep and circadian rhythms. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Lardone, P.J.; Alvarez-Sanchez, S.N.; Guerrero, J.M.; Carrillo-Vico, A. Melatonin and glucose metabolism: Clinical relevance. Curr. Pharm. Des. 2014, 20, 4841–4853. [Google Scholar] [CrossRef]
- Bonnefond, A.; Froguel, P. Disentangling the Role of Melatonin and its Receptor MTNR1B in Type 2 Diabetes: Still a Long Way to Go? Curr. Diab. Rep. 2017, 17, 122. [Google Scholar] [CrossRef]
- Samanta, S. Physiological and pharmacological perspectives of melatonin. Arch. Physiol. Biochem. 2020, 1–22. [Google Scholar] [CrossRef]
- Sutradhar, S.; Deb, A.; Singh, S.S. Melatonin attenuates diabetes-induced oxidative stress in spleen and suppression of splenocyte proliferation in laboratory mice. Arch. Physiol. Biochem. 2020, 1–12. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef]
- Nogueira, T.C.; Lellis-Santos, C.; Jesus, D.S.; Taneda, M.; Rodrigues, S.C.; Amaral, F.G.; Lopes, A.M.S.; Cipolla-Neto, J.; Bordin, S.; Anhê, G.F. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology 2011, 152, 1253–1263. [Google Scholar] [CrossRef]
- Elbe, H.; Esrefoglu, M.; Vardi, N.; Taslidere, E.; Ozerol, E.; Tanbek, K. Melatonin, quercetin and resveratrol attenuates oxidative hepatocellular injury in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2015, 34, 859–868. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef]
- Rubio-Sastre, P.; Scheer, F.A.J.L.; Gómez-Abellán, P.; Madrid, J.A.; Garaulet, M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 2014, 37, 1715–1719. [Google Scholar] [CrossRef]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A.J.L. Melatonin Effects on Glucose Metabolism: Time To Unlock the Controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef]
- Mahlberg, R.; Tilmann, A.; Salewski, L.; Kunz, D. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology 2006, 31, 634–641. [Google Scholar] [CrossRef]
- Greendale, G.A.; Witt-Enderby, P.; Karlamangla, A.S.; Munmun, F.; Crawford, S.; Huang, M.; Santoro, N. Melatonin Patterns and Levels during the Human Menstrual Cycle and After Menopause. J. Endocr. Soc. 2020, 4, bvaa115. [Google Scholar] [CrossRef]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, Sweet, Salty, Sour and Umami Taste Perception Decreases with Age: Sex-Specific Analysis, Modulation by Genetic Variants and Taste-Preference Associations in 18 to 80 Year-Old Subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef]
- Ortega-Azorín, C.; Coltell, O.; Asensio, E.M.; Sorlí, J.V.; González, J.I.; Portolés, O.; Saiz, C.; Estruch, R.; Ramírez-Sabio, J.B.; Pérez-Fidalgo, A.; et al. Candidate Gene and Genome-Wide Association Studies for Circulating Leptin Levels Reveal Population and Sex-Specific Associations in High Cardiovascular Risk Mediterranean Subjects. Nutrients 2019, 11, 2751. [Google Scholar] [CrossRef]
- Coltell, O.; Asensio, E.M.; Sorlí, J.V.; Barragán, R.; Fernández-Carrión, R.; Portolés, O.; Ortega-Azorín, C.; Martínez-LaCruz, R.; González, J.I.; Zanón-Moreno, V.; et al. Genome-Wide Association Study (GWAS) on Bilirubin Concentrations in Subjects with Metabolic Syndrome: Sex-Specific GWAS Analysis and Gene-Diet Interactions in a Mediterranean Population. Nutrients 2019, 11, 90. [Google Scholar] [CrossRef]
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016, 1, 2. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Corella, D.; Demissie, S.; Cupples, L.A.; Couture, P.; Coltell, O.; Wilson, P.W.F.; Schaefer, E.J.; Tucker, K.L. Dietary fat intake determines the effect of a common polymorphism in the hepatic lipase gene promoter on high-density lipoprotein metabolism: Evidence of a strong dose effect in this gene-nutrient interaction in the Framingham Study. Circulation 2002, 106, 2315–2321. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Peschke, E. Melatonin, endocrine pancreas and diabetes. J. Pineal Res. 2008, 44, 26–40. [Google Scholar] [CrossRef]
- Boden, G.; Ruiz, J.; Urbain, J.L.; Chen, X. Evidence for a circadian rhythm of insulin secretion. Am. J. Physiol. Endocrinol. Metab. 1996, 271, E246–E252. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62, e12377. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Holzapfel, C.; Siegrist, M.; Rank, M.; Langhof, H.; Grallert, H.; Baumert, J.; Irimie, C.; Klopp, N.; Wolfarth, B.; Illig, T.; et al. Association of a MTNR1B gene variant with fasting glucose and HOMA-B in children and adolescents with high BMI-SDS. Eur. J. Endocrinol. 2011, 164, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, Y.-K.; Qin, L.-Y.; Wei, Q.; Liu, N.; Jiang, M.; Yu, H.-P.; Yu, X.-Y. A functional polymorphism rs10830963 in melatonin receptor 1B associated with the risk of gestational diabetes mellitus. Biosci. Rep. 2019, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet. Med. 2014, 31, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Diabetes in Pregnancy Working Group; Maternal Medicine Clinical Study Group; Royal College of Obstetricians and Gynaecologists, UK. Gestational diabetes: Opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020, 8, 793–800. [Google Scholar] [CrossRef]
- Garcia-Gil, C.; Cortés-Majó, M.; Garcia Nieto, A.; Rosado Martín, M.; Nájera, E. Epidemiological appraisal of the active role of women in the decline of infant mortality in Spain during the twentieth century. Soc. Sci. Med. 1989, 29, 1351–1362. [Google Scholar] [CrossRef]
- Dashti, H.S.; Vetter, C.; Lane, J.M.; Smith, M.C.; Wood, A.R.; Weedon, M.N.; Rutter, M.K.; Garaulet, M.; Scheer, F.A.J.L.; Saxena, R. Assessment of MTNR1B Type 2 Diabetes Genetic Risk Modification by Shift Work and Morningness-Eveningness Preference in the UK Biobank. Diabetes 2020, 69, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Nisa, H.; Qi, K.H.T.; Leng, J.; Zhou, T.; Liu, H.; Li, W.; Wang, L.; Li, N.; Hu, G.; Qi, L. The Circadian Rhythm-Related MTNR1B Genotype, Gestational Weight Gain, and Postpartum Glycemic Changes. J. Clin. Endocrinol. Metab. 2018, 103, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 1378) | Men (n = 543) | Women (n = 835) | p | |
|---|---|---|---|---|
| Age (years) | 41.3 ± 14.0 | 40.3 ± 13.7 | 42.0 ± 14.2 | 0.030 |
| Weight (Kg) | 73.6 ± 16.5 | 83.5 ± 15.9 | 67.1 ± 13.5 | <0.001 |
| BMI (Kg/m2) | 26.3 ± 5.2 | 27.2 ± 4.9 | 25.7 ± 5.2 | <0.001 |
| Waist circumference (cm) | 88.7 ± 14.8 | 95.7 ± 13.7 | 84.1 ± 13.6 | <0.001 |
| SBP (mm Hg) | 124.5 ± 17.3 | 130.9 ± 16.0 | 120.2 ± 16.8 | <0.001 |
| DBP (mm Hg) | 77.7 ± 10.3 | 80.3 ± 10.8 | 76.1 ± 9.5 | <0.001 |
| Total cholesterol (mg/dL) | 204.5 ± 39.7 | 200.0 ± 39.5 | 207.5 ± 9.6 | 0.001 |
| LDL-C (mg/dL) | 130.9 ± 33.2 | 131.5 ± 33.7 | 130.7 ± 32.8 | 0.681 |
| HDL-C (mg/dL) | 59.9 ± 14.3 | 52.4 ± 11.3 | 64.8 ± 13.9 | <0.001 |
| Triglycerides (mg/dL) | 103.3 ± 58.3 | 117.7 ± 69.9 | 93.9 ± 47.1 | <0.001 |
| Fasting glucose (mg/dL) | 92.1 ± 1 6.9 | 94.0 ± 17.9 | 90.8 ± 16.2 | 0.001 |
| Type-2 diabetes: n, % | 53 (3.8) | 23 (4.2) | 30 (3.6) | 0.544 |
| Obesity: n, % | 301 (21.8) | 134 (24.7) | 167 (20.0) | 0.040 |
| MTNR1B-rs10830963: n, % | 0.409 | |||
| CC | 665 (48.3) | 270 (49.7) | 395 (47.3) | |
| CG | 565 (41.0) | 211 (38.9) | 345 (41.3) | |
| GG | 148 (10.7) | 62 (11.4) | 86 (10.3) |
| Fasting Glucose (mg/dL) | |||||||
|---|---|---|---|---|---|---|---|
| Total Population (n = 1378) | ≤ 41 years (n = 684) | > 41 years (n = 694) | pinteraction | ||||
| CC | CG | GG | CC | CG | GG | ||
| Codominant model | 84.38 ± 0.47 | 86.54 ± 0.51 | 91.65 ± 1.45 | 98.06 ± 1.29 | 98.48 ± 1.10 | 96.39 ± 1.40 | |
| p1: 2.60 × 10−9 | p1: 0.739 | pint-1: 0.008 | |||||
| p2: 2.60 × 10−9 | p2: 0.985 | pint-2: 0.006 | |||||
| p3: 2.10 × 0−9 | p3: 0.969 | pint-3: 0.004 | |||||
| Additive model | Regression coefficient (B ± SE) per G allele | Regression coefficient (B ± SE) per G allele | |||||
| B1: 3.16 ± 0.52 | p1: 1.58 × 10−9 | B1: −0.38 ± 1.16 | p1: 0.744 | pint-1: 0.005 | |||
| B2: 2.99 ± 0.43 | p2: 5.90 × 10−10 | B2: −0.12 ± 0.87 | p2: 0.805 | pint-2: 0.002 | |||
| B3: 3.00 ± 0.47 | p3: 4.61 × 10−10 | B3: −0.21 ± 0.86 | p3: 0.870 | pint-3: 0.001 | |||
| Non-diabetic subjects (n = 1325) | ≤ 41 years (n = 682) | > 41 years (n = 643) | pinteraction | ||||
| CC | CG | GG | CC | CG | GG | ||
| Codominant model | 84.39 ± 0.46 | 86.54 ± 0.51 | 90.46 ± 0.96 | 93.64 ± 0.62 | 94.87 ± 0.65 | 95.36 ± 1.29 | |
| p1: 2.04 × 10−8 | p1: 0.276 | pint-1: 0.043 | |||||
| p2: 2.06 × 10−8 | p2: 0.166 | pint-2: 0.049 | |||||
| p3: 1.77 × 10−8 | p3: 0.187 | pint-3: 0.039 | |||||
| Additive model | Regression coefficient (B ± SE) per G allele | Regression coefficient (B ± SE) per G allele | |||||
| B1: 2.81 ± 0.58 | p1: 5.04 × 10−9 | B1: 0.99 ± 0.64 | p1: 0.119 | pint-1: 0.021 | |||
| B2: 2.79 ± 0.49 | p2: 4.02 × 10−9 | B2: 1.12 ± 0.62 | p2: 0.079 | pint-2: 0.029 | |||
| B3: 2.79 ± 0.47 | p3: 3.29 × 10−9 | B3: 1.00 ± 0.61 | p3: 0.109 | pint-3: 0.020 | |||
| Fasting Glucose (mg/dL) | ||||||
|---|---|---|---|---|---|---|
| Total Population (n = 1001) | Non-Diabetic Subjects (n = 537) | Diabetic Subjects (n = 464) | ||||
| CC | CG | GG | CC | CG | GG | |
| Codominant model | 99.77 ± 1.15 | 101.18 ± 1.31 | 99.67 ± 3.09 | 144.10 ± 2.92 | 144.39 ± 3.12 | 144.05 ± 6.24 |
| p1: 0.688 | p2: 0.997 | |||||
| Additive model | Regression coefficient (B ± SE) per G allele | Regression coefficient (B ± SE) per G allele | ||||
| B1: 0.65 ± 1.23 | p1: 0.614 | B2: 0.08 ± 3.01 | p2: 0.979 | |||
| Strata | Genotypes (%) | |||||
|---|---|---|---|---|---|---|
| CC | CG | GG | ||||
| Non-diabetic subjects | 52.3 | 40.8 | 6.9 | |||
| Type-2 diabetic subjects | 47.8 | 41.6 | 10.6 | OR and 95% CI | p | |
| ptrend: 0.046 | Model 1: | 1.22 (1.03–1.48) | 0.046 | |||
| Model 2: | 1.22 (1.01–1.49) | 0.046 | ||||
| Model 3: | 1.22 (1.03–1.48) | 0.048 | ||||
| Fasting Glucose (mg/dL) | ||||||
|---|---|---|---|---|---|---|
| Total Population (n = 444) | Non-Diabetic Subjects (n = 271) | Diabetic Subjects (n = 173) | ||||
| CC | CG | GG | CC | CG | GG | |
| Codominant model | 99.87 ± 1.21 | 100.36 ± 1.23 | 99.96 ± 2.68 | 133.35 ± 3.91 | 130.84 ± 4.14 | 121.23 ± 7.12 |
| p1: 0.940 | p1: 0.798 | |||||
| Additive model | Regression coefficient (B ± SE) per G allele | Regression coefficient (B ± SE) per G allele | ||||
| B1: 0.22 ± 1.23 | p1: 0.857 | B2: 5.08 ± 3.69 | p2: 0.170 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorlí, J.V.; Barragán, R.; Coltell, O.; Portolés, O.; Pascual, E.C.; Ortega-Azorín, C.; González, J.I.; Estruch, R.; Saiz, C.; Pérez-Fidalgo, A.; et al. Chronological Age Interacts with the Circadian Melatonin Receptor 1B Gene Variation, Determining Fasting Glucose Concentrations in Mediterranean Populations. Additional Analyses on Type-2 Diabetes Risk. Nutrients 2020, 12, 3323. https://doi.org/10.3390/nu12113323
Sorlí JV, Barragán R, Coltell O, Portolés O, Pascual EC, Ortega-Azorín C, González JI, Estruch R, Saiz C, Pérez-Fidalgo A, et al. Chronological Age Interacts with the Circadian Melatonin Receptor 1B Gene Variation, Determining Fasting Glucose Concentrations in Mediterranean Populations. Additional Analyses on Type-2 Diabetes Risk. Nutrients. 2020; 12(11):3323. https://doi.org/10.3390/nu12113323
Chicago/Turabian StyleSorlí, Jose V., Rocío Barragán, Oscar Coltell, Olga Portolés, Eva C. Pascual, Carolina Ortega-Azorín, José I. González, Ramon Estruch, Carmen Saiz, Alejandro Pérez-Fidalgo, and et al. 2020. "Chronological Age Interacts with the Circadian Melatonin Receptor 1B Gene Variation, Determining Fasting Glucose Concentrations in Mediterranean Populations. Additional Analyses on Type-2 Diabetes Risk" Nutrients 12, no. 11: 3323. https://doi.org/10.3390/nu12113323
APA StyleSorlí, J. V., Barragán, R., Coltell, O., Portolés, O., Pascual, E. C., Ortega-Azorín, C., González, J. I., Estruch, R., Saiz, C., Pérez-Fidalgo, A., Ordovas, J. M., & Corella, D. (2020). Chronological Age Interacts with the Circadian Melatonin Receptor 1B Gene Variation, Determining Fasting Glucose Concentrations in Mediterranean Populations. Additional Analyses on Type-2 Diabetes Risk. Nutrients, 12(11), 3323. https://doi.org/10.3390/nu12113323









