Inulin Supplementation Disturbs Hepatic Cholesterol and Bile Acid Metabolism Independent from Housing Temperature
Abstract
1. Introduction
2. Materials and Methods
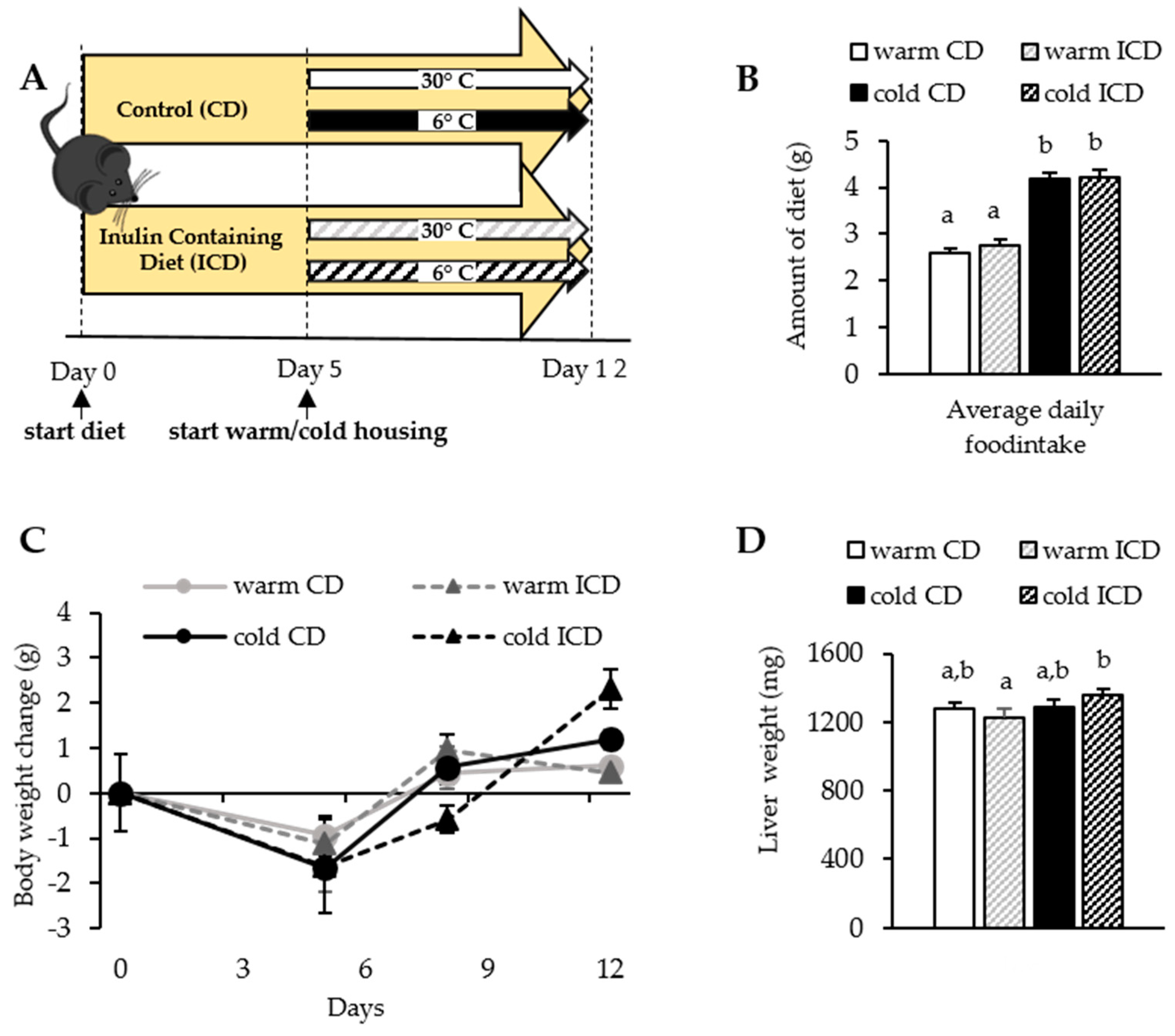
2.1. Experimental Animals, Housing Conditions, Diets, and Animal Experiment
2.2. Plasma Analysis
2.3. Quantification of Fecal Cholesterol
2.4. Liver Lipids
2.5. Gene Expression Analysis
2.6. Bile Acid Measurement
2.7. Histology
2.8. GC-FID Based Analysis of SCFAs
2.9. Satistical Analysis
3. Results
3.1. Short-Term Dietary Inulin Supplementation Does Not Affect Food Intake and Body Weight Gain
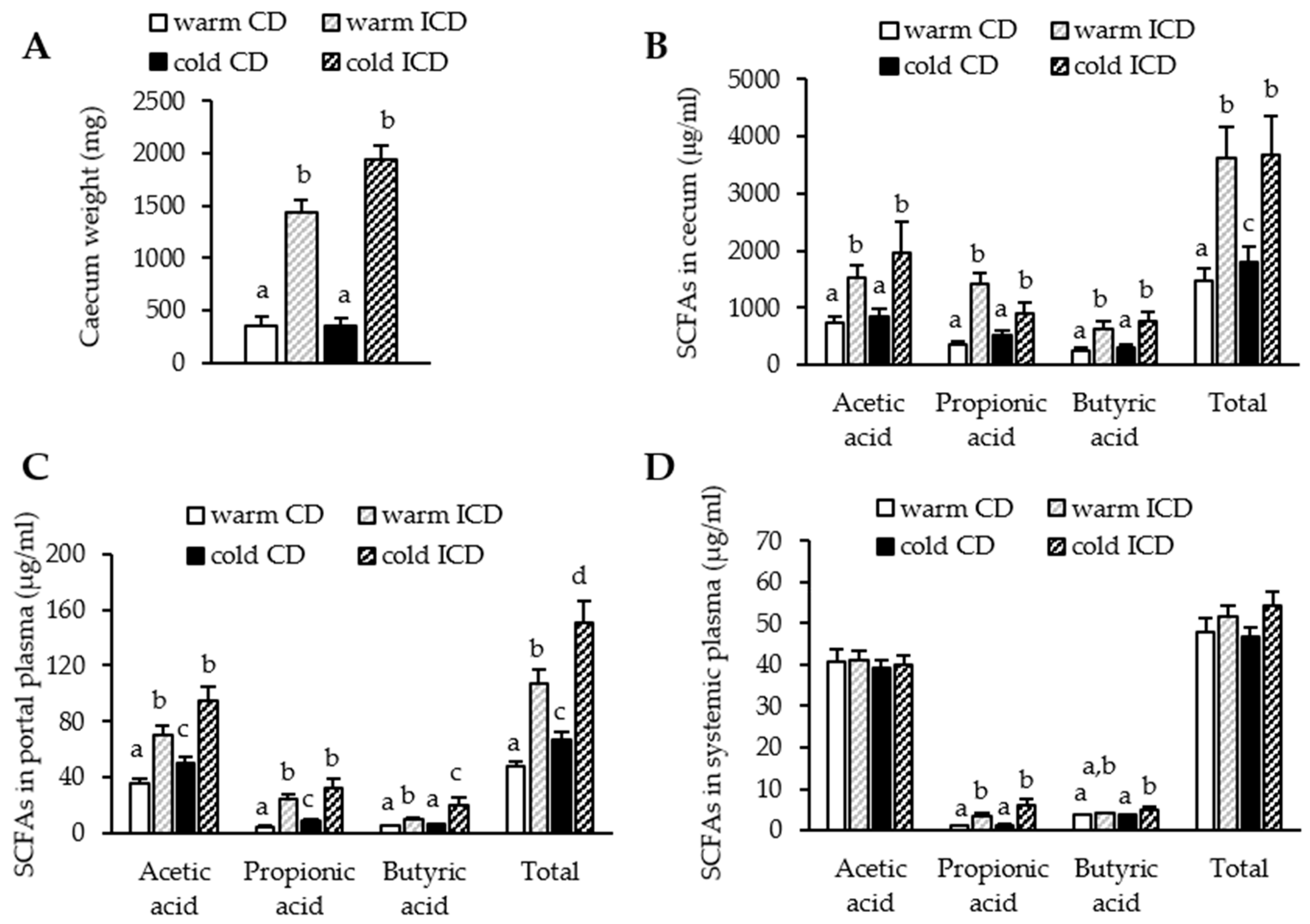
3.2. Short-Term Dietary Inulin Supplementation Leads to an Increased Production of SCFAs
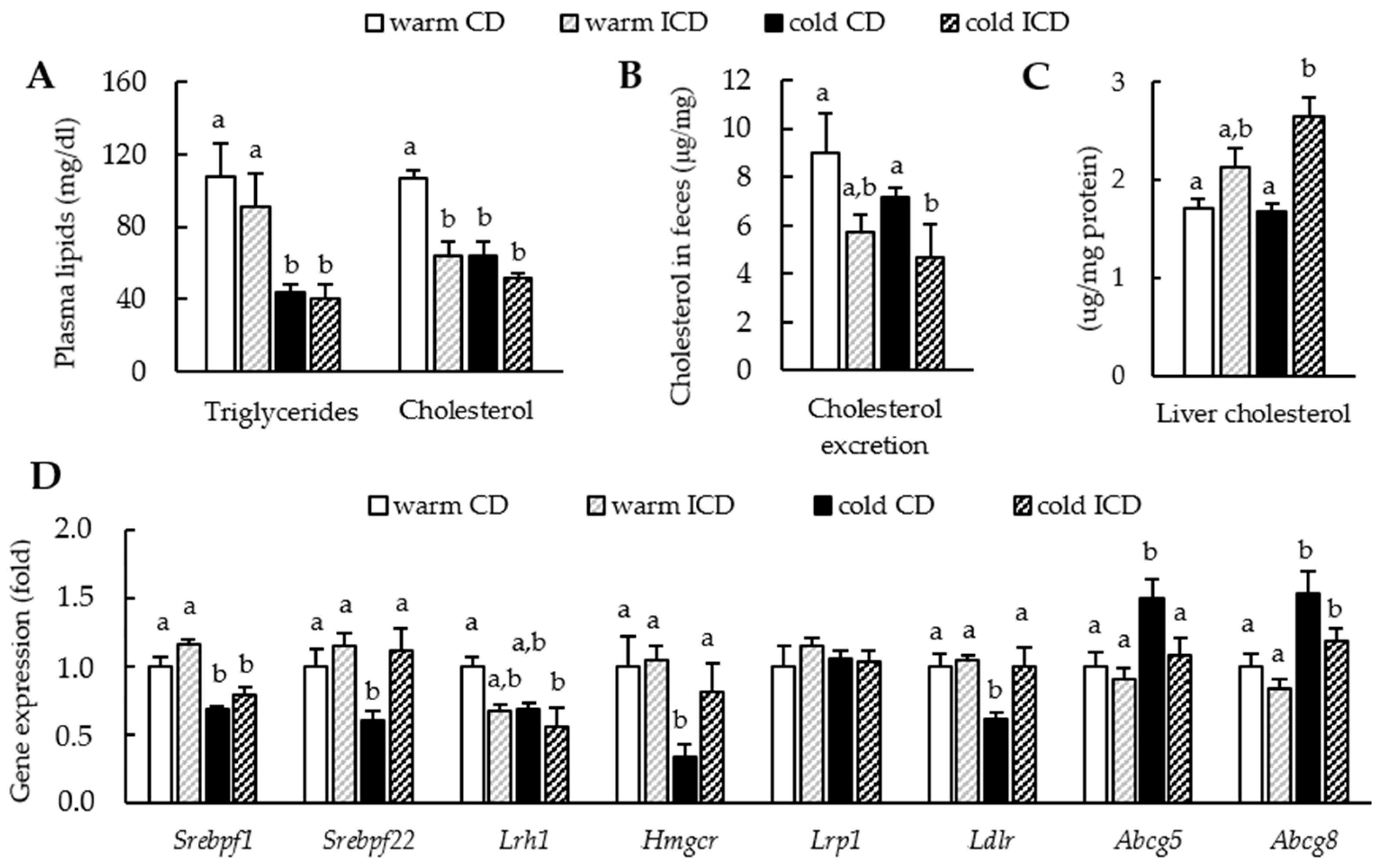
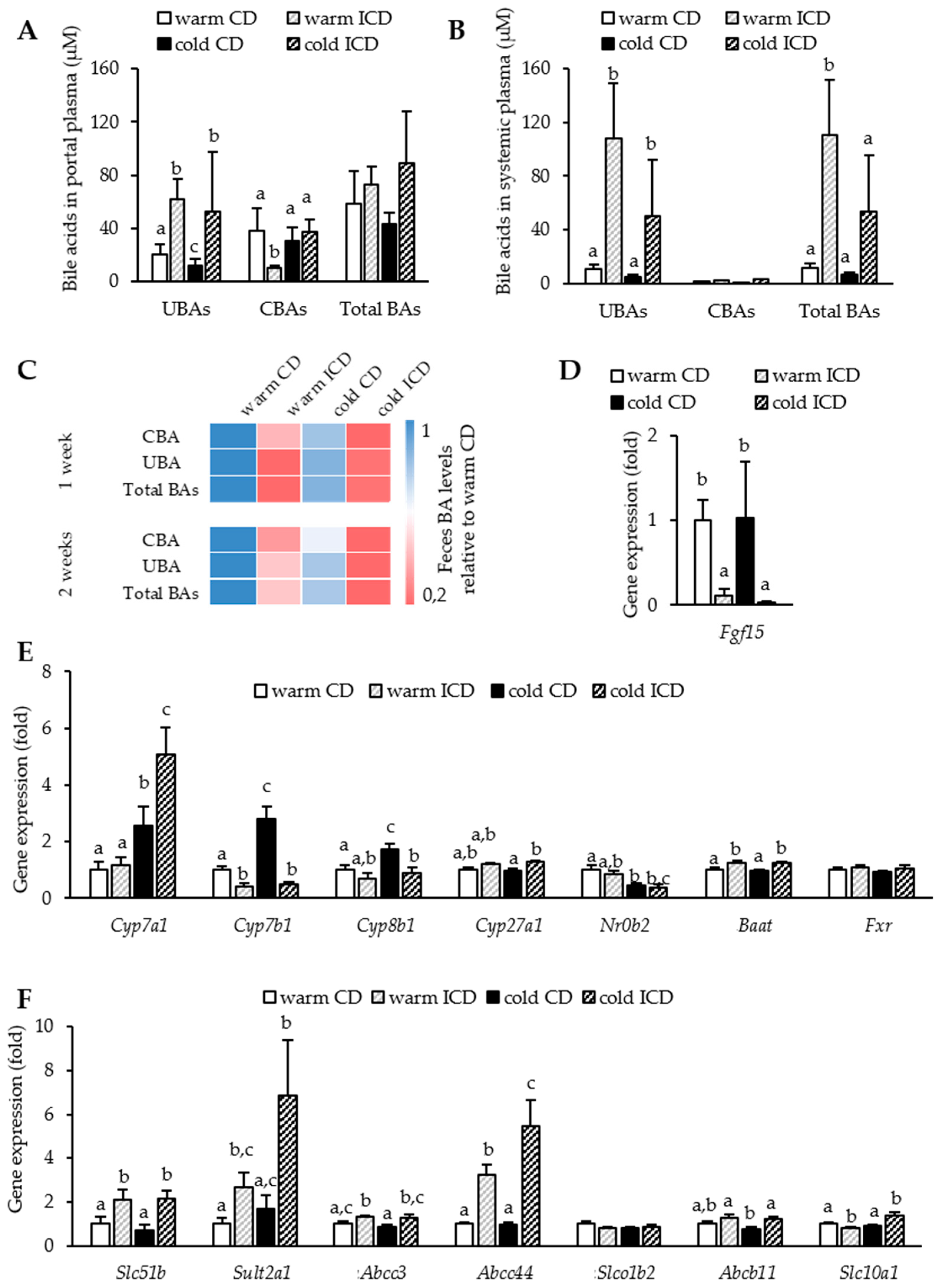
3.3. Effects of Short-Term Inulin Supplementation on Cholesterol and Bile Acid Metabolism
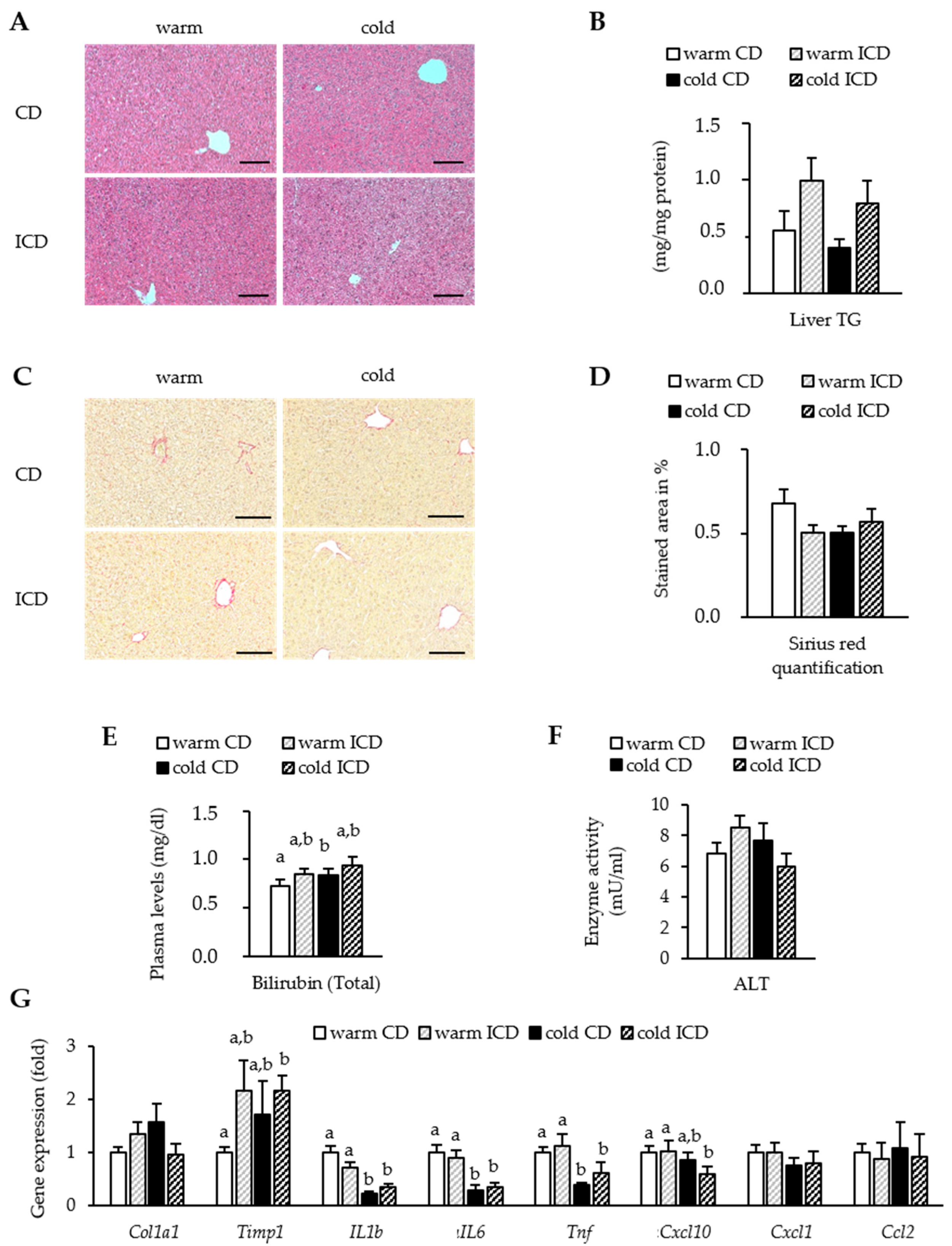
3.4. Effects of Short-Term Inulin Supplementation on Liver Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanford, K.I.; Middelbeek, R.J. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Bruns, O.T. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, A.E.; Johnson, J.W. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Worthmann, A.; John, C. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Berbee, J.F.; Boon, M.R. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Rieckhoff, D. Dietary inulin lowers plasma cholesterol and triacylglycerol and alters biliary bile acid profile in hamsters. J. Nutr. 1998, 128, 1937–1943. [Google Scholar] [CrossRef]
- Hiel, S.; Neyrinck, A.M. Inulin Improves Postprandial Hypertriglyceridemia by Modulating Gene Expression in the Small Intestine. Nutrients 2018, 10, 532. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 712–722. [Google Scholar] [CrossRef]
- Zou, J.; Chassaing, B. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53 e44. [Google Scholar] [CrossRef]
- Liber, A.; Szajewska, H. Effects of inulin-type fructans on appetite, energy intake, and body weight in children and adults: Systematic review of randomized controlled trials. Ann. Nutr. Metab. 2013, 63, 42–54. [Google Scholar] [CrossRef]
- Guess, N.D.; Dornhorst, A. A randomized controlled trial: The effect of inulin on weight management and ectopic fat in subjects with prediabetes. Nutr. Metab. 2015, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.; Viardot, A. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol. Metab. 2017, 6, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.X. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARgamma-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Jin, C.J.; Sellmann, C. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br. J. Nutr. 2015, 114, 1745–1755. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Miles, J.P.; Zou, J. Supplementation of Low- and High-fat Diets with Fermentable Fiber Exacerbates Severity of DSS-induced Acute Colitis. Inflamm. Bowel Dis. 2017, 23, 1133–1143. [Google Scholar] [CrossRef]
- Hoving, L.R.; de Vries, M.R. The Prebiotic Inulin Aggravates Accelerated Atherosclerosis in Hypercholesterolemic APOE*3-Leiden Mice. Nutrients 2018, 10, 172. [Google Scholar] [CrossRef]
- Janssen, A.W.F.; Houben, T. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: A potential role for bile acids. J. Lipid Res. 2017, 58, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Yeoh, B.S. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694 e622. [Google Scholar] [CrossRef]
- Mistry, R.H.; Gu, F. Effect of the prebiotic fiber inulin on cholesterol metabolism in wildtype mice. Sci. Rep. 2018, 8, 13238. [Google Scholar] [CrossRef]
- Hoving, L.R.; Katiraei, S. The prebiotic inulin modulates gut microbiota but does not ameliorate atherosclerosis in hypercholesterolemic APOE*3-Leiden.CETP mice. Sci. Rep. 2018, 8, 16515. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S. Vancomycin prevents fermentable fiber-induced liver cancer in mice with dysbiotic gut microbiota. Gut Microbes 2020, 11, 1077–1091. [Google Scholar] [CrossRef]
- Bartelt, A.; Orlando, P. Altered endocannabinoid signalling after a high-fat diet in Apoe(-/-) mice: Relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia 2011, 54, 2900–2910. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Wegner, K.; Just, S. Rapid analysis of bile acids in different biological matrices using LC-ESI-MS/MS for the investigation of bile acid transformation by mammalian gut bacteria. Anal. Bioanal. Chem. 2017, 409, 1231–1245. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, R. Hepatocellular Carcinoma: Causes, Mechanism of Progression and Biomarkers. Curr. Chem. Genom. Transl. Med. 2018, 12, 9–26. [Google Scholar] [CrossRef]
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Halilbasic, E.; Claudel, T. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 2013, 58, 155–168. [Google Scholar] [CrossRef]
- Navarro, V.J.; Khan, I. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Li, X. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Zhao, T.T. Sodium Butyrate Improves Liver Glycogen Metabolism in Type 2 Diabetes Mellitus. J. Agric. Food Chem. 2019, 67, 7694–7705. [Google Scholar] [CrossRef]
- Nilsson, N.E.; Kotarsky, K. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauly, M.J.; Rohde, J.K.; John, C.; Evangelakos, I.; Koop, A.C.; Pertzborn, P.; Tödter, K.; Scheja, L.; Heeren, J.; Worthmann, A. Inulin Supplementation Disturbs Hepatic Cholesterol and Bile Acid Metabolism Independent from Housing Temperature. Nutrients 2020, 12, 3200. https://doi.org/10.3390/nu12103200
Pauly MJ, Rohde JK, John C, Evangelakos I, Koop AC, Pertzborn P, Tödter K, Scheja L, Heeren J, Worthmann A. Inulin Supplementation Disturbs Hepatic Cholesterol and Bile Acid Metabolism Independent from Housing Temperature. Nutrients. 2020; 12(10):3200. https://doi.org/10.3390/nu12103200
Chicago/Turabian StylePauly, Mira J., Julia K. Rohde, Clara John, Ioannis Evangelakos, Anja Christina Koop, Paul Pertzborn, Klaus Tödter, Ludger Scheja, Joerg Heeren, and Anna Worthmann. 2020. "Inulin Supplementation Disturbs Hepatic Cholesterol and Bile Acid Metabolism Independent from Housing Temperature" Nutrients 12, no. 10: 3200. https://doi.org/10.3390/nu12103200
APA StylePauly, M. J., Rohde, J. K., John, C., Evangelakos, I., Koop, A. C., Pertzborn, P., Tödter, K., Scheja, L., Heeren, J., & Worthmann, A. (2020). Inulin Supplementation Disturbs Hepatic Cholesterol and Bile Acid Metabolism Independent from Housing Temperature. Nutrients, 12(10), 3200. https://doi.org/10.3390/nu12103200



