Srebf2 Locus Overexpression Reduces Body Weight, Total Cholesterol and Glucose Levels in Mice Fed with Two Different Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Diets
2.2. Food Intake Experiment
2.3. Metabolic Measurements
2.4. Enzyme-Linked Immuno Sorbent Assay (ELISA)
2.5. Liver, Pancreas and Fat Immunostainings
2.6. Adipocyte Quantification in Adipose Tissue: Size and Number
2.7. RNA Extraction and Reverse Transcription from Tissue Samples
2.8. mRNA Quantification by Real-Time Quantitative PCR
2.9. Statistical Analysis
3. Results
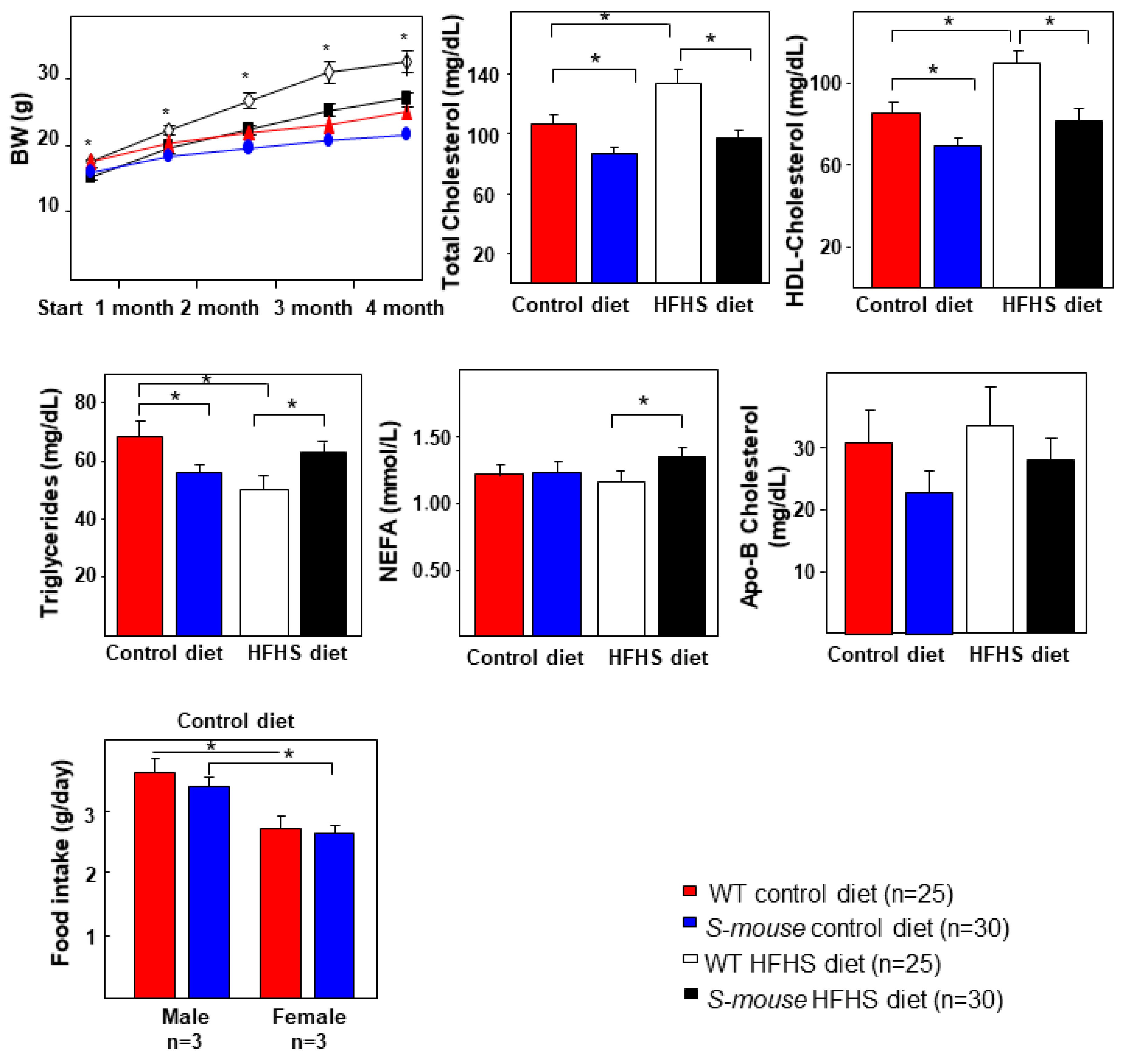
3.1. Body Weight, Food Intake and Lipid Metabolism Characterisation of WT and S-mice Fed a High-Fat, High-Sucrose or Control Diet
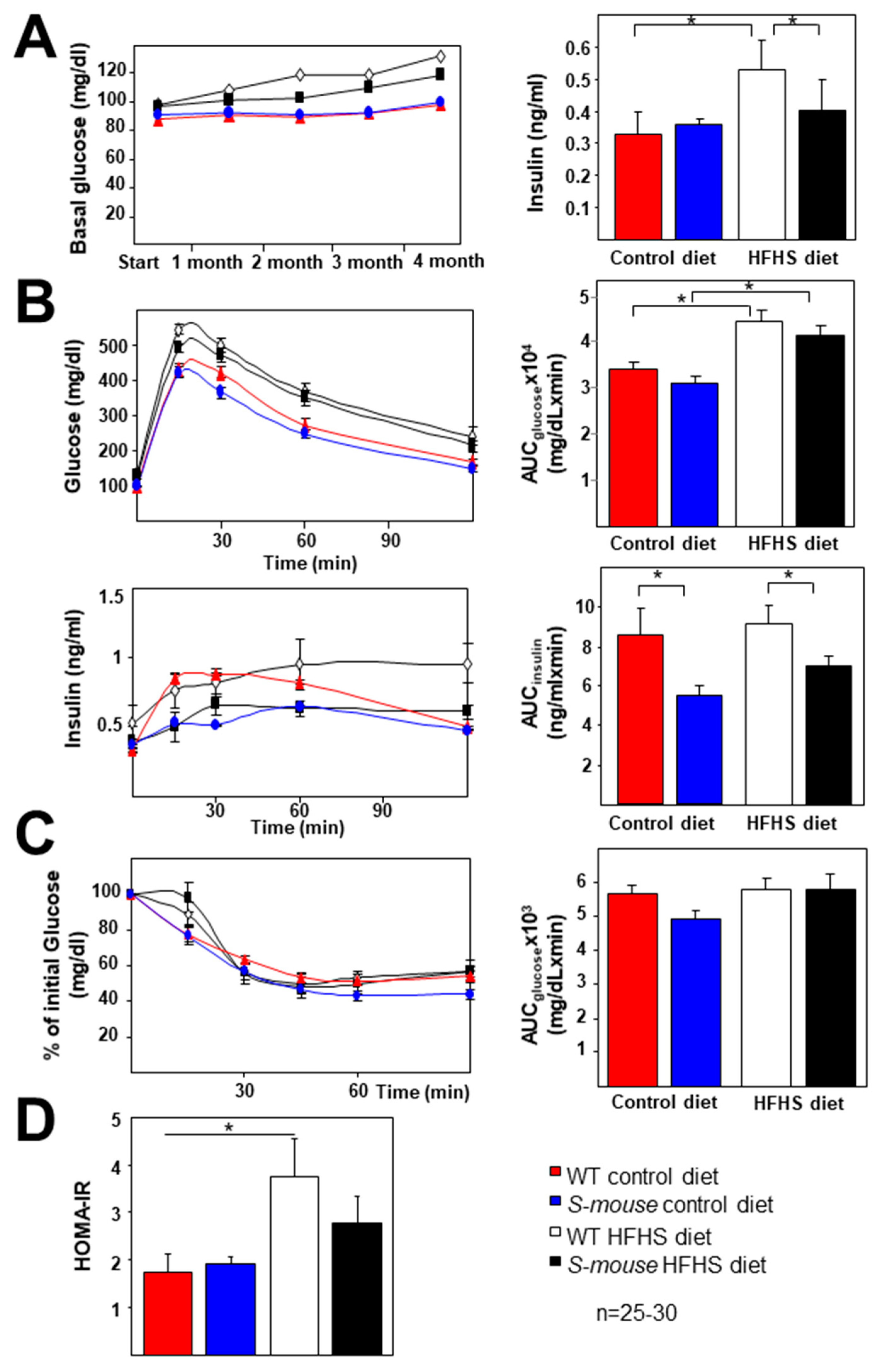
3.2. Carbohydrate Metabolism Characterisation of WT and S-mice Fed a High-Fat, High-Sucrose or Control Diet
3.3. Increased Dosage of Srebf2 Showed Low Pancreatic β-Cell Numbers in S-mice Fed a High-Fat, High-Sucrose and Control Diet
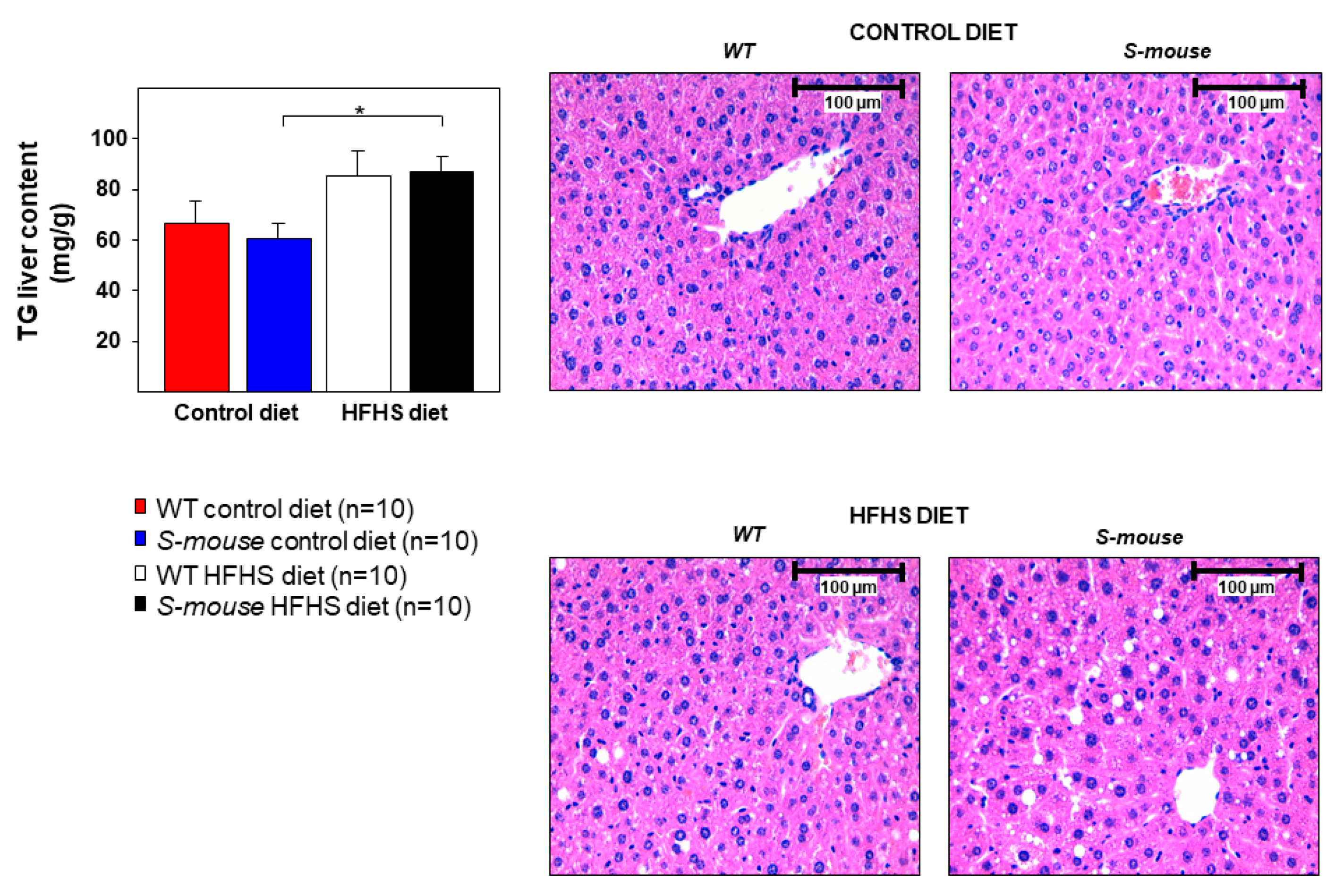
3.4. S-mice Fed a High-Fat, High-Sucrose Diet Have Increased Hepatic Triglyceride Content Compared with S-mice Fed Control Diet
3.5. Adipose Tissue Characterization in WT and S-mice Fed a High-Fat, High-Sucrose or Control Diet
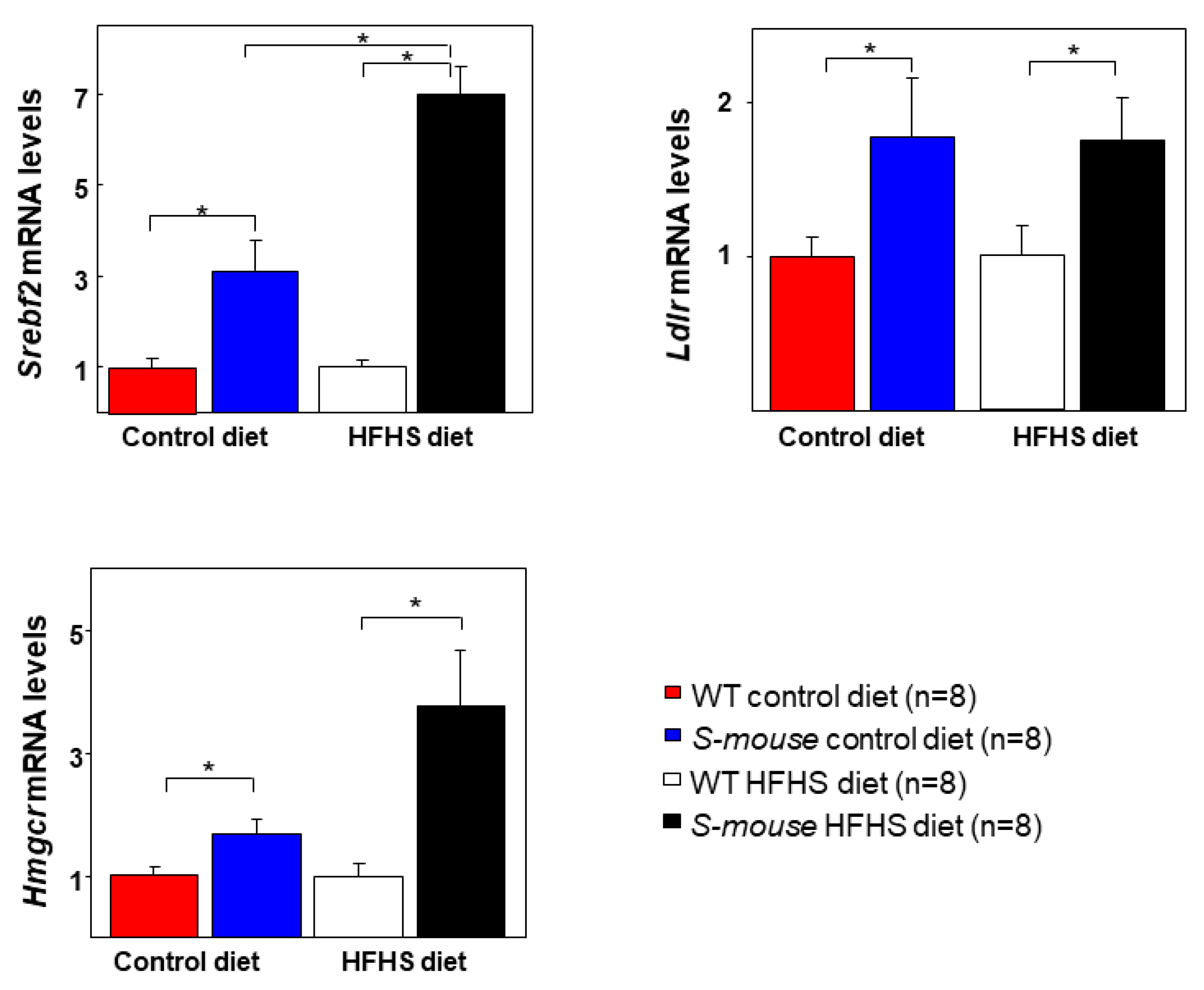
3.6. Increased Activation of Srebf2, Ldlr and Hmgcr in S-mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nunn, A.V.; Bell, J.D.; Guy, G.W. Lifestyle-induced metabolic inflexibility and accelerated ageing syndrome: Insulin resistance, friend or foe? Nutr. Metab. 2009, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Zambon, S.; Zanoni, S.; Romanato, G.; Corti, C.M.; Noale, M.; Sartori, L.; Musacchio, E.; Baggio, G.; Crepaldi, G.; Manzato, E. Metabolic Syndrome and All-Cause and Cardiovascular Mortality in an Italian Elderly Population. Diabetes Care 2009, 32, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Scholmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Ellacott, K.L.; Morton, G.J.; Woods, S.C.; Tso, P.; Schwartz, M.W. Assessment of feeding behavior in laboratory mice. Cell Metab. 2011, 12, 10–17. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Sakanaka, M.; Kimura, Y. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J. Nutr. 2006, 136, 582–587. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Osborne, T.; Espenshade, P.J. Evolutionary conservation and adaptation in the mechanism that regulates SREBP action: What a long, strange tRIP it’s been. Genes Dev. 2009, 23, 2578–2591. [Google Scholar] [CrossRef]
- Bengoechea-Alonso, M.T.; Ericsson, J. SREBP in signal transduction: Cholesterol metabolism and beyond. Curr. Opin. Cell Biol. 2007, 19, 215–222. [Google Scholar] [CrossRef]
- Durst, R.; Jansen, A.; Erez, G.; Bravdo, R.; Butbul, E.; Avi, L.B.; Shpitzen, S.; Lotan, C.; Leitersdorf, E.; Defesche, J.; et al. The discrete and combined effect of SREBP-2 and SCAP isoforms in the control of plasma lipids among familial hypercholesterolaemia patients. Atherosclerosis 2006, 189, 443–450. [Google Scholar] [CrossRef]
- Liu, F.-H.; Song, J.-Y.; Meng, X.-R.; Ma, J.; Wang, H.-J. The gene-gene interaction of INSIG-SCAP-SREBP pathway on the risk of obesity in Chinese children. BioMed Res. Int. 2014, 2014, 538564. [Google Scholar] [CrossRef]
- Shao, W.; Espenshade, P.J. Expanding roles for SREBP in metabolism. Cell Metab. 2012, 16, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Xia, Z.-P.; Adams, C.M.; Rawson, R.B. Three mutations in sterol-sensing domain of SCAP block interaction with insig and render SREBP cleavage insensitive to sterols. Proc. Natl. Acad. Sci. USA 2002, 99, 16672–16677. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, J.Y.; Wang, S.; Liu, F.H.; Zhang, Y.N.; Shang, X.R.; Wang, H.J.; Ma, J. Genetic variations in sterol regulatory element binding protein cleavage-activating protein (SCAP) are associated with blood pressure in overweight/obese Chinese children. PLoS ONE 2017, 12, e0177973. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vergnes, L.; Chin, R.G.; de Aguiar Vallim, T.; Fong, L.G.; Osborne, T.F.; Young, S.G.; Reue, K. SREBP-2-deficient and hypomorphic mice reveal roles for SREBP-2 in embryonic development and SREBP-1c expression. J. Lipid Res. 2016, 57, 410–421. [Google Scholar] [CrossRef]
- Muller, P.; Miserez, A.R. Identification of mutations in the gene encoding sterol regulatory element binding protein (SREBP)-2 in hypercholesterolaemic subjects. J. Med. Genet. 2002, 39, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Bo, S.; De Michieli, F.; Gambino, R. Sterol regulatory element-binding factor 2 (SREBF-2) predicts 7-year NAFLD incidence and severity of liver disease and lipoprotein and glucose dysmetabolism. Diabetes 2013, 62, 1109–1120. [Google Scholar] [CrossRef]
- Davalos, A.; Goedeke, L.; Smibert, P.; Ramirez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef]
- Najafi-Shoushtari, S.H.; Kristo, F.; Li, Y.; Shioda, T.; Cohen, D.E.; Gerszten, R.E.; Näär, A.M. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010, 328, 1566–1569. [Google Scholar] [CrossRef]
- Ramírez, C.M.; Goedeke, L.; Rotllan, N.; Yoon, J.-H.; Cirera-Salinas, D.; Mattison, J.A.; Suárez, Y.; de Cabo, R.; Gorospe, M.; Fernández-Hernando, C. MicroRNA 33 regulates glucose metabolism. Mol. Cell. Biol. 2013, 33, 2891–2902. [Google Scholar] [CrossRef]
- Wijesekara, N.; Zhang, L.-h.; Kang, M.H.; Abraham, T.; Bhattacharjee, A.; Warnock, G.L.; Verchere, C.B.; Hayden, M.R. miR-33a modulates ABCA1 expression, cholesterol accumulation, and insulin secretion in pancreatic islets. Diabetes 2012, 61, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Blesa, S.; Olivares, M.D.; Alic, A.S.; Serrano, A.; Lendinez, V.; González-Albert, V.; Olivares, L.; Martínez-Hervás, S.; Juanes, J.M.; Marín, P.; et al. Easy One-Step Amplification and Labeling Procedure for Copy Number Variation Detection. Clin. Chem. 2020, 66, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Navarro, H.; Nabah, Y.N.A.; Vinue, A.; Andres-Manzano, M.J.; Collado, M.; Serrano, M.; Andres, V. p19(ARF) deficiency reduces macrophage and vascular smooth muscle cell apoptosis and aggravates atherosclerosis. J. Am. Coll. Cardiol. 2010, 55, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Navarro, H.; Vila-Caballer, M.; Pastor, M.F.; Vinue, A.; White, M.F.; Burks, D.; Andres, V. Plasma insulin levels predict the development of atherosclerosis when IRS2 deficiency is combined with severe hypercholesterolemia in apolipoprotein E-null mice. Front. Biosci. 2007, 12, 2291–2298. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Navarro, H.; Vinue, A.; Vila-Caballer, M.; Fortuno, A.; Beloqui, O.; Zalba, G.; Burks, D.; Diez, J.; Andres, V. Molecular mechanisms of atherosclerosis in metabolic syndrome: Role of reduced IRS2-dependent signaling. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2187–2194. [Google Scholar] [CrossRef]
- Norris, A.W.; Chen, L.; Fisher, S.J.; Szanto, I.; Ristow, M.; Jozsi, A.C.; Hirshman, M.F.; Rosen, E.D.; Goodyear, L.J.; Gonzalez, F.J.; et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J. Clin. Investig. 2003, 112, 608–618. [Google Scholar] [CrossRef]
- Osman, S.O.; Selway, J.L.; Kępczyńska, M.A.; Stocker, C.J.; O’Dowd, J.F.; Cawthorne, M.A.; Arch, J.R.S.; Jassim, S.; Langlands, K. A novel automated image analysis method for accurate adipocyte quantification. Adipocyte 2013, 2, 160–164. [Google Scholar] [CrossRef]
- Horton, J.D.; Shimomura, I.; Brown, M.S.; Hammer, R.E.; Goldstein, J.L.; Shimano, H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J. Clin. Investig. 1998, 101, 2331–2339. [Google Scholar] [CrossRef]
- Ishikawa, M.; Iwasaki, Y.; Yatoh, S.; Kato, T.; Kumadaki, S.; Inoue, N.; Yamamoto, T.; Matsuzaka, T.; Nakagawa, Y.; Yahagi, N.; et al. Cholesterol accumulation and diabetes in pancreatic beta-cell-specific SREBP-2 transgenic mice: A new model for lipotoxicity. J. Lipid Res. 2008, 49, 2524–2534. [Google Scholar] [CrossRef]
- Landa, V.; Zidek, V.; Mlejnek, P.; Simakova, M.; Silhavy, J.; Trnovska, J.; Kazdova, L.; Pravenec, M. Sterol regulatory element binding protein 2 overexpression is associated with reduced adipogenesis and ectopic fat accumulation in transgenic spontaneously hypertensive rats. Physiol. Res. 2014, 63, 587–590. [Google Scholar]
- Ma, K.; Malhotra, P.; Soni, V.; Hedroug, O.; Annaba, F.; Dudeja, A.; Shen, L.; Turner, J.R.; Khramtsova, E.A.; Saksena, S.; et al. Overactivation of intestinal SREBP2 in mice increases serum cholesterol. PLoS ONE 2014, 9, e84221. [Google Scholar] [CrossRef] [PubMed]
- Gerin, I.; Clerbaux, L.A.; Haumont, O.; Lanthier, N.; Das, A.K.; Burant, C.F.; Leclercq, I.A.; MacDougald, O.A.; Bommer, G.T. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J. Biol. Chem. 2010, 285, 33652–33661. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Ono, K.; Nishi, H.; Horiguchi, M.; Nakamura, T.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Marusawa, H.; Iwanaga, Y.; et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 17321–17326. [Google Scholar] [CrossRef] [PubMed]
- Marquart, T.J.; Allen, R.M.; Ory, D.S.; Baldan, A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 12228–12232. [Google Scholar] [CrossRef] [PubMed]
- Brunham, L.R.; Kruit, J.K.; Verchere, C.B.; Hayden, M.R. Cholesterol in islet dysfunction and type 2 diabetes. J. Clin. Investig. 2008, 118, 403–408. [Google Scholar] [CrossRef]
- Hao, M.; Head, W.S.; Gunawardana, S.C.; Hasty, A.H.; Piston, D.W. Direct effect of cholesterol on insulin secretion: A novel mechanism for pancreatic beta-cell dysfunction. Diabetes 2007, 56, 2328–2338. [Google Scholar] [CrossRef]
- Malhotra, A.A.; Aloman, C.; Khadra, H.; Ooka, K.; Gill, R.K.; Saksena, S.; Dudeja, P.K.; Alrefai, W.A. Overactivation of intestinal sterol response element-binding protein 2 promotes diet-induced nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G376–G385. [Google Scholar] [CrossRef]
- Ide, S.H.; Yahagi, N.; Matsuzaka, T.; Nakakuki, M.; Yamamoto, T.; Nakagawa, Y.; Takahashi, A.; Suzuki, H.; Sone, H.; Toyoshima, H.; et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat. Cell Biol. 2004, 6, 351–357. [Google Scholar] [CrossRef]
- Rong, S.; Cortés, V.A.; Rashid, S.; Anderson, N.N.; McDonald, J.G.; Liang, G.; Moon, Y.A.; Hammer, R.E.; Horton, J.D. Expression of SREBP-1c Requires SREBP-2-mediated Generation of a Sterol Ligand for LXR in Livers of Mice. eLife 2017, 6, e25015. [Google Scholar] [CrossRef]
- Merath, K.; Chang, B.; Dubielzig, R.; Jeannotte, R.; Sidjanin, D.J. A spontaneous mutation in Srebf2 leads to cataracts and persistent skin wounds in the lens opacity 13 (lop13) mouse. Mamm. Genome 2011, 22, 661–673. [Google Scholar] [CrossRef]
- Le Lay, K.S.; Farnier, C.; Lefrère, I.; Le Liepvre, X.; Bazin, R.; Ferré, P.; Dugail, I. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J. Biol. Chem. 2001, 276, 16904–16910. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Perreault, M.; Klaman, L.D.; Tobin, J.F.; Smith, E.; Gimeno, R.E. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology 2007, 148, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Ludgero-Correia, A., Jr.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Faria, T.S. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition 2012, 28, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, K.I.; Powers, C.A. Signals in the pancreatic islet microenvironment influence β-cell proliferation. Diabetes Obes. Metab. 2017, 19 (Suppl. S1), 124–136. [Google Scholar] [CrossRef]
- Bock, T.; Pakkenberg, B.; Buschard, K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes 2003, 52, 1716–1722. [Google Scholar] [CrossRef]
- Hull, R.L.; Kodama, K.; Utzschneider, K.M.; Carr, D.B.; Prigeon, R.L.; Kahn, S.E. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: Evidence for specificity of impaired beta cell adaptation. Diabetologia 2005, 48, 1350–1358. [Google Scholar] [CrossRef]
- Peyot, M.-L.; Pepin, E.; Lamontagne, J.; Latour, M.G.; Zarrouki, B.; Lussier, R.; Pineda, M.; Jetton, T.L.; Madiraju, S.R.M.; Joly, E.; et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: Secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 2010, 59, 2178–2187. [Google Scholar] [CrossRef]
- Shah, S.; Yoon, G.H.; Chung, S.S.; Abid, M.N.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer’s disease neuropathological deficits. Mol. Psychiatry 2017, 22, 407–416. [Google Scholar] [CrossRef]
- Xiao, X.; Song, B.L. SREBP: A novel therapeutic target. Acta Biochim. Biophys. Sin. 2013, 45, 2–10. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, X.; Zhou, Y.P.; Lu, C.; Thu, P.M.; Qian, C.; Zhang, M.; Li, P.; Li, H.J.; Xu, X. Anhydroicaritin, a SREBPs inhibitor, inhibits RANKL-induced osteoclastic differentiation and improves diabetic osteoporosis in STZ-induced mice. Eur. J. Pharmacol. 2017, 15, 156–162. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés-Blasco, I.; Blesa, S.; Vinué, Á.; González-Navarro, H.; Real, J.T.; Martínez-Hervás, S.; Carretero, J.; Ferrández-Izquierdo, A.; Chaves, F.J.; García-García, A.-B. Srebf2 Locus Overexpression Reduces Body Weight, Total Cholesterol and Glucose Levels in Mice Fed with Two Different Diets. Nutrients 2020, 12, 3130. https://doi.org/10.3390/nu12103130
Andrés-Blasco I, Blesa S, Vinué Á, González-Navarro H, Real JT, Martínez-Hervás S, Carretero J, Ferrández-Izquierdo A, Chaves FJ, García-García A-B. Srebf2 Locus Overexpression Reduces Body Weight, Total Cholesterol and Glucose Levels in Mice Fed with Two Different Diets. Nutrients. 2020; 12(10):3130. https://doi.org/10.3390/nu12103130
Chicago/Turabian StyleAndrés-Blasco, Irene, Sebastian Blesa, Ángela Vinué, Herminia González-Navarro, José Tomás Real, Sergio Martínez-Hervás, Julián Carretero, Antonio Ferrández-Izquierdo, Felipe Javier Chaves, and Ana-Bárbara García-García. 2020. "Srebf2 Locus Overexpression Reduces Body Weight, Total Cholesterol and Glucose Levels in Mice Fed with Two Different Diets" Nutrients 12, no. 10: 3130. https://doi.org/10.3390/nu12103130
APA StyleAndrés-Blasco, I., Blesa, S., Vinué, Á., González-Navarro, H., Real, J. T., Martínez-Hervás, S., Carretero, J., Ferrández-Izquierdo, A., Chaves, F. J., & García-García, A.-B. (2020). Srebf2 Locus Overexpression Reduces Body Weight, Total Cholesterol and Glucose Levels in Mice Fed with Two Different Diets. Nutrients, 12(10), 3130. https://doi.org/10.3390/nu12103130





