A Randomized, Double-Blinded, Placebo-Controlled, Clinical Study of the Effects of a Nutraceutical Combination (LEVELIP DUO®) on LDL Cholesterol Levels and Lipid Pattern in Subjects with Sub-Optimal Blood Cholesterol Levels (NATCOL Study)
Abstract
1. Introduction
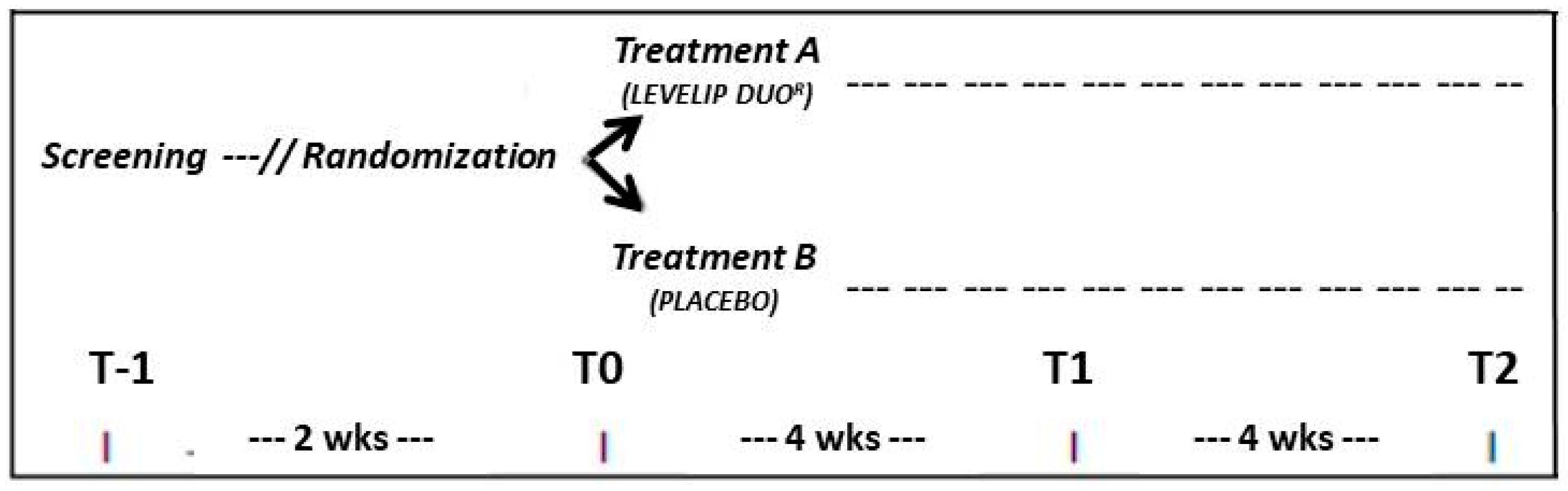
2. Materials and Methods
- Personal history of cardiovascular disease or risk equivalents;
- Triglycerides (TG) ≥400 mg/dL;
- Obesity (Body Mass Index > 32 kg/m2);
- Assumption of lipid-lowering drugs or supplements affecting lipid metabolism;
- Uncontrolled diabetes mellitus;
- Known thyroid, liver, renal or muscle diseases.
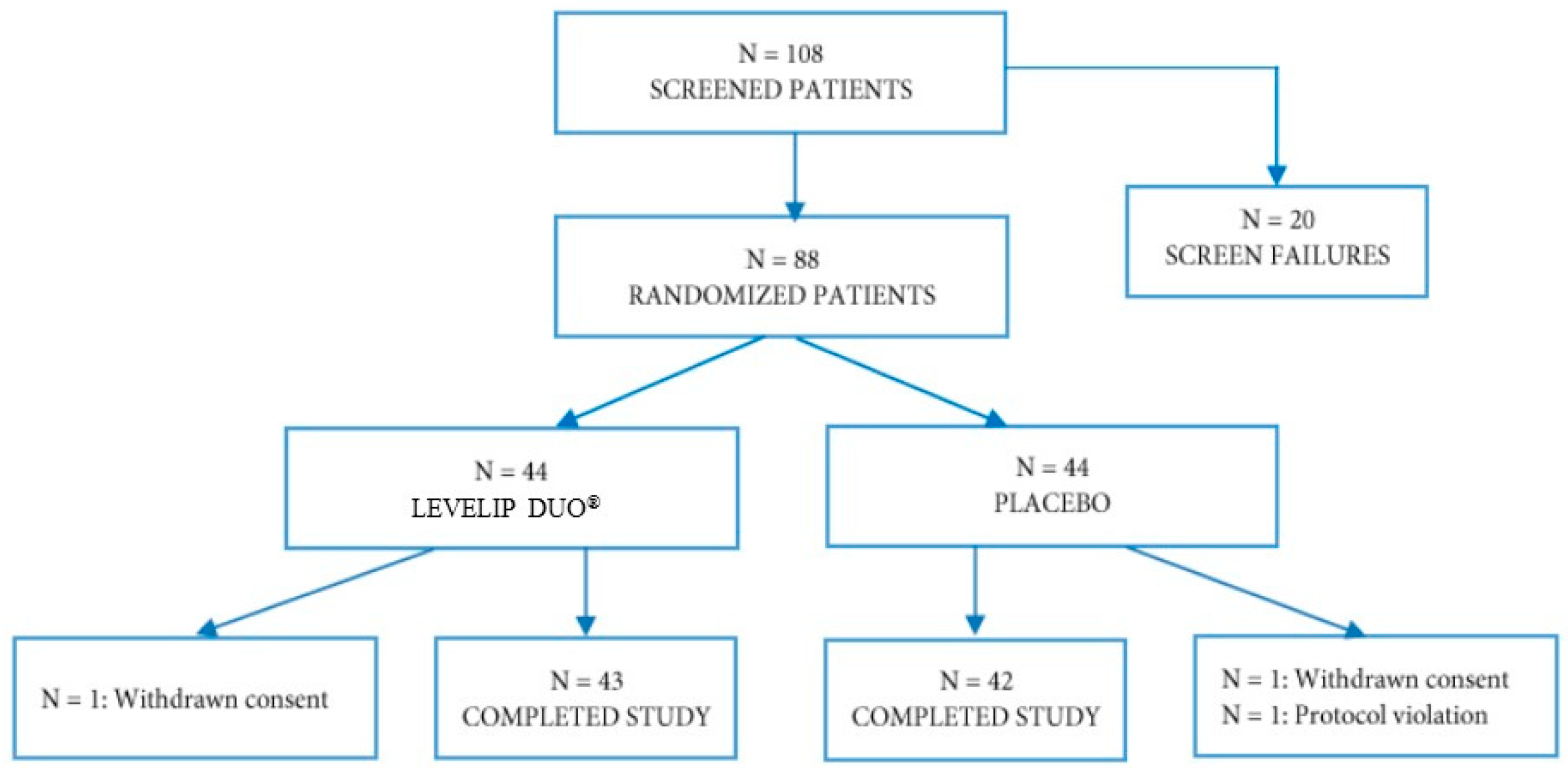
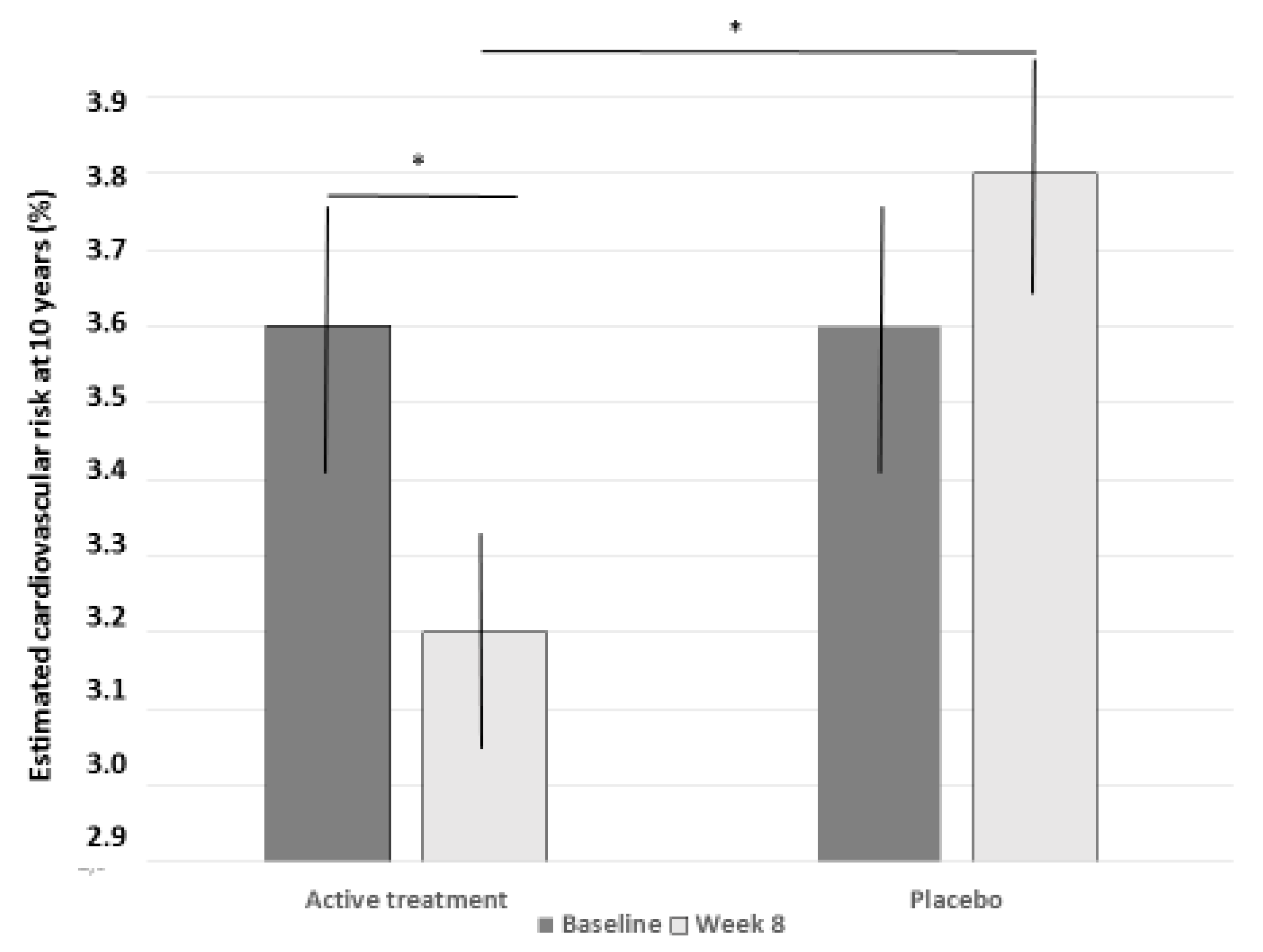
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bittner, V.A. The New 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation. 2019. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; McEvoy, J.W.; Blumenthal, R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019, 381, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, R.A.; Fischer, N.M.; Xun, H.; Michos, E.D. Nutrition and physical activity recommendations from the United States and European cardiovascular guidelines: A comparative review. Curr. Opin. Cardiol. 2020, 35, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef]
- Cicero, A.F.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef]
- Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Goldstein, M.R. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis. Am. J. Med. 2000, 109, 72–73. [Google Scholar] [CrossRef]
- Cedó, L.; Farràs, M.; Lee-Rueckert, M.; Escolà-Gil, J.C. Molecular Insights into the Mechanisms Underlying the Cholesterol-Lowering Effects of Phytosterols. Curr. Med. Chem. 2019, 26, 6704–6723. [Google Scholar] [CrossRef]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef]
- Han, S.; Jiao, J.; Xu, J.; Zimmermann, D.; Actis-Goretta, L.; Guan, L.; Zhao, Y.; Qin, L. Effects of plant stanol or sterol-enriched diets on lipid profiles in patients treated with statins: Systematic review and meta-analysis. Sci. Rep. 2016, 6, 31337. [Google Scholar] [CrossRef]
- Fumeron, F.; Bard, J.M.; Lecerf, J.M. Interindividual variability in the cholesterol-lowering effect of supplementation with plant sterols or stanols. Nutr. Rev. 2017, 75, 134–145. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the modification of the authorisation of a health claim related to plant sterol esters and lowering blood LDL-cholesterol; high blood LDL-cholesterol is a risk factor in the development of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006, following a request in accordance with Article 19 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3577. [Google Scholar]
- Cicero, A.F.; Fogacci, F.; Banach, M. Red Yeast Rice for Hypercholesterolemia. Methodist Debakey Cardiovasc. J. 2019, 15, 192–199. [Google Scholar] [PubMed]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.; Gerdes, V.E. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL-cholesterol concentrations (ID 1648, 1700) pursuant to Article 13 of Regulation (EC) No 1924/20061; EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), European Food Safety Authority (EFSA), Parma, Italy. EFSA J. 2011, 9, 2304. [Google Scholar]
- EFSA. Scientific opinion on the safety of monacolins in red yeast rice. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). European Food Safety Authority (EFSA), Parma, Italy. EFSA J. 2018, 16, 5368. [Google Scholar]
- Fogacci, F.; Banach, M.; Mikhailidis, D.P.; Bruckert, E.; Toth, P.P.; Watts, G.F.; Reiner, Ž.; Mancini, J.; Rizzo, M.; Mitchenko, O.; et al. Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 143, 1–16. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytother. Res. 2019, 33, 2094–2101. [Google Scholar] [CrossRef]
- Nasi, M.; Patrizi, G.; Pizzi, C.; Landolfo, M.; Boriani, G.; Dei Cas, A.; Cicero, A.F.; Fogacci, F.; Rapezzi, C.; Sisca, G.; et al. The role of physical activity in individuals with cardiovascular risk factors: An opinion paper from Italian Society of Cardiology-Emilia Romagna-Marche and SIC-Sport. J. Cardiovasc. Med. 2019, 20, 631–639. [Google Scholar] [CrossRef]
- Cicero, A.F.; Derosa, G.; Pisciotta, L.; Barbagallo, C.; SISA-PUFACOL Study Group. Testing the Short-Term Efficacy of a Lipid-Lowering Nutraceutical in the Setting of Clinical Practice: A Multicenter Study. J. Med. Food 2015, 18, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Brancaleoni, M.; Laghi, L.; Donati, F.; Mino, M. Antihyperlipidaemic effect of a Monascus purpureus brand dietary supplement on a large sample of subjects at low risk for cardiovascular disease: A pilot study. Complement. Ther. Med. 2005, 13, 273–278. [Google Scholar] [CrossRef]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: A pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 2013, 52, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Fogacci, F.; Bove, M.; Veronesi, M.; Rizzo, M.; Giovannini, M.; Borghi, C. Short-Term Effects of a Combined Nutraceutical on Lipid Level, Fatty Liver Biomarkers, Hemodynamic Parameters, and Estimated Cardiovascular Disease Risk: A Double-Blind, Placebo-Controlled Randomized Clinical Trial. Adv. Ther. 2017, 34, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Fogacci, F.; Morbini, M.; Colletti, A.; Bove, M.; Veronesi, M.; Giovannini, M.; Borghi, C. Nutraceutical Effects on Glucose and Lipid Metabolism in Patients with Impaired Fasting Glucose: A Pilot, Double-Blind, Placebo-Controlled, Randomized Clinical Trial on a Combined Product. High. Blood Press Cardiovasc. Prev. 2017, 24, 283–288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palmieri, L.; Donfrancesco, C.; Giampaoli, S.; Trojani, M.; Panico, S.; Vanuzzo, D.; Pilotto, L.; Cesana, G.; Ferrario, M.; Chiodini, P.; et al. Favorable cardiovascular risk profile and 10-year coronary heart disease incidence in women and men: Results from the Progetto CUORE. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 562–570. [Google Scholar] [CrossRef]
- Cicero, A.F.; Colletti, A. Combinations of phytomedicines with different lipid lowering activity for dyslipidemia management: The available clinical data. Phytomedicine 2016, 23, 1113–1118. [Google Scholar] [CrossRef]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Rosticci, M.; Parini, A.; Giovannini, M.; Veronesi, M.; D’Addato, S.; Borghi, C. Effect of a short-term dietary supplementation with phytosterols, red yeast rice or both on lipid pattern in moderately hypercholesterolemic subjects: A three-arm, double-blind, randomized clinical trial. Nutr. Metab. 2017, 14, 61. [Google Scholar] [CrossRef]
- Baila-Rueda, L.; Pérez-Ruiz, M.R.; Jarauta, E.; Tejedor, M.T.; Mateo-Gallego, R.; Lamiquiz-Moneo, I.; de Castro-Orós, I.; Cenarro, A.; Civeira, F. Cosegregation of serum cholesterol with cholesterol intestinal absorption markers in families with primary hypercholesterolemia without mutations in LDLR, APOB, PCSK9 and APOE genes. Atherosclerosis 2016, 246, 202–207. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to niacin and energy-yielding metabolism (ID 43, 49, 54), function of the nervous system (ID 44, 53), maintenance of the skin and mucous membranes (ID 45, 48, 50, 52), maintenance of normal LDL-cholesterol, HDL-cholesterol and triglyceride concentrations (ID 46), maintenance of bone (ID 50), maintenance of teeth (ID 50), maintenance of hair (ID 50, 2875) and maintenance of nails (ID 50, 2875) pursuant to Article 13 of Regulation (EC) No 1924/2006 on request from European Commission. EFSA J. 2009, 7, 1224. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Active Treatment | Placebo | |
|---|---|---|
| Age (years) | 51.3 ± 9.6 | 51.8 ± 10.7 |
| Height (m) | 1.69 ± 0.09 | 1.69 ± 0.10 |
| Weight (kg) | 70.3 ± 12.0 | 71.2 ± 14.8 |
| Body Mass Index (kg/m2) | 24.5 ± 3.2 | 24.7 ± 3.1 |
| Waist circumference (cm) | 89.5 ± 10.6 | 91.6 ± 11.1 |
| Systolic Blood Pressure (mmHg) | 124 ± 16 | 124 ± 15 |
| Diastolic Blood Pressure (mmHg) | 77 ± 10 | 78 ± 9 |
| Heart Rate (bpm) | 70 ± 10 | 70 ± 9 |
| Total cholesterol (mg/dL) | 229.1 ± 27.9 | 232.6 ± 21.6 |
| LDL cholesterol (mg/dL) | 155.1 ± 19.9 | 161.5 ± 21.3 |
| HDL cholesterol (mg/dL) | 51.0 ± 13.5 | 49.0 ± 11.3 |
| Non-HDL cholesterol (mg/dL) | 178.1 ± 25.5 | 183.6 ± 22.2 |
| Triglycerides (mg/dL) | 114.9 ± 46.2 | 110.4 ± 46.3 |
| Apolipoprotein B (mg/dL) | 106.9 ± 13.6 | 110.8 ± 15.8 |
| TC/HDL-Cholesterol | 4.76 ± 1.22 | 4.98 ± 1.19 |
| LDL-C/HDL-Cholesterol | 3.25 ± 0.99 | 3.48 ± 0.93 |
| Fasting Glucose (mg/dL) | 89.1 ± 11.6 | 88.8 ± 11.4 |
| Alanine aminotransferase (U/L) | 22.5 ± 8.6 | 23.4 ± 10.3 |
| Aspartate aminotransferase (U/L) | 20.9 ± 5.6 | 21.7 ± 4.0 |
| Gamma-glutamyl transferase (U/L) | 22.1 ± 13.6 | 21.2 ± 13.1 |
| Serum uric acid (mg/dL) | 4.4 ± 1.3 | 4.2 ± 0.9 |
| Serum creatinine (mg/dL) | 0.94 ± 0.13 | 0.96 ± 0.16 |
| Estimated GFR (ml/min/1.73 m2) | 77.3 ± 8.3 | 76.1 ± 11.9 |
| Creatine phosphokinase (U/L) | 110 ± 65 | 131 ± 77 |
| Variable | Active Treatment | Placebo | ||||
|---|---|---|---|---|---|---|
| Baseline (T0) | Day 28 (T1) | Day 56 (T2) | Baseline (T0) | Day 28 (T1) | Day 56 (T2) | |
| TC (mg/dL) | 229.1 ± 27.9 | 196.7 ± 24.1 * ° | 197.1 ± 28.3 * ° | 232.6 ± 21.6 | 237.5 ± 23.6 | 239.8 ± 23.6 |
| LDL-C (mg/dL) | 155.1 ± 19.9 | 124.0 ± 22.3 * ° | 122.6 ± 24.7 * ° | 161.5 ± 21.3 | 163.3 ± 22.3 | 164.0 ± 20.4 |
| HDL-C (mg/dL) | 51.0 ± 13.5 | 52.0 ± 13.1 | 51.8 ± 12.1 | 49.0 ± 11.3 | 50.3 ± 13.9 | 50.1 ± 11.9 |
| Non-HDL-C (mg/dL) | 178.1 ± 25.5 | 144.7 ± 23.6 * ° | 145.3 ± 26.5 * ° | 183.6 ± 22.2 | 187.2 ± 24.3 | 189.6 ± 24.0 |
| Triglycerides (mg/dL) | 114.9 ± 46.2 | 103.7 ± 53.9 | 113.5 ± 46.8 | 110.4 ± 46.3 | 119.5 ± 71.9 | 127.9 ± 79.6 |
| Apo B (mg/dL) | 106.9 ± 13.6 | 88.5 ± 14.6 *° | 92.1 ± 17.0 * ° | 110.8 ± 15.8 | 111.8 ± 15.9 | 112.4 ± 19.6 |
| TC/HDL-C | 4.76 ± 1.22 | 4.01 ± 1.07 * ° | 3.97 ± 0.92 * ° | 4.98 ± 1.19 | 5.02 ± 1.34 | 5.05 ± 1.28 |
| LDL-C/HDL-C | 3.25 ± 0.99 | 2.54 ± 0.81 * ° | 2.49 ± 0.74 * ° | 3.48 ± 0.93 | 3.47 ± 1.05 | 3.46 ± 0.95 |
| FPG (mg/dL) | 89.1 ± 11.6 | 88.5 ± 12.7 | 88.2 ± 12.5 | 88.8 ± 11.4 | 88.7 ± 12.2 | 87.2 ± 12.2 |
| ALT (U/L) | 22.5 ± 8.6 | 26.3 ± 12.6 | 27.1 ± 13.8 | 23.4 ± 10.3 | 27.2 ± 17.1 | 26.3 ± 14.6 |
| AST (U/L) | 20.9 ± 5.6 | 23.6 ± 7.6 | 21.8 ± 5.6 | 21.7 ± 4.0 | 21.7 ± 5.5 | 22.6 ± 4.8 |
| γ-GT (U/L) | 22.1 ± 13.6 | 22.9 ± 16.4 | 23.2 ± 17.5 | 21.2 ± 13.1 | 24.4 ± 25.8 | 24.3 ± 21.2 |
| SUA (mg/dL) | 4.4 ± 1.3 | 4.1 ± 0.9 | 4.1 ± 1.0 | 4.2 ± 0.9 | 4.2 ± 0.9 | 4.2 ± 0.9 |
| Serum creatinine (mg/dL) | 0.94 ± 0.13 | 0.95 ± 0.13 | 0.94 ± 0.11 | 0.96 ± 0.16 | 0.99 ± 0.14 | 0.96 ± 0.15 |
| sGFR (mL/min/1.73 m2) | 77.3 ± 8.3 | 77.2 ± 7.9 | 77.4 ± 7.8 | 76.1 ± 11.9 | 77.2 ± 10.9 | 76.9 ± 9.3 |
| CPK (U/L) | 110 ± 65 | 125 ± 85 | 112 ± 61 | 131 ± 77 | 124 ± 76 | 124 ± 61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicero, A.F.G.; D’Addato, S.; Borghi, C. A Randomized, Double-Blinded, Placebo-Controlled, Clinical Study of the Effects of a Nutraceutical Combination (LEVELIP DUO®) on LDL Cholesterol Levels and Lipid Pattern in Subjects with Sub-Optimal Blood Cholesterol Levels (NATCOL Study). Nutrients 2020, 12, 3127. https://doi.org/10.3390/nu12103127
Cicero AFG, D’Addato S, Borghi C. A Randomized, Double-Blinded, Placebo-Controlled, Clinical Study of the Effects of a Nutraceutical Combination (LEVELIP DUO®) on LDL Cholesterol Levels and Lipid Pattern in Subjects with Sub-Optimal Blood Cholesterol Levels (NATCOL Study). Nutrients. 2020; 12(10):3127. https://doi.org/10.3390/nu12103127
Chicago/Turabian StyleCicero, Arrigo F.G., Sergio D’Addato, and Claudio Borghi. 2020. "A Randomized, Double-Blinded, Placebo-Controlled, Clinical Study of the Effects of a Nutraceutical Combination (LEVELIP DUO®) on LDL Cholesterol Levels and Lipid Pattern in Subjects with Sub-Optimal Blood Cholesterol Levels (NATCOL Study)" Nutrients 12, no. 10: 3127. https://doi.org/10.3390/nu12103127
APA StyleCicero, A. F. G., D’Addato, S., & Borghi, C. (2020). A Randomized, Double-Blinded, Placebo-Controlled, Clinical Study of the Effects of a Nutraceutical Combination (LEVELIP DUO®) on LDL Cholesterol Levels and Lipid Pattern in Subjects with Sub-Optimal Blood Cholesterol Levels (NATCOL Study). Nutrients, 12(10), 3127. https://doi.org/10.3390/nu12103127







