Energy Availability in Male and Female Elite Wheelchair Athletes over Seven Consecutive Training Days
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Body Composition and Resting Energy Expenditure
2.3. Self-Reported Food and Training Diary
2.4. Calculations
2.5. Guidelines of Macronutrient Intake
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Energy Availability and Energy Intake
4.2. Macronutrient Intake before and after Training Sessions
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burke, L.M.; Lundy, B.; Fahrenholtz, I.L.; Melin, A.K. Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Logue, D.; Madigan, S.M.; Delahunt, E.; Heinen, M.; Donnell, S.J.M.; Corish, C.A. Low energy availability in athletes: A review of prevalence, dietary patterns, physiological health, and sports performance. Sports Med. 2018, 48, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Nazem, T.G.; Ackerman, K.E. The female athlete triad. Sports Health 2012, 4, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Yeager, K.K.; Agostini, R.; Nattiv, A.; Drinkwater, B. The female athlete triad: Disordered eating, amenorrhea, osteoporosis. Med. Sci. Sports Exerc. 1993, 25, 775–777. [Google Scholar] [CrossRef]
- Brook, E.M.; Tenforde, A.S.; Broad, E.M.; Matzkin, E.G.; Yang, H.Y.; Collins, J.E.; Blauwet, C.A. Low energy availability, menstrual dysfunction, and impaired bone health: A survey of elite para athletes. Scand. J. Med. Sci. Sports 2019, 29, 678–685. [Google Scholar] [CrossRef]
- Williams, N.I.; Statuta, S.M.; Austin, A. Female athlete triad: Future directions for energy availability and eating disorder research and practice. Clin. Sports Med. 2017, 36, 671–686. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International olympic committee (IOC) consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Barrack, M.T.; Nattiv, A.; Fredericson, M. Parallels with the female athlete triad in male athletes. Sports Med. 2016, 46, 171–182. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29, S7–S15. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Holtzman, B.; Cooper, K.M.; Flynn, E.F.; Bruinvels, G.; Tenforde, A.S.; Popp, K.L.; Simpkin, A.J.; Parziale, A.L. Low energy availability surrogates correlate with health and performance consequences of relative energy deficiency in sport. Br. J. Sports Med. 2019, 53, 628–633. [Google Scholar] [CrossRef]
- Blauwet, C.A.; Brook, E.M.; Tenforde, A.S.; Broad, E.; Hu, C.H.; Abdu-Glass, E.; Matzkin, E.G. Low energy availability, menstrual dysfunction, and low bone mineral density in individuals with a disability: Implications for the para athlete population. Sports Med. 2017, 47, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Figel, K.; Pritchett, K.; Pritchett, R.; Broad, E. Energy and nutrient issues in athletes with spinal cord injury: Are they at risk for low energy availability? Nutrients 2018, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; Tenforde, A.S.; Parziale, A.L.; Holtzman, B.; Ackerman, K.E. Endocrine effects of relative energy deficiency in sport. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Capling, L.; Beck, K.L.; Gifford, J.A.; Slater, G.; Flood, V.M.; O’Connor, H. Validity of dietary assessment in athletes: A systematic review. Nutrients 2017, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Melin, A.K.; Heikura, I.A.; Tenforde, A.; Mountjoy, M. Energy availability in athletics: Health, performance, and physique. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 152–164. [Google Scholar] [CrossRef]
- Stellingwerff, T. Case Study: Body composition periodization in an olympic-level female middle-distance runner over a 9-year career. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 428–433. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Morton, J.P.; Burke, L.M. A framework for periodized nutrition for athletics. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 141–151. [Google Scholar] [CrossRef]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Pelly, F.E.; Broad, E.M.; Stuart, N.; Holmes, M.A. Resting energy expenditure in male athletes with a spinal cord injury. J. Spinal Cord Med. 2018, 41, 208–215. [Google Scholar] [CrossRef]
- Nana, A.; Slater, G.J.; Stewart, A.D.; Burke, L.M. Methodology review: Using dual-energy X-ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 198–215. [Google Scholar] [CrossRef]
- Keil, M.; de Zepetnek, J.O.T.; Brooke-Wavell, K.; Goosey-Tolfrey, V.L. Measurement precision of body composition variables in elite wheelchair athletes, using dual-energy X-ray absorptiometry. Eur. J. Sport Sci. 2016, 16, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Camenzind-Frey, E.; Zuberbühler, C. Schweizerisches Fotobuch-Livre Photo Suisse-Manuale Fotografico Svizzero. menuCH; Bundesamt für Gesundheit BAG und Bundesamt für Lebensmittelsicherheit und Veterinäwesen BLV: Bern, Switzerland, 2014. [Google Scholar]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Hottenrott, K.; Neumann, G. Trainingswissenschaft: Ein Lehrbuch in 14 Lektionen; Meyer & Meyer Verlag: Aachen, Germany, 2010. [Google Scholar]
- Ginis, K.M.; Latimer, A.E. The Leisure Time Physical Activity Questionnaire for People with Spinal Cord Injury (LTPAQ-SCI). 2007. Available online: http://sciactioncanada.ca/ (accessed on 25 October 2020).
- Black, A.E.; Coward, W.A.; Cole, T.J.; Prentice, A.M. Human energy expenditure in affluent societies: An analysis of 574 doubly-labelled water measurements. Eur. J. Clin. Nutr. 1996, 50, 72–92. [Google Scholar]
- Conger, S.A.; Bassett, D.R. A compendium of energy costs of physical activities for individuals who use manual wheelchairs. Adapt. Phys. Activ. Q 2011, 28, 310–325. [Google Scholar] [CrossRef]
- Jequier, E. Pathways to obesity. Int. J. Obes. Relat. Metab. Disord. 2002, 26 (Suppl. 2), 12–17. [Google Scholar] [CrossRef]
- Broad, E. Sports Nutrition for Paralympic Athletes; Taylor & Francis Group; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Muia, E.N.; Wright, H.H.; Onywera, V.O.; Kuria, E.N. Adolescent elite Kenyan runners are at risk for energy deficiency, menstrual dysfunction and disordered eating. J. Sports Sci. 2016, 34, 598–606. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, A.B.; Skouby, S.; Moller, S.S.; Sundgot-Borgen, J.; Faber, J.; Sidelmann, J.J.; Aziz, M.; Sjodin, A. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sports 2015, 25, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, S.; Meloche, R.D. Energy availability of female varsity volleyball players. Int. J. Sport Nutr. Exerc. Metab. 2012, 23, 24–30. [Google Scholar]
- Heikura, I.A.; Uusitalo, A.L.T.; Stellingwerff, T.; Bergland, D.; Mero, A.A.; Burke, L.M. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 403–411. [Google Scholar] [CrossRef]
- Koehler, K.; Achtzehn, S.; Braun, H.; Mester, J.; Schaenzer, W. Comparison of self-reported energy availability and metabolic hormones to assess adequacy of dietary energy intake in young elite athletes. Appl. Physiol. Nutr. Metab. 2013, 38, 725–733. [Google Scholar] [CrossRef]
- Day, J.; Wengreen, H.; Heath, E. Prevalence of low energy availability in collegiate female runners and implementation of nutrition education intervention. Sports Nutr. Ther. 2016, 1, 1. [Google Scholar] [CrossRef]
- Keay, N.; Francis, G.; Hind, K. Low energy availability assessed by a sport-specific questionnaire and clinical interview indicative of bone health, endocrine profile and cycling performance in competitive male cyclists. BMJ Open Sport Exerc. Med. 2018, 4, e000424. [Google Scholar] [CrossRef] [PubMed]
- Krempien, J.L.; Barr, S.I. Risk of nutrient inadequacies in elite Canadian athletes with spinal cord injury. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 417–425. [Google Scholar] [CrossRef]
- Tomey, K.M.; Chen, D.M.; Wang, X.; Braunschweig, C.L. Dietary intake and nutritional status of urban community-dwelling men with paraplegia. Arch. Phys. Med. Rehabil. 2005, 86, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Gerrish, H.R.; Broad, E.; Lacroix, M.; Ogan, D.; Pritchett, R.C.; Pritchett, K. Nutrient intake of elite canadian and american athletes with spinal cord injury. Int. J. Exerc. Sci. 2017, 10, 1018–1028. [Google Scholar] [PubMed]
- Madden, R.F.; Shearer, J.; Parnell, J.A. Evaluation of dietary intakes and supplement use in paralympic athletes. Nutrients 2017, 9, 1266. [Google Scholar] [CrossRef]
- Sabour, H.; Javidan, A.N.; Vafa, M.R.; Shidfar, F.; Nazari, M.; Saberi, H.; Rahimi, A.; Razavi, H.E. Calorie and macronutrients intake in people with spinal cord injuries: An analysis by sex and injury-related variables. Nutrition 2012, 28, 143–147. [Google Scholar] [CrossRef]
- Krempien, J.L.; Barr, S.I. Eating attitudes and behaviours in elite Canadian athletes with a spinal cord injury. Eat. Behav. 2012, 13, 36–41. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Meredith, K.; Cox, G.R.; Hopkins, W.G.; Burke, L.M. Variability in estimation of self-reported dietary intake data from elite athletes resulting from coding by different sports dietitians. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 152–165. [Google Scholar] [CrossRef]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Collins, E.G.; Gater, D.; Kiratli, J.; Butler, J.; Hanson, K.; Langbein, W.E. Energy cost of physical activities in persons with spinal cord injury. Med. Sci. Sports Exerc. 2010, 42, 691–700. [Google Scholar] [CrossRef]
| Energy Expenditure (kcal kg−1 min) | ||||
|---|---|---|---|---|
| Sport | Recovery | GA 1 | GA 2 | Intensive/Race |
| Handcycling or arm cranking | 2.8 | 4.4 | 6.0 | 9.3 |
| Wheelchair racing | 2.4 | 4.3 | 6.1 | 7.9 |
| Badminton/tennis session | 1.6 | 2.5 | 3.3 | 4.1 |
| Shooting | Game | |||
| Basketball session | 3.2 | 6.1 | ||
| Resistance | Circuit | |||
| Strength training session | 2.2 | 2.3 | ||
| Participants | |||
|---|---|---|---|
| All (n = 14) | Male (n = 8) | Female (n = 6) | |
| Age [years] | 34.9 ± 9.4 | 35.8 ± 7.6 | 33.8 ± 12.1 |
| Height [m] | 1.71 ± 0.14 | 1.80 ± 0.08 | 1.59 ± 0.10 * |
| Body mass [kg] | 64.9 ± 12 | 66.5 ± 12.1 | 62.8 ± 12.6 |
| Body-mass index [kg m−2] | 22.3 ± 4.4 | 20.4 ± 2.7 | 24.9 ± 5.1 |
| Fat-free mass [kg] | 46.2 ± 9.7 | 51.7 ± 8.8 | 38.9 ± 4.9 * |
| Fat mass [kg] | 18.7 ± 2.3 | 14.8 ± 4.2 | 23.9 ± 7.9 * |
| REE [kcal d−1] | 1368 ± 259 | 1506 ± 222 | 1184 ± 185 * |
| Disability | |||
| Years since injury | 19.6 ± 7.8 | 18.4 ± 6.8 | 21.2 ± 9.5 |
| Paraplegia | 11 (79%) | 6 (75%) | 5 (83%) |
| Tetraplegia | 2 (14%) | 2 (25%) | 0 (0%) |
| AIS A | 9 (64%) | 5 (63%) | 4 (67%) |
| AIS B-D | 4 (29%) | 3 (38%) | 1 (17%) |
| Participant | Sport | FFM (kg) | EA Day No. 1 | EA Day No. 2 | EA Day No. 3 | EA Day No. 4 | EA Day No. 5 | EA Day No. 6 | EA Day No. 7 | Weekly Mean of EA | No. of Days of LEA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M01 | Badminton | 52.5 | 41.3 | 31.7 | 41.8 | 33.4 | 22.3 | 27.0 | 29.3 | 32.4 ± 7.2 | 3 |
| M02 | Paracycling | 57.6 | 37.9 | 52.8 | 28.8 | 41.3 | 17.2 | 41.2 | 44.4 | 37.7 ± 11.5 | 2 |
| M03 | Paracycling | 46.6 | 55.1 | 54.1 | 61.0 | 58.8 | 40.1 | 50.0 | 33.0 | 50.3 ± 10.2 | 0 |
| M04 | Paracycling | 36.5 | 32.4 | 51.0 | 38.1 | 23.6 | 50.1 | 39.4 | 40.0 | 39.2 ± 9.6 | 1 |
| M05 | Paracycling | 47.4 | 32.8 | 49.0 | 26.9 | 37.9 | 32.4 | 42.8 | 29.3 | 35.9 ± 7.8 | 2 |
| M06 | Athletics | 53.9 | 41.7 | 23.6 | 29.5 | 33.5 | 22.9 | 28.0 | 36.2 | 30.8 ± 6.8 | 4 |
| M07 | Paracycling | 52.4 | 25.5 | 34.2 | 38.9 | 28.2 | 40.0 | 33.0 | 37.3 | 33.9 ± 5.4 | 2 |
| M08 | Basketball | 66.6 | 36.6 | 37.8 | 20.4 | 36.1 | 17.3 | 33.4 | 19.7 | 28.8 ± 9.1 | 3 |
| Male athletes | 36.1 ± 6.7 * | 30.4% | |||||||||
| F01 | Badminton | 44.7 | 28.1 | 33.3 | 12.7 | 26.3 | 64.3 | 23.2 | 21.9 | 30.0 ± 16.4 | 5 |
| F02 | Badminton | 42.0 | 24.8 | 19.7 | 14.8 | 20.8 | 20.2 | 10.9 | 20.5 | 18.8 ± 4.5 | 7 |
| F03 | Athletics | 30.3 | 38.9 | 19.8 | 43.4 | 42.3 | 3.9 | 19.1 | n/a | 27.9 ± 16.0 | 3 |
| F04 | Athletics | 37.9 | 43.6 | 16.1 | 10.2 | 24.4 | 17.6 | 6.6 | −0.7 | 16.8 ± 14.3 | 6 |
| F05 | Tennis | 39.5 | 19.7 | 26.1 | 12.7 | 20.1 | 23.8 | 24.4 | 26.5 | 21.9 ± 4.9 | 7 |
| F06 | Paracycling | 39.2 | 49.4 | 36.6 | 39.7 | 32.7 | 44.2 | 22.8 | 21.9 | 35.3 ± 10.3 | 2 |
| Female athletes | 25.1 ± 7.1 | 73.2% | |||||||||
| All athletes | 31.4 ± 8.7 | 48.6% |
| Participants | |||
|---|---|---|---|
| Energy Balance | All (n = 14) | Male (n = 8) | Female (n = 6) |
| Weekly training [h] | 13.0 ± 4.9 | 13.3 ± 6.4 | 12.8 ± 2.2 |
| EEE [kcal·d−1] | 423 ± 142 | 440 ± 152 | 400.0 ± 138 |
| EI [kcal·d−1] | 1891 ± 574 | 2276 ± 368 | 1377 ± 337 * |
| TEE [kcal·d−1] | 1918 ± 366 | 2107 ± 355 | 1666 ± 196 * |
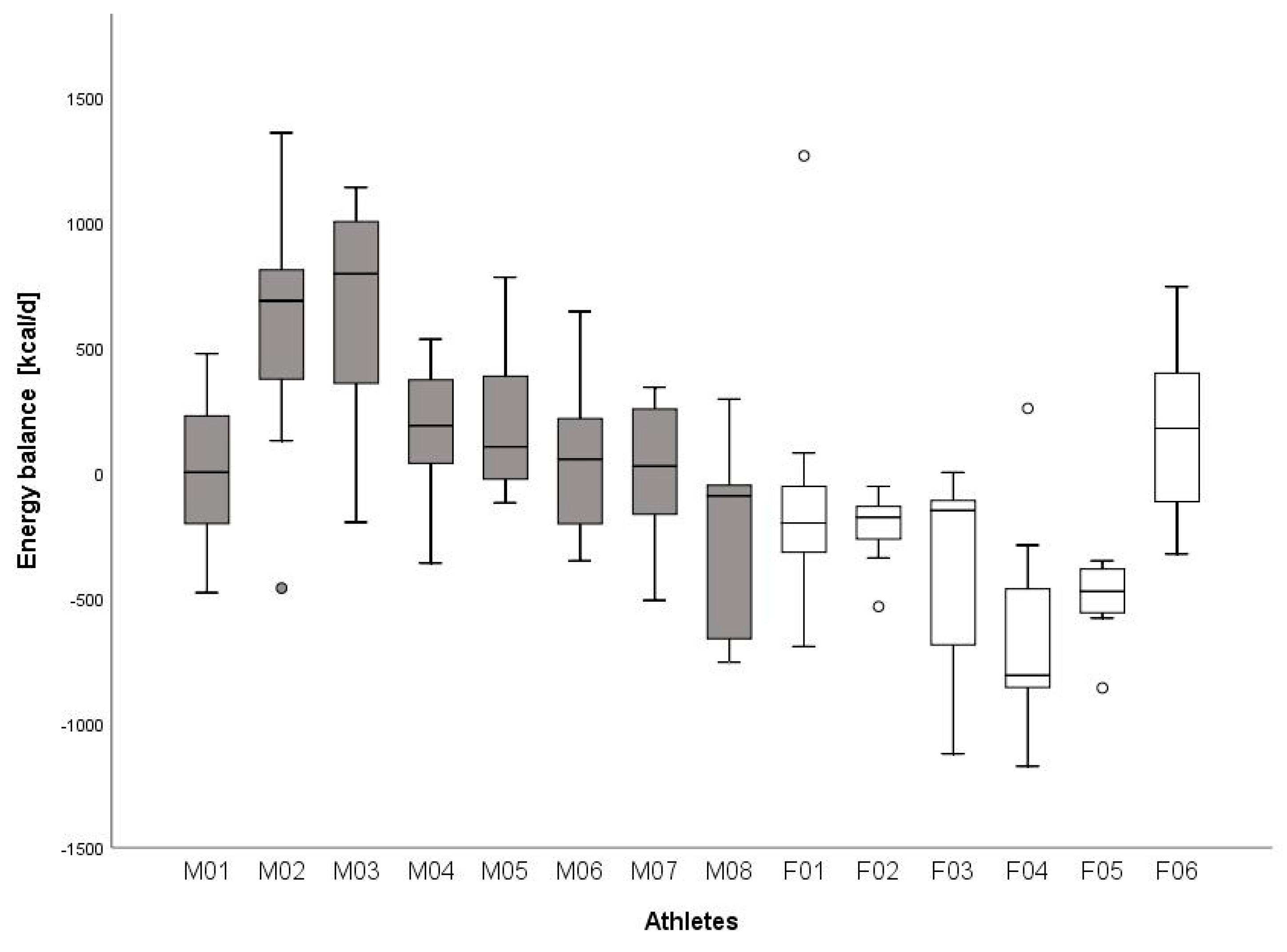
| Energy balance [kcal·d−1] | −27 ± 376 | 169 ± 305 | −289 ± 305 * |
| EA classified (n (%)) | |||
| >45 kcal kg−1 FFM day−1 | 1/14 (7%) | 1/8 (13%) | 0/6 (0%) |
| 30–45 kcal kg−1 FFM day−1 | 7/14 (50%) | 6/8 (75%) | 1/6 (17%) |
| <30 kcal kg−1 FFM day−1 | 6/14 (43%) | 1/8 (13%) | 5/6 (83%) |
| Participants | |||
|---|---|---|---|
| Total Daily Macronutrient Intake | All (n = 14) | Male (n = 8) | Female (n = 6) |
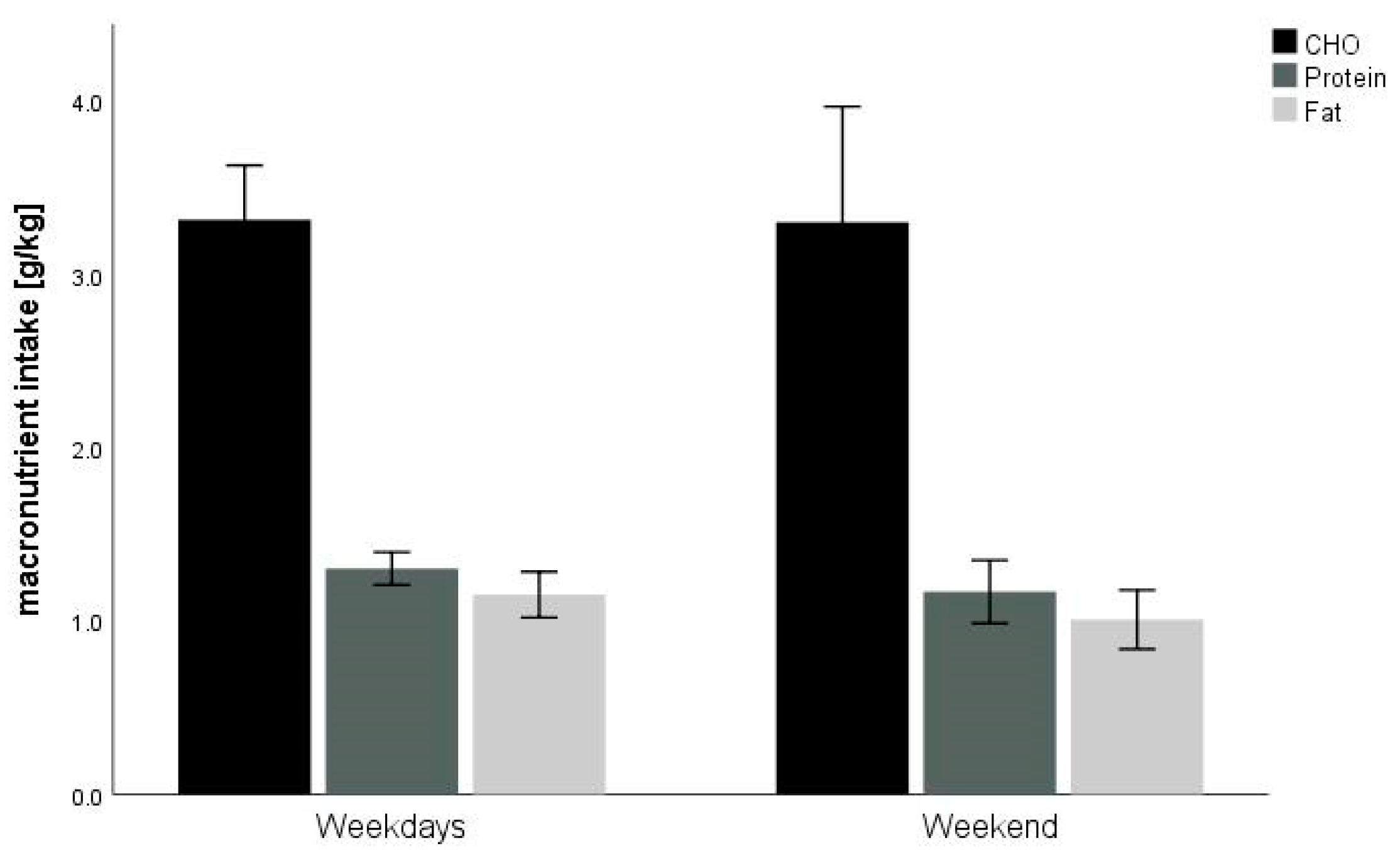
| Carbohydrate, g per day | 211 ± 70 | 260 ± 16 | 146 ± 13 * |
| g kg−1 BM | 3.3 ± 1.2 | 4.0 ± 0.3 | 2.4 ± 0.3 * |
| Protein, g per day | 82 ± 17 | 91 ± 5 | 70 ± 6 * |
| g kg−1 BM | 1.3 ± 0.2 | 1.4 ± 0.4 | 1.1 ± 0.1 * |
| Fat, g per day | 72 ± 27 | 86 ± 8 | 52 ± 8 * |
| g kg−1 BM | 1.1 ± 0.4 | 1.3 ± 0.1 | 0.9 ± 0.2 * |
| Number of days, where guidelines for total daily protein and carbohydrate intake were met (n (%)) | All (n = 97 days) | Male (n = 56 days) | Female (n = 41 days) |
| Guidelines | No. days | No. days | No. days |
| Protein intake ≥ 1.2 g kg−1 BM | 55 (57%) | 39 (70%) | 16 (39%) |
| Carbohydrate intake ≥ 3 g kg−1 BM | 55 (57%) | 46 (82%) | 9 (22%) |
| Number of sessions, where pre- and post-training guidelines were met (n (%)) | All (n = 74 sessions) | Male (n = 40 sessions) | Female (n = 34 sessions) |
| Guidelines | No. sessions | No. sessions | No. sessions |
| Protein intake: 20–25 g after intensive or strength training session | 36 (49%) | 22 (55%) | 14 (41%) |
| Carbohydrate intake: 1–4 g kg−1 BM before endurance training session | 49 (66%) | 25 (63%) | 24 (71%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egger, T.; Flueck, J.L. Energy Availability in Male and Female Elite Wheelchair Athletes over Seven Consecutive Training Days. Nutrients 2020, 12, 3262. https://doi.org/10.3390/nu12113262
Egger T, Flueck JL. Energy Availability in Male and Female Elite Wheelchair Athletes over Seven Consecutive Training Days. Nutrients. 2020; 12(11):3262. https://doi.org/10.3390/nu12113262
Chicago/Turabian StyleEgger, Thomas, and Joelle Leonie Flueck. 2020. "Energy Availability in Male and Female Elite Wheelchair Athletes over Seven Consecutive Training Days" Nutrients 12, no. 11: 3262. https://doi.org/10.3390/nu12113262
APA StyleEgger, T., & Flueck, J. L. (2020). Energy Availability in Male and Female Elite Wheelchair Athletes over Seven Consecutive Training Days. Nutrients, 12(11), 3262. https://doi.org/10.3390/nu12113262






