The Cardiotonic Steroid Marinobufagenin Is a Predictor of Increased Left Ventricular Mass in Obesity: The African-PREDICT Study
Abstract
1. Introduction
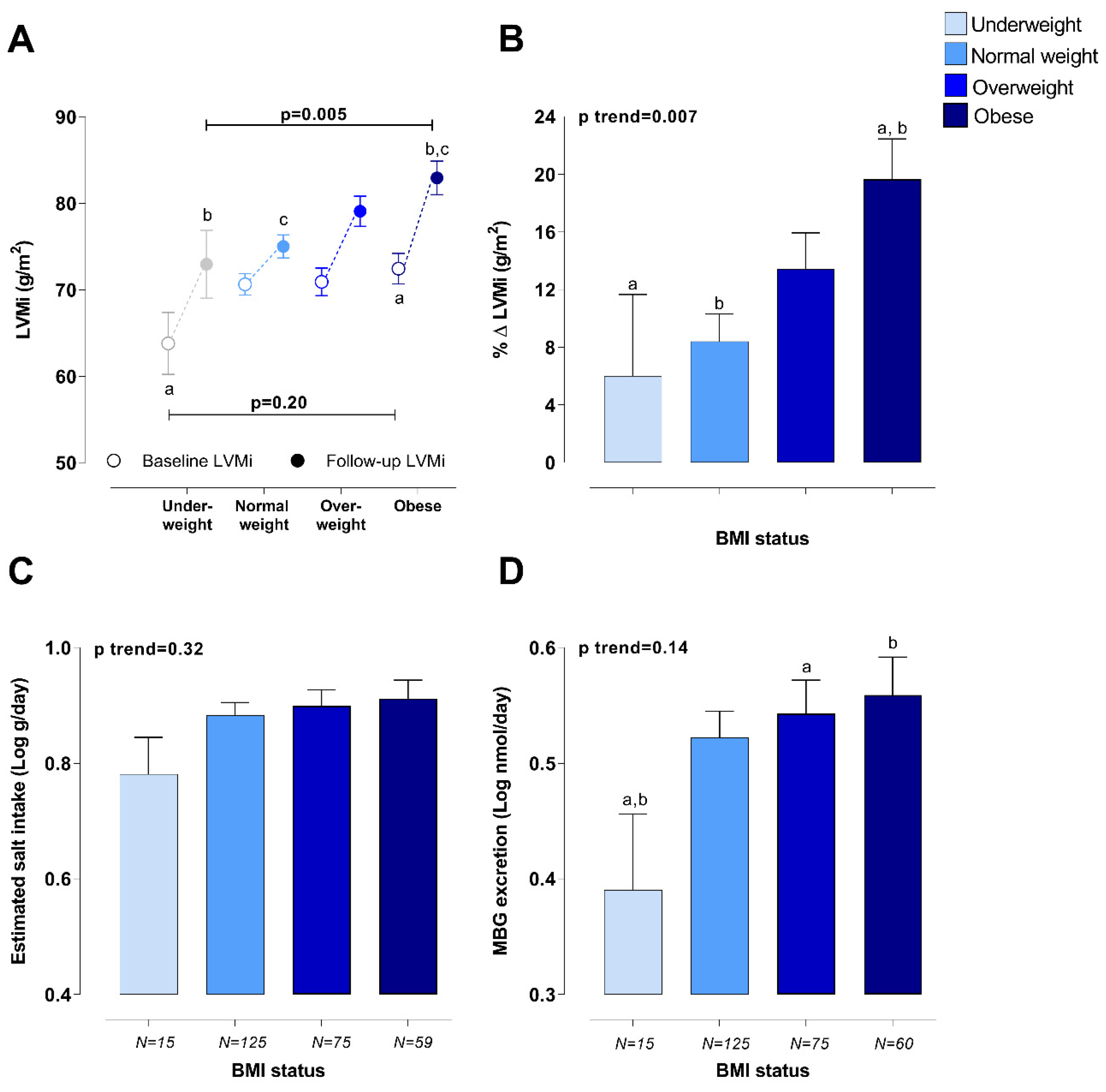
2. Results
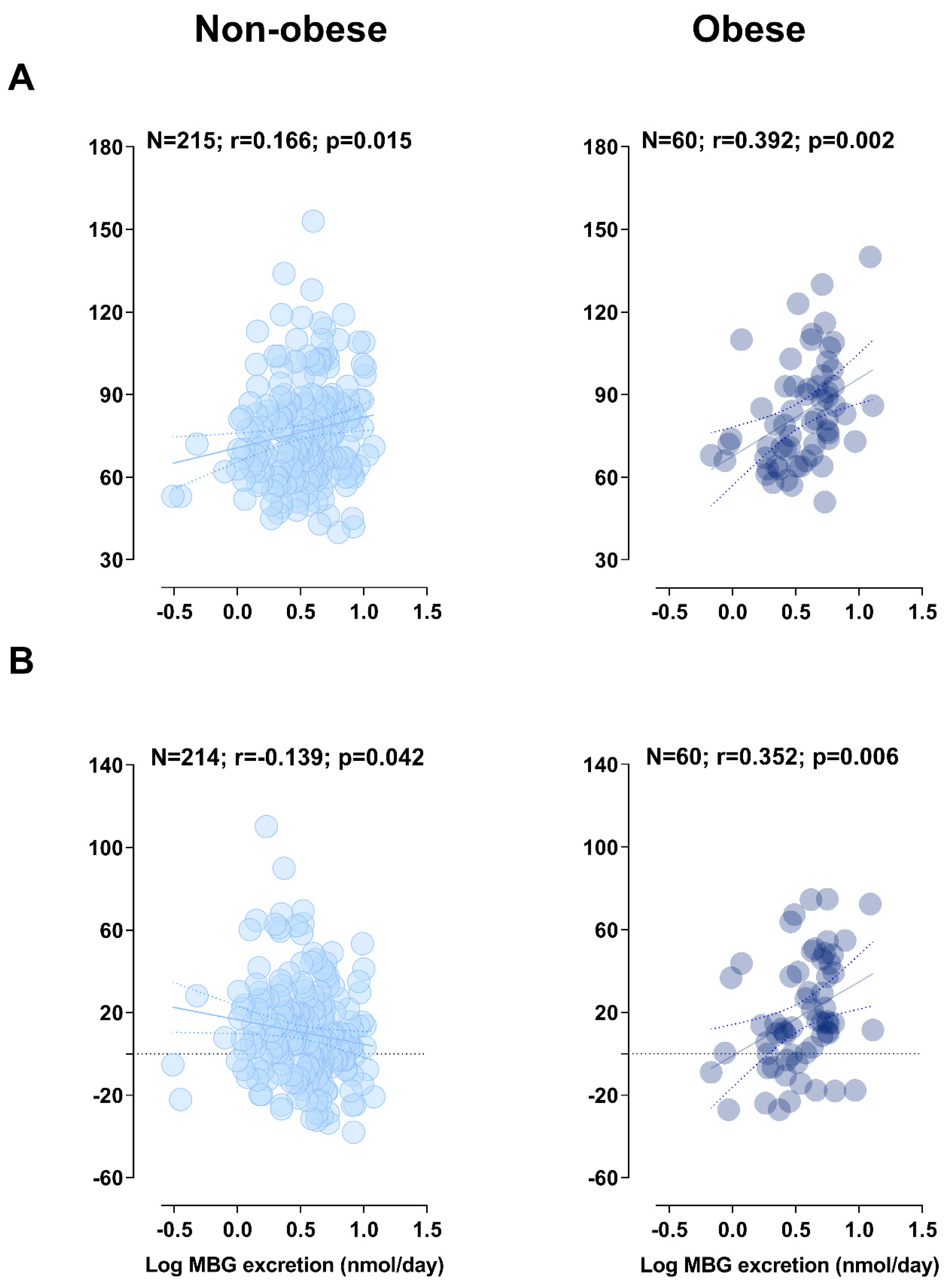
2.1. Pearson, Partial, and Multiple Regression Analyses
2.2. Sensitivity Analyses
2.2.1. Estimated Salt Intake
2.2.2. Estradiol
3. Discussion
4. Strengths and Limitations
5. Materials and Methods
5.1. Study Design and Methodology
5.2. Questionnaire and Anthropometric Data
5.3. Cardiovascular Measurements
5.4. Biochemical Sampling and Biochemical Analyses
5.5. Statistical Analyses
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Na+/K+-ATPase | sodium-potassium adenosine triphosphatase |
| PAHO/WHO | Pan American Health Organization/World Health Organization |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| LVMi | Left ventricular mass index |
| WHtR | Waist/height ratio |
| EDVi | End diastolic volume index |
| TGF-β | Transforming growth factor beta |
| ANP | Atrial natriuretic peptide |
| BMI | Body mass index |
| BSA | Body surface area |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| ECG | Echocardiogram |
| GGT | Gamma glutamyl transferase |
| HDL | High density lipoprotein |
| LDL | Low density lipoprotein |
| MBG | Marinobufagenin |
| SBP | Systolic blood pressure |
| SVi | Stroke volume index |
| ER | Estradiol receptor |
| WC | Waist circumference |
References
- Non-communicable Disease Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metab. Clin. Exp. 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, V.; Stabouli, S.; Bouldin, M.; Low, A.; Toumanidis, S.; Zakopoulos, N. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension 2005, 45, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Li, Y.; Soliman, E.Z. Association of obesity phenotypes with electrocardiographic left ventricular hypertrophy in the general population. J. Electrocardiol. 2018, 51, 1125–1130. [Google Scholar] [CrossRef]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol 2018, 3, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Carmo, J.; Silva, A.; Juncos, L.; Wang, Z.; Hall, J. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 75–88. [Google Scholar] [CrossRef]
- Strazzullo, P.; Barbato, A.; Galletti, F.; Barba, G.; Siani, A.; Iacone, R.; D’Elia, L.; Russo, O.; Versiero, M.; Farinaro, E.; et al. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the Olivetti Heart study. J. Hypertens. 2006, 24, 1633–1639. [Google Scholar] [CrossRef][Green Version]
- Aurigemma, G.P.; Simone, G.D.; Fitzgibbons, T.P. Cardiac remodeling in obesity. Circ. Cardiovasc. Imaging 2013, 6, 142–152. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt reduction to prevent hypertension and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef]
- Strauss, M.; Smith, W.; Fedorova, O.V.; Schutte, A.E. The Na(+)K(+)-ATPase inhibitor marinobufagenin and early cardiovascular risk in humans: A review of recent evidence. Curr. Hypertens. Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Zernetkina, V.I.; Shilova, V.Y.; Grigorova, Y.N.; Juhasz, O.; Wei, W.; Marshall, C.A.; Lakatta, E.G.; Bagrov, A.Y. Synthesis of an endogenous steroidal Na pump inhibitor marinobufagenin, implicated in human cardiovascular diseases, is initiated by CYP27A1 via bile acid pathway. Circ. Cardiovasc. Genet. 2015, 8, 736–745. [Google Scholar] [CrossRef]
- Strauss, M.; Smith, W.; Wei, W.; Bagrov, A.Y.; Fedorova, O.V.; Schutte, A.E. Large artery stiffness is associated with marinobufagenin in young adults: The African-PREDICT study. J. Hypertens. 2018, 36, 2333. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Talan, M.I.; Agalakova, N.I.; Lakatta, E.G.; Bagrov, A.Y. Endogenous ligand of α1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride–dependent hypertension. Circulation 2002, 105, 1122–1127. [Google Scholar] [CrossRef]
- Elkareh, J.; Kennedy, D.J.; Yashaswi, B.; Vetteth, S.; Shidyak, A.; Kim, E.G.R.; Smaili, S.; Periyasamy, S.M.; Hariri, I.M.; Fedorova, L.; et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension 2007, 49, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Elkareh, J.; Periyasamy, S.M.; Shidyak, A.; Vetteth, S.; Schroeder, J.; Raju, V.; Hariri, I.M.; El-Okdi, N.; Gupta, S.; Fedorova, L. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase c and fli-1: Implications for uremic cardiomyopathy. Am. J. Physiol. Renal Physiol. 2009, 296, F1219–F1226. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Shilova, V.; Petrashevskaya, N.N.; Zernetkina, V.I.; Grigorova, Y.N.; Marshall, C.A.; Fenner, R.C.; Lehrmann, E.; Wood, W.H.; et al. Monoclonal antibody to marinobufagenin downregulates TGFβ; profibrotic signaling in left ventricle and kidney and reduces tissue remodeling in salt-sensitive hypertension. JAHA 2019, 8, e012138. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.D.; Brickman, C.; Nawab, A.; Cottrill, C.; Snoad, B.; Lakhani, H.V.; Jelcick, A.; Henderson, B.; Bhardwaj, N.N.; Sanabria, J.R.; et al. The adipocyte Na/K-ATPase oxidant amplification loop is the central regulator of western diet-induced obesity and associated comorbidities. Sci. Rep. 2019, 9, 7927. [Google Scholar] [CrossRef]
- Strauss, M.; Smith, W.; Kruger, R.; Wei, W.; Fedorova, O.V.; Schutte, A.E. Marinobufagenin and left ventricular mass in young adults: The african-PREDICT study. Eur. J. Prev. Cardiol. 2018, 25, 1587–1595. [Google Scholar] [CrossRef]
- Obradovic, M.; Zafirovic, S.; Jovanovic, A.; Milovanovic, E.S.; Mousa, S.A.; Labudovic-Borovic, M.; Isenovic, E.R. Effects of 17β-estradiol on cardiac Na+/K+-ATPase in high fat diet fed rats. Mol. Cell. Endocrinol. 2015, 416, 46–56. [Google Scholar] [CrossRef]
- Liu, C.; Bai, Y.; Chen, Y.; Wang, Y.; Sottejeau, Y.; Liu, L.; Li, X.; Lingrel, J.B.; Malhotra, D.; Cooper, C.J. Reduction of na/k-atpase potentiates marinobufagenin-induced cardiac dysfunction and myocyte apoptosis. J. Biol. Chem. 2012, 287, 16390–16398. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Bjelogrlic, P.; Rizzo, M.; Katsiki, N.; Haidara, M.; Stewart, A.J.; Jovanovic, A.; Isenovic, E.R. Effects of obesity and estradiol on Na+/K+-ATPase and their relevance to cardiovascular diseases. J. Endocrinol. 2013, 218, R13–R23. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000; p. 9. ISBN 9241208945. [Google Scholar]
- Ekoru, K.; Murphy, G.; Young, E.; Delisle, H.; Jerome, C.; Assah, F.; Longo-Mbenza, B.; Nzambi, J.; On’Kin, J.; Buntix, F.; et al. Deriving an optimal threshold of waist circumference for detecting cardiometabolic risk in Sub-Saharan Africa. Int. J. Obes. 2017, 42. [Google Scholar] [CrossRef]
- Iannello, S.; Milazzo, P.; Belfiore, F. Animal and human tissue Na, K-ATPase in obesity and diabetes: A new proposed enzyme regulation. Am. J. Med. Sci. 2007, 333, 1–9. [Google Scholar] [CrossRef]
- Dzurba, A.; Ziegelhöffer, A.; Vrbjar, N.; Styk, J.; Slezák, J. Estradiol modulates the sodium pump in the heart sarcolemma. Mol. Cell. Biochem. 1997, 176, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The role of estrogen receptors in cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 4314. [Google Scholar] [CrossRef] [PubMed]
- Belo, N.; Sairam, M.; Reis, A. Impairment of the natriuretic peptide system in follitropin receptor knockout mice and reversal by estradiol: Implications for obesity-associated hypertension in menopause. Endocrinology 2008, 149, 1399–1406. [Google Scholar] [CrossRef]
- Jankowski, M.; Rachelska, G.; Donghao, W.; McCann, S.M.; Gutkowska, J. Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proc. Natl. Acad. Sci. USA 2001, 98, 11765–11770. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Kashkin, V.A.; Zakharova, I.O.; Lakatta, E.G.; Bagrov, A.Y. Age-associated increase in salt sensitivity is accompanied by a shift in the atrial natriuretic peptide modulation of the effect of marinobufagenin on renal and vascular sodium pump. J. Hypertens. 2012, 30, 1817. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Talan, M.I.; Agalakova, N.I.; Lakatta, E.G.; Bagrov, A.Y. Coordinated shifts in Na/K-ATPase isoforms and their endogenous ligands during cardiac hypertrophy and failure in NaCl-sensitive hypertension. J. Hypertens. 2004, 22, 389–397. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Agalakova, N.I.; Kashkin, V.A.; Fedorova, O.V. Endogenous cardiotonic steroids and differential patterns of sodium pump inhibition in NaCl-loaded salt-sensitive and normotensive rats. Am. J. Hypertens. 2009, 22, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Shrestha, K.; Sheehey, B.; Li, X.S.; Guggilam, A.; Wu, Y.; Finucan, M.; Gabi, A.; Medert, C.M.; Westfall, K.; et al. Elevated plasma marinobufagenin, an endogenous cardiotonic steroid, is associated with right ventricular dysfunction and nitrative stress in heart failure. Circ. Heart. Fail. 2015, 8, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Chen, Y.; Huang, W.; Viterna, J.; Liu, J.; Westfall, K.; Tian, J.; Bartlett, D.J.; Tang, W.H.; Xie, Z.; et al. CD36 and Na/K-ATPase-α1 form a proinflammatory signaling loop in kidney. Hypertension 2013, 61, 216–224. [Google Scholar] [CrossRef]
- Vasan, R.S. Cardiac function and obesity. Heart 2003, 89, 1127–1129. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012; ISBN 9789241504836. [Google Scholar]
- Fedorova, O.; Doris, P.; Bagrov, A. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin. Exp. Hypertens. 1998, 20, 581–591. [Google Scholar] [CrossRef]
- Grigorova, Y.N.; Wei, W.; Petrashevskaya, N.; Zernetkina, V.; Juhasz, O.; Fenner, R.; Gilbert, C.; Lakatta, E.G.; Shapiro, J.I.; Bagrov, A.Y. Dietary sodium restriction reduces arterial stiffness, vascular TGF-β-dependent fibrosis and marinobufagenin in young normotensive rats. Int. J. Mol. Sci. 2018, 19, 3168. [Google Scholar] [CrossRef]
- Strauss, M.; Smith, W.; Wei, W.; Fedorova, O.V.; Schutte, A.E. Autonomic activity and its relationship with the endogenous cardiotonic steroid marinobufagenin: The african-PREDICT study. Nutr. Neurosci. 2019, 1–11. [Google Scholar] [CrossRef]
- Strauss-Kruger, M.; Smith, W.; Wei, W.; Bagrov, A.Y.; Fedorova, O.V.; Schutte, A.E. Microvascular function in non-dippers: Potential involvement of the salt sensitivity biomarker, marinobufagenin—The african-PREDICT study. J. Clin. Hypertens. 2020, 22, 86–94. [Google Scholar] [CrossRef]
- Jablonski, K.L.; Fedorova, O.V.; Racine, M.L.; Geolfos, C.J.; Gates, P.E.; Chonchol, M.; Fleenor, B.S.; Lakatta, E.G.; Bagrov, A.Y.; Seals, D.R. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin. J. Am. Soc. Nephrol. 2013, 8, 1952–1959. [Google Scholar] [CrossRef]
- Ogihara, T.; Asano, T.; Ando, K.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Onishi, Y.; Fujishiro, M.; Abe, M.; et al. High-salt diet enhances insulin signaling and induces insulin resistance in dahl salt-sensitive rats. Hypertension 2002, 40, 83–89. [Google Scholar] [CrossRef]
- Schutte, A.E.; Gona, P.N.; Delles, C.; Uys, A.S.; Burger, A.; Mels, C.M.; Kruger, R.; Smith, W.; Fourie, C.M.; Botha, S.; et al. The African Prospective study on the Early Detection and Identification of Cardiovascular Disease and Hypertension (African-PREDICT): Design, recruitment and initial examination. Eur. J. Prev. Cardiol. 2019, 26, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and bmi for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Einhorn, P.T.; Cushman, W.C.; Whelton, P.K.; Bello, N.A.; Drawz, P.E.; Green, B.B.; Jones, D.W.; Juraschek, S.P.; Margolis, K.L.; et al. Blood pressure assessment in adults in clinical practice and clinic-based research: JACC scientific expert panel. J. Am. Coll. Cardiol. 2019, 73, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and the Pan American Health Organization Group for Cardiovascular Disease Prevention through Population-Wide Dietary Salt Reduction. Protocol for Population Level Sodium Determination in 24-h Urine Samples. 2010. Available online: https://www.paho.org/hq/dmdocuments/2013/24h-urine-Protocol-eng.pdf (accessed on 10 September 2020).
- Fedorova, O.V.; Simbirtsev, A.S.; Kolodkin, N.I.; Kotov, A.Y.; Agalakova, N.I.; Kashkin, V.A.; Tapilskaya, N.I.; Bzhelyansky, A.; Reznik, V.A.; Frolova, E.V. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J. Hypertens. 2008, 26, 2414. [Google Scholar] [CrossRef]
- Stevens, L.A.; Claybon, M.A.; Schmid, C.H.; Chen, J.; Horio, M.; Imai, E.; Nelson, R.G.; Van Deventer, M.; Wang, H.-Y.; Zuo, L.; et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011, 79, 555–562. [Google Scholar] [CrossRef] [PubMed]
| Baseline | Follow-Up | Difference | p | |
|---|---|---|---|---|
| Men, N (%) | 125 (45.5) | 125 (45.5) | ||
| Black, N (%) | 138 (50.2) | 138 (50.2) | ||
| Age (years) | 25.4 ± 3.16 | 30.0 ± 3.21 | 4.60 (4.49; 4.70) | <0.001 |
| Anthropometric measurements | ||||
| Height (m) | 1.68 ± 0.09 | 1.68 ± 0.09 | 0.00 (−0.001; 0.001) | 0.68 |
| Weight (kg) | 73.3 ± 18.3 | 77.9 ± 20.2 | 4.64 (3.51; 5.77) | <0.001 |
| Waist circumference (cm) | 81.5 ± 14.2 | 83.2 ± 14.5 | 1.66 (0.91; 2.41) | <0.001 |
| BMI (kg/m2) | 25.8 ± 5.79 | 27.3 ± 6.57 | 1.51 (1.21; 1.82) | <0.001 |
| WHtR | 0.48 ± 0.08 | 0.49 ± 0.09 | 0.01 (0.01; 0.01) | <0.001 |
| Frequency of obesity based on: | ||||
| BMI, N (%) | 60 (21.8) | 82 (29.8) | 22 (36.7) | <0.001 |
| WC, N (%) | 95 (34.5) | 109 (39.6) | 14 (14.7) | 0.014 |
| WHtR, N (%) | 94 (34.2) | 103 (37.5) | 9 (0.9) | 0.11 |
| Composite obesity criteria, N (%) * | 55 (20.0) | 74 (26.9) | 19 (34.5) | <0.001 |
| Blood pressure | ||||
| Clinic SBP (mmHg) | 120 ± 12.4 | 116 ± 12.7 | −3.76 (−4.97; −2.55) | <0.001 |
| Clinic DBP (mmHg) | 78.9 ± 8.07 | 79.3 ± 9.45 | 0.30 (−0.63; 1.24) | 0.52 |
| Central SBP (mmHg) | 109 ± 9.48 | 110 ± 10.4 | 0.87 (−0.12; 1.86) | 0.085 |
| Hypertension, N (%) # | 39 (14.2) | 42 (15.3) | 3 (7) | 0.76 |
| Hypertension medication, N (%) | 0 (0.0) | 3 (1.0) | 0.25 | |
| Echocardiography | ||||
| LVMi (g/m2) | 70.7 ± 15.7 | 77.8 ± 18.7 | 7.02 (5.03; 9.00) | <0.001 |
| IVSd (cm/m) | 0.47 ± 0.10 | 0.53 ± 0.09 | 0.06 (0.05; 0.08) | <0.001 |
| LVIDd (cm/m) | 2.84 ± 0.25 | 2.78 ± 0.24 | −0.07 (−0.09; −0.04) | <0.001 |
| PWTd (cm/m) | 0.50 ± 0.09 | 0.54 ± 0.01 | 0.05 (0.03; 0.06) | <0.001 |
| EDVi (mL/m) | 64.0 ± 13.7 | 60.3 ± 13.0 | −3.70 (−4.94; −2.46) | <0.001 |
| SVi (ml/m2.04) | 25.1 ± 5.52 | 23.1 ± 4.96 | −2.03 (−2.61; −1.45) | <0.001 |
| Urinary profile | ||||
| eGFR (ml/min/1.73 m2) | 111 ± 16.4 | 108 ± 16.6 | −3.15 (−4.90; −1.39) | <0.001 |
| 24 h MBG excretion (nmol/day) | 3.38 (1.12; 9.13) | - | ||
| Estimated salt intake (g/day) ꝉ | 7.73 (2.80; 19.4) | 7.13 (1.61; 24.4) | −0.22 (10.3) | 0.47 |
| Biochemical profile | ||||
| Glucose (mmol/L) | 4.63 ± 0.76 | 4.08 ± 0.65 | −0.55 (−0.66; −0.44) | <0.001 |
| HDL-C (mmol/L) | 1.34 ± 0.39 | 1.25 ± 0.34 | −0.09 (−0.12; −0.05) | <0.001 |
| LDL-C (mmol/L) | 2.80 ± 0.92 | 2.66 ± 0.91 | −0.14 (−0.22; −0.06) | 0.001 |
| C-reactive protein (mg/L) | 1.04 (0.11; 9.38) | 1.07 (0.15; 10.3) | 0.01 (1.46) | 0.56 |
| γ-glutamyl transferase (U/L) | 21.9 (8.74; 61.1) | 21.4 (7.21; 63.5) | −0.48 (9.12) | 0.46 |
| MBG Excretion (nmol/Day) | ||||||
|---|---|---|---|---|---|---|
| Non-Obese 18.6–29.9 kg/m2 N = 211 | Obese BMI > 30 kg/m2 N = 56 | |||||
| Dependent Variable | Adj R2 | Std. β | p | Adj R2 | Std. β | p |
| LVMi (g/m2) | 0.39 | NS | 0.35 | 0.311 | 0.007 | |
| % Δ LVMi | 0.21 | NS | 0.4 | 0.336 | 0.003 | |
| Sensitivity analysis additionally adjusted for estimated salt intake | ||||||
| LVMi (g/m2) | 0.39 | NS | 0.35 | 0.311 | 0.008 | |
| % Δ LVMi | 0.21 | NS | 0.4 | 0.337 | 0.003 | |
| Sensitivity analysis additionally adjusted for estradiol | ||||||
| LVMi (g/m2) | 0.39 | NS | 0.47 | 0.305 | 0.007 | |
| % Δ LVMi | 0.21 | NS | 0.5 | 0.344 | 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strauss-Kruger, M.; Kruger, R.; Smith, W.; Gafane-Matemane, L.F.; Mokwatsi, G.; Wei, W.; Fedorova, O.V.; Schutte, A.E. The Cardiotonic Steroid Marinobufagenin Is a Predictor of Increased Left Ventricular Mass in Obesity: The African-PREDICT Study. Nutrients 2020, 12, 3185. https://doi.org/10.3390/nu12103185
Strauss-Kruger M, Kruger R, Smith W, Gafane-Matemane LF, Mokwatsi G, Wei W, Fedorova OV, Schutte AE. The Cardiotonic Steroid Marinobufagenin Is a Predictor of Increased Left Ventricular Mass in Obesity: The African-PREDICT Study. Nutrients. 2020; 12(10):3185. https://doi.org/10.3390/nu12103185
Chicago/Turabian StyleStrauss-Kruger, Michél, Ruan Kruger, Wayne Smith, Lebo F. Gafane-Matemane, Gontse Mokwatsi, Wen Wei, Olga V. Fedorova, and Aletta E. Schutte. 2020. "The Cardiotonic Steroid Marinobufagenin Is a Predictor of Increased Left Ventricular Mass in Obesity: The African-PREDICT Study" Nutrients 12, no. 10: 3185. https://doi.org/10.3390/nu12103185
APA StyleStrauss-Kruger, M., Kruger, R., Smith, W., Gafane-Matemane, L. F., Mokwatsi, G., Wei, W., Fedorova, O. V., & Schutte, A. E. (2020). The Cardiotonic Steroid Marinobufagenin Is a Predictor of Increased Left Ventricular Mass in Obesity: The African-PREDICT Study. Nutrients, 12(10), 3185. https://doi.org/10.3390/nu12103185








