Inducible Nitric Oxide Regulates Na-Glucose Co-Transport in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Ileitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
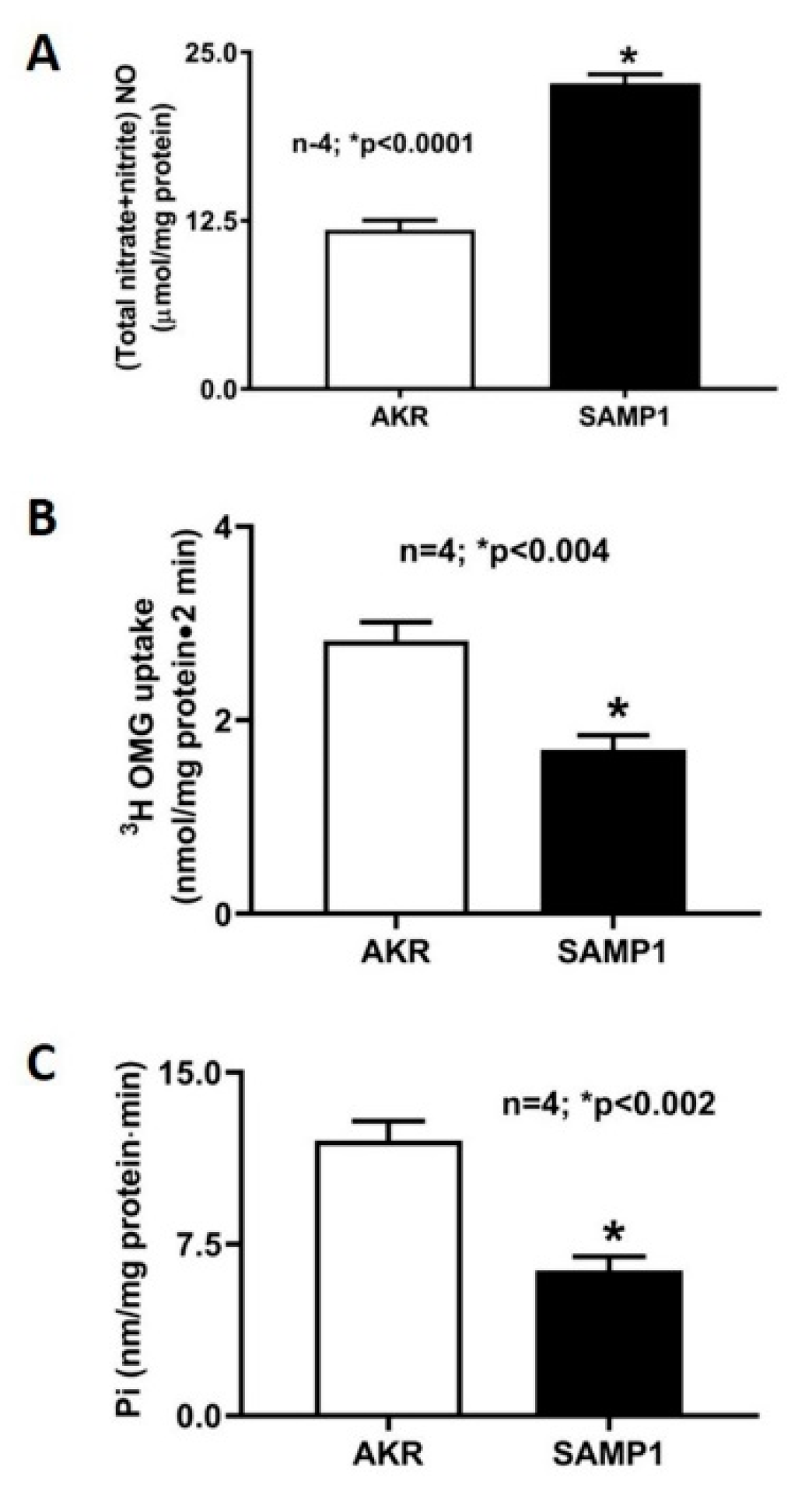
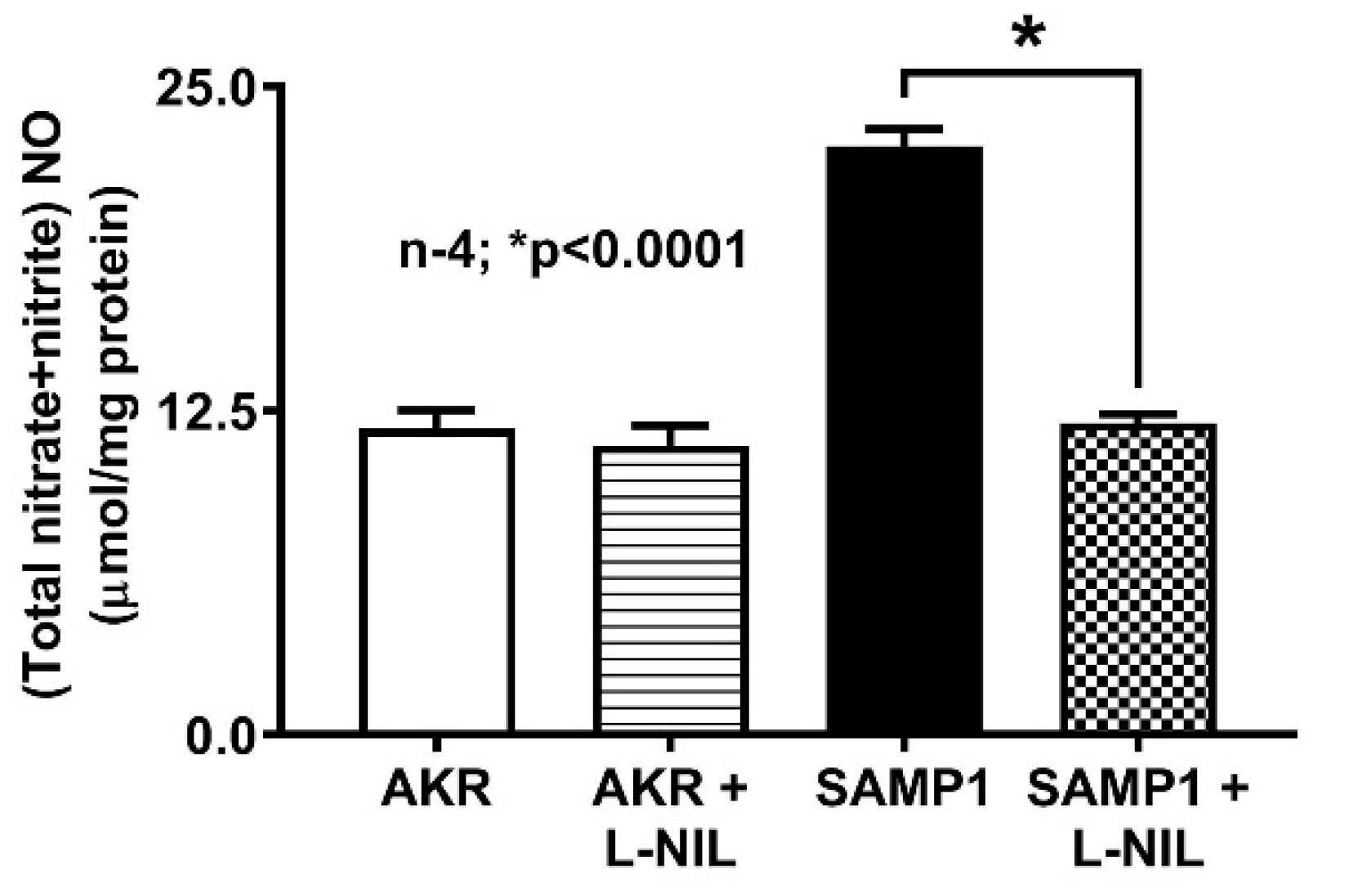
2.2. Measurement of NO
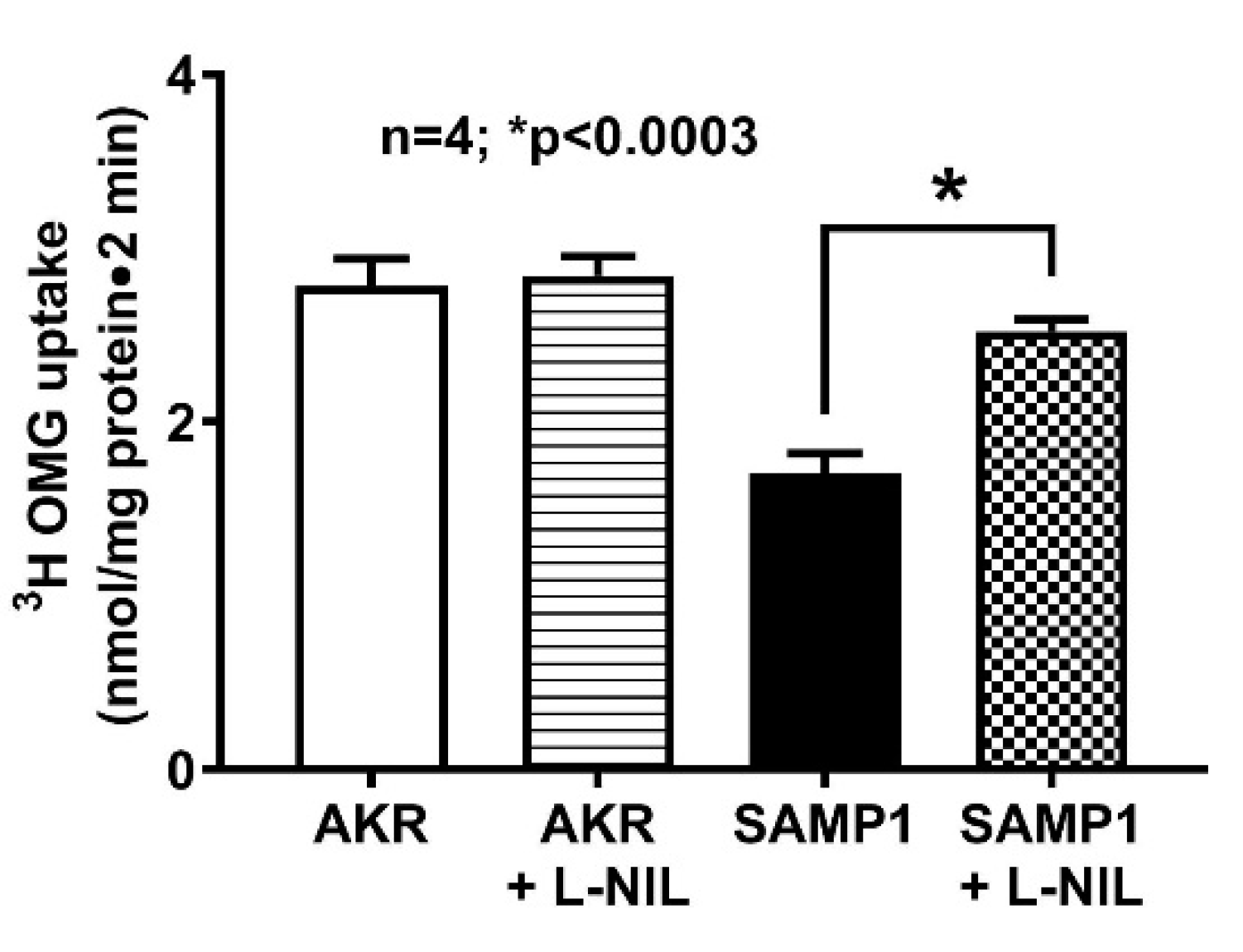
2.3. Na-Glucose Co-Transport Uptake Studies in Intact Villus Cells and BBMV
2.4. Na+/K+-ATPase Enzyme Measurement
2.5. RT-qPCR Analysis
2.6. Western Blot Analysis
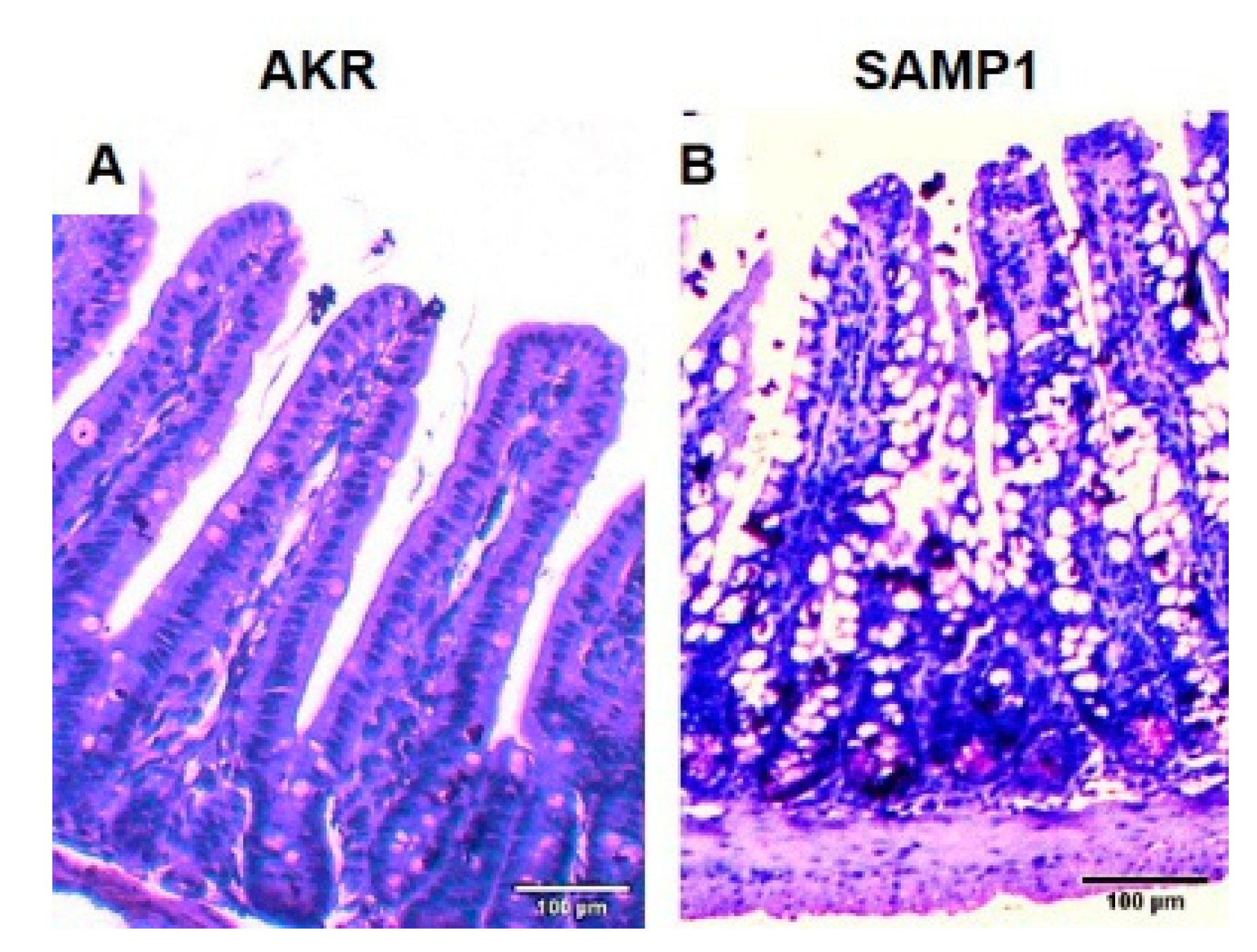
2.7. Histology and Immunofluorescence Study
2.8. Protein Quantification
2.9. Statistics
3. Results
3.1. Effect of L-NIL on Intracellular NO Levels
3.2. Effect of L-NIL Treatment on Na-Glucose Co-Transport in Intact Villus Cells
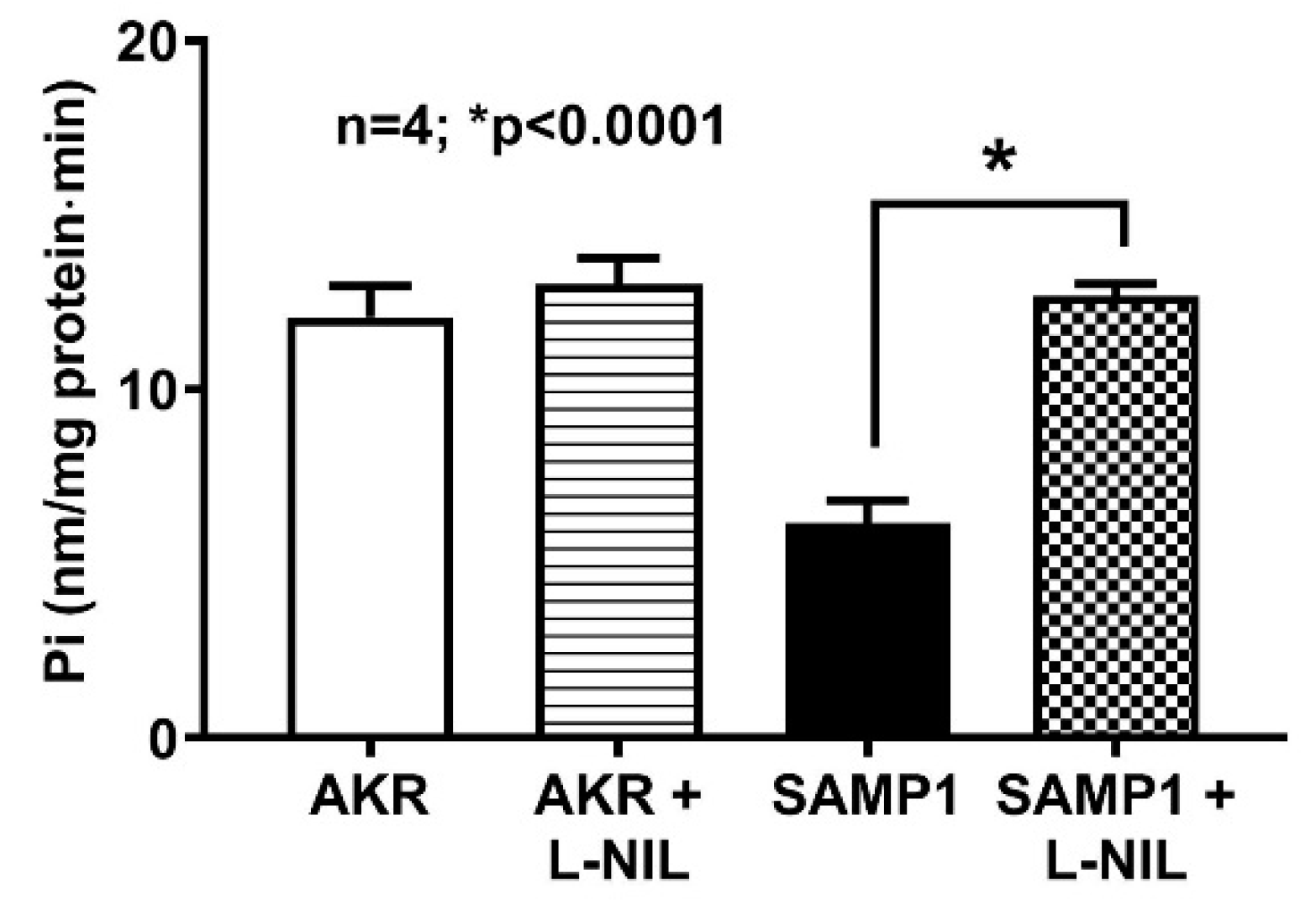
3.3. Effect of L-NIL on Na+/K+-ATPase Activity
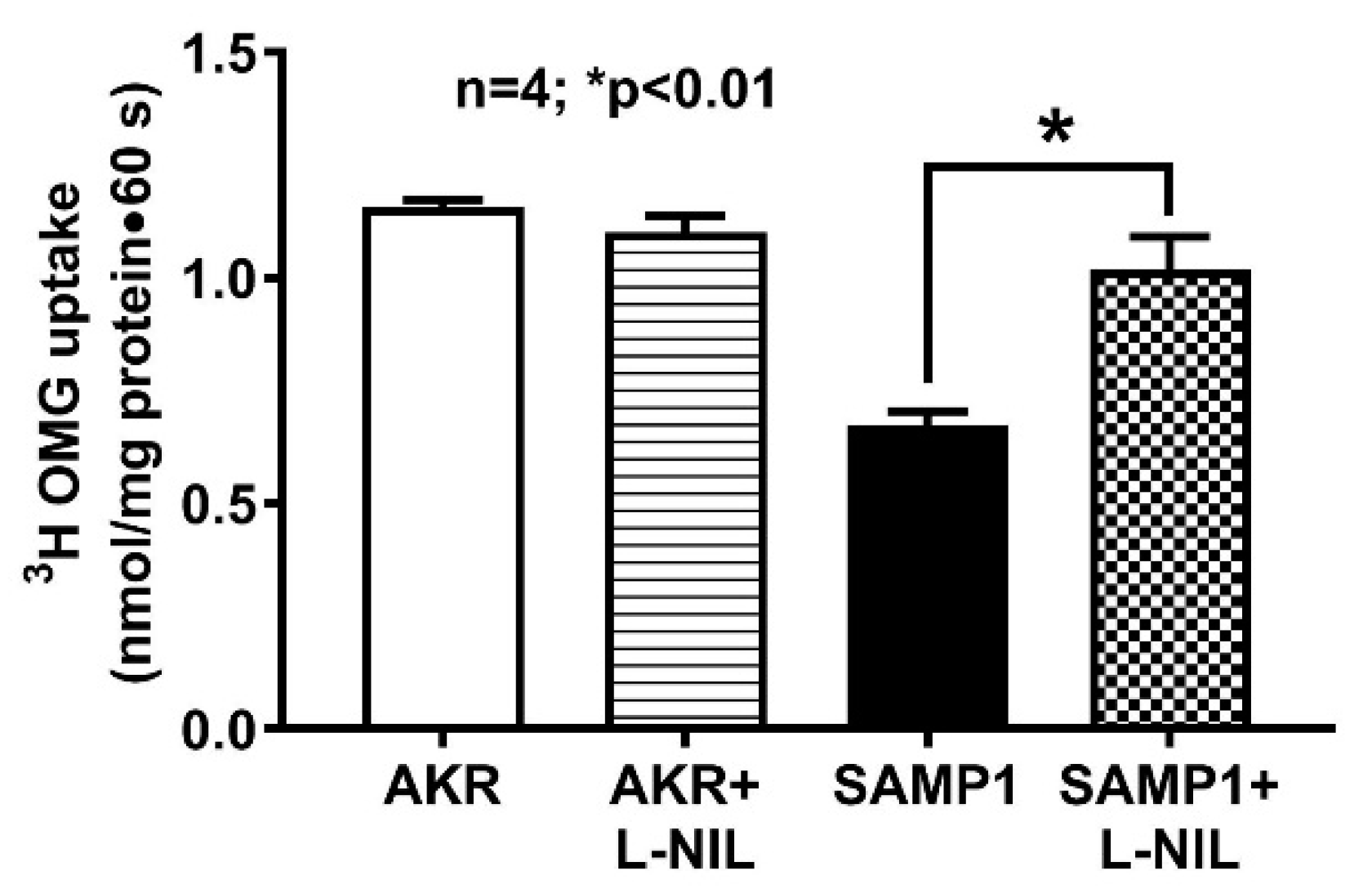
3.4. Effect of L-NIL on Na-Glucose Co-Transport in the BBMV of Ileal Villus Cells
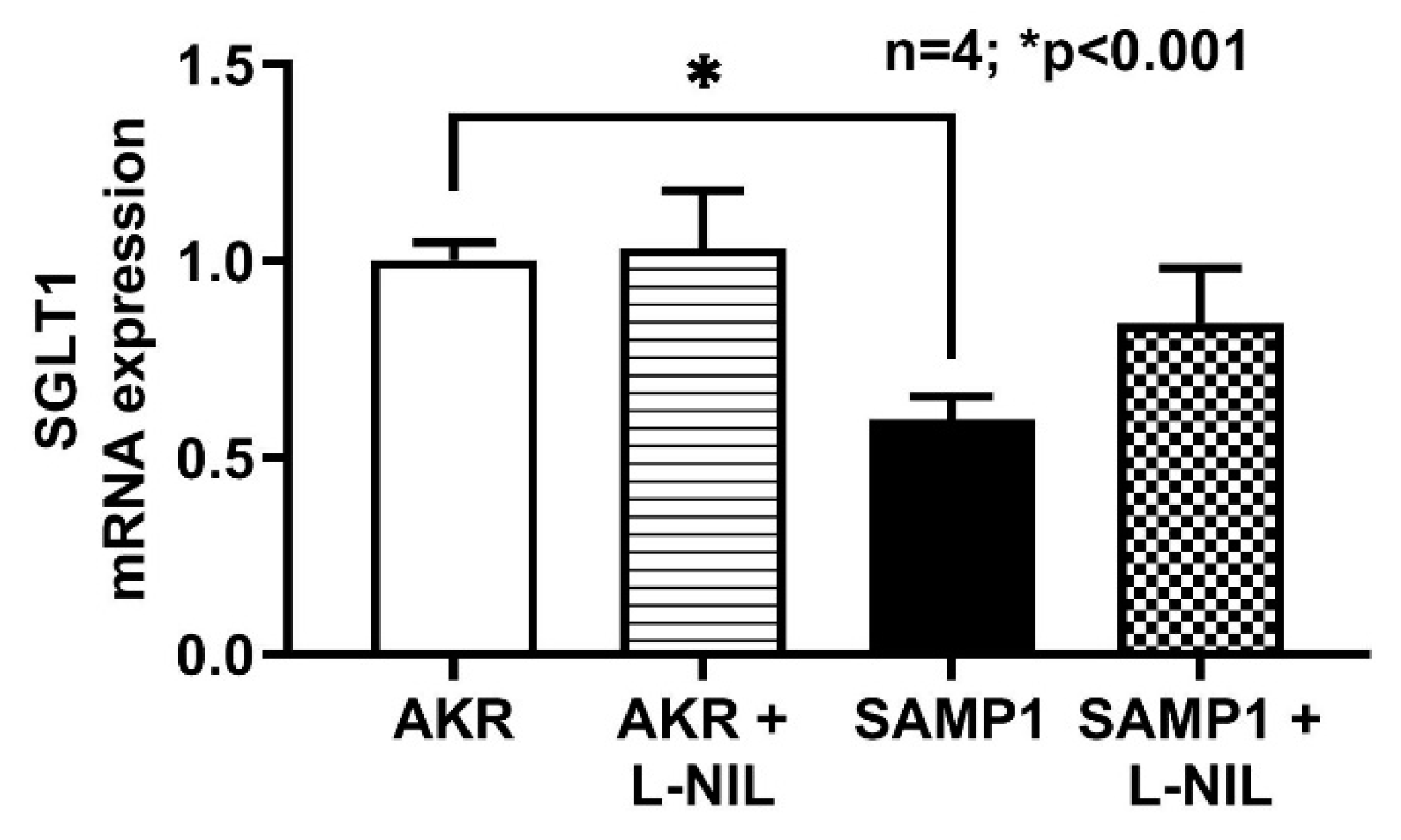
3.5. SGLT1 mRNA Expressions by RT-qPCR
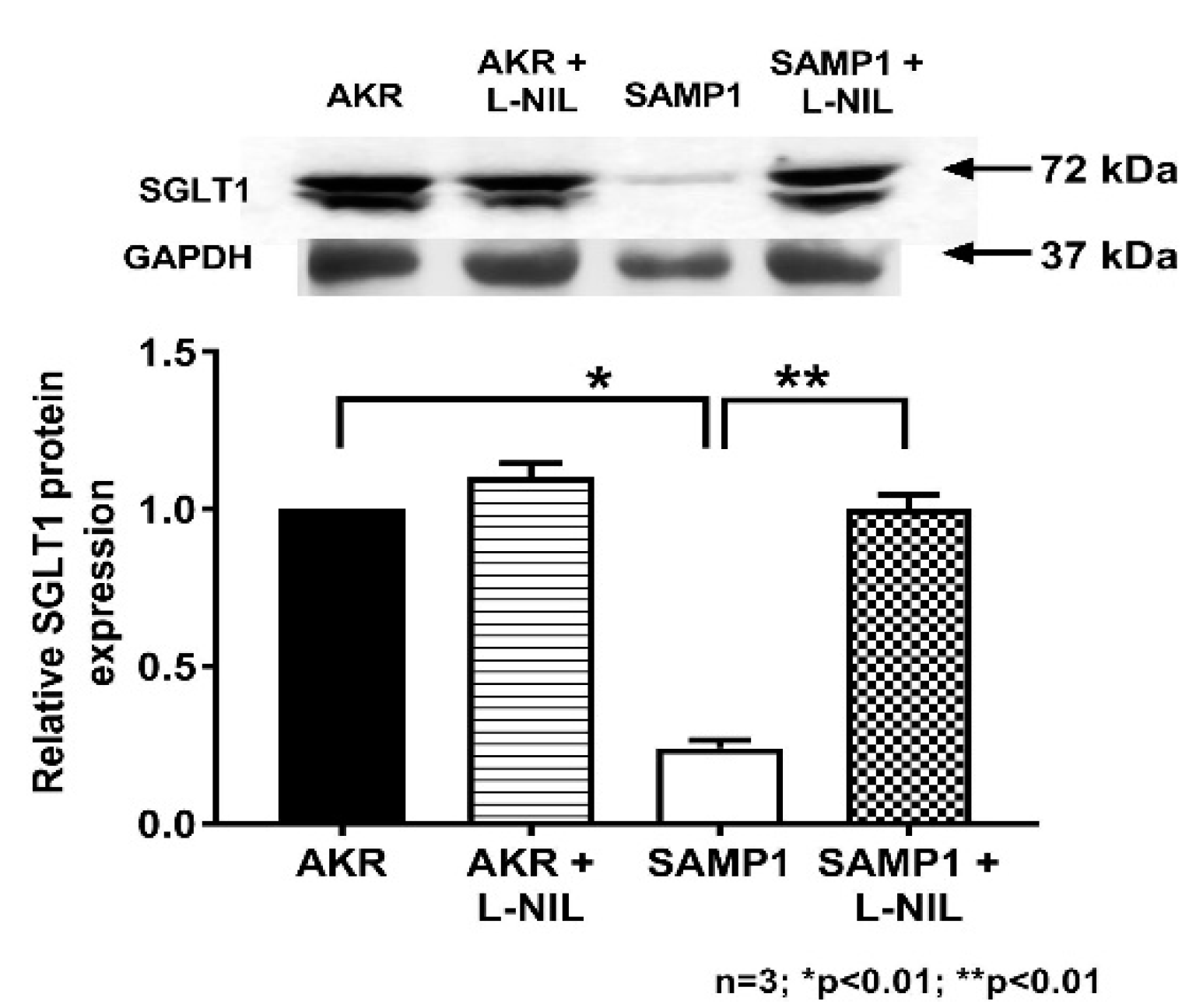
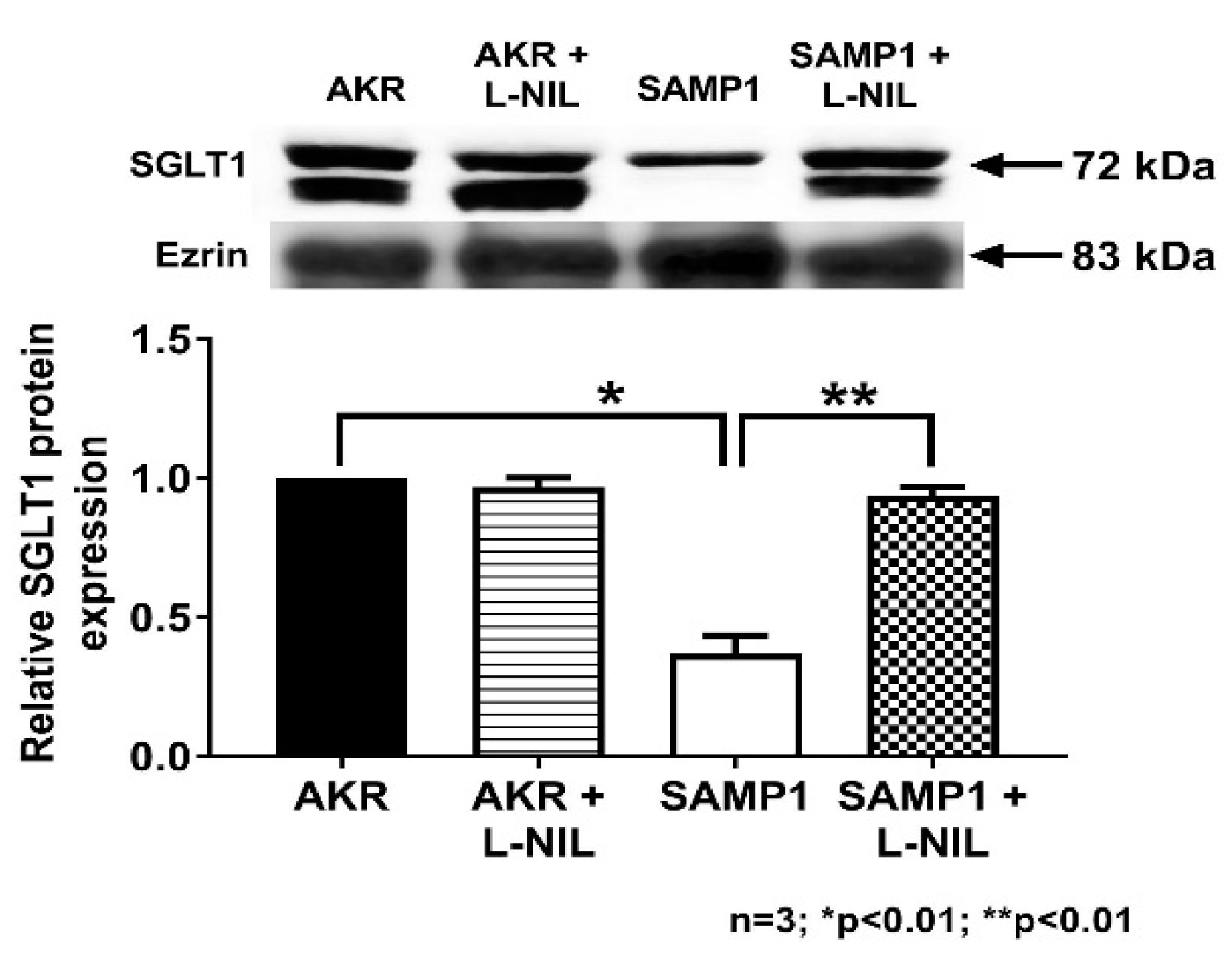
3.6. Quantitation of SGLT1 Protein by Western Blot Analysis
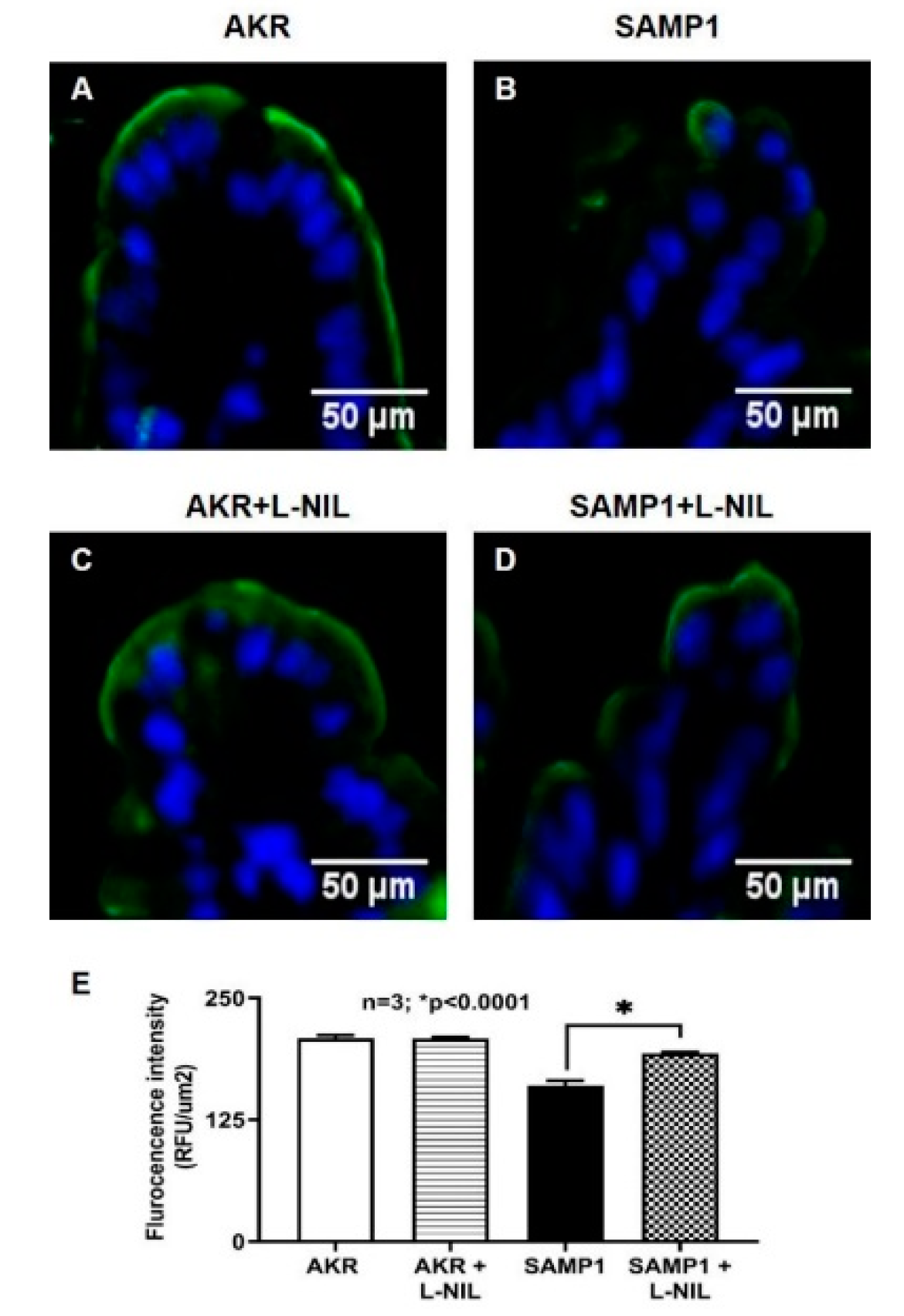
3.7. Immunohistochemistry of SGLT1 in SAMP1 Mice Intestine
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wright, E.M.; Hirayama, B.A.; Loo, D.F. Active sugar transport in health and disease. J. Intern. Med. 2007, 261, 32–43. [Google Scholar] [CrossRef]
- Wright, E.; Loo, D.; Hirayama, B.; Turk, E. Sugar Absorption. In Physiology of Gastrointestinal Tract, 4th ed.; Leonard Johnson, K.B., Ghishan, F., Merchant, J., Said, H., Wood, J., Eds.; Elsevier/Academic Press: San Diego, CA, USA, 2006; pp. 1653–1666. [Google Scholar]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Dahlhamer, J.M.; Zammitti, E.P.; Ward, B.W.; Wheaton, A.G.; Croft, J.B. Prevalence of Inflammatory Bowel Disease among Adults Aged >/=18 Years—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1166–1169. [Google Scholar] [CrossRef]
- Kaser, A.; Lee, A.-H.; Franke, A.; Glickman, J.N.; Zeissig, S.; Tilg, H.; Nieuwenhuis, E.E.; Higgins, D.E.; Schreiber, S.; Glimcher, L.H. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef]
- Wallace, J.L.; Chin, B.C. Inflammatory mediators in gastrointestinal defense and injury. Proc. Soc. Exp. Biol. Med. 1997, 214, 192–203. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Saha, P.; Arthur, S.; Kekuda, R.; Sundaram, U. Na-glutamine co-transporters B 0 AT1 in villus and SN2 in crypts are differentially altered in chronically inflamed rabbit intestine. Biochim. Biophys. Acta Biomembr. 2012, 1818, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, U.; Wisel, S.; Coon, S. Neutral Na-amino acid cotransport is differentially regulated by glucocorticoids in the normal and chronically inflamed rabbit small intestine. Am. J. Physiol. Gastrointest. 2007, 292, G467–G474. [Google Scholar] [CrossRef]
- Coon, S.; Kekuda, R.; Saha, P.; Sundaram, U. Glucocorticoids differentially regulate Na-bile acid cotransport in normal and chronically inflamed rabbit ileal villus cells. Am. J. Physiol. 2010, 298, G675–G682. [Google Scholar] [CrossRef]
- Sundaram, U.; Coon, S.; Wisel, S.; West, A.B. Corticosteroids reverse the inhibition of Na-glucose cotransport in the chronically inflamed rabbit ileum. Am. J. Physiol. 1999, 276, G211–G218. [Google Scholar] [CrossRef]
- Brosnan, C.; Lee, S.; Liu, J. Regulation of Inducible Nitric Oxide Synthase Expression in Human Glia: Implications for Inflammatory Central Nervous System Diseases; Portland Press Limited: London, UK, 1997. [Google Scholar]
- Kröncke, K.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; McCafferty, D.-M. Nitric oxide and intestinal inflammation. Am. J. Med. Sci. 2000, 109, 150–158. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, D.M.; Mudgett, J.S.; Swain, M.G.; Kubes, P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology 1997, 112, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Tan, S.; Wang, Z.; Deng, B.; Li, J.; Wang, Q.; Zhou, C.; Kang, X.; Yu, Z.; Zhuang, S. Chronic Kidney Disease Elicits an Intestinal Inflammation Resulting in Intestinal Dysmotility Associated with the Activation of Inducible Nitric Oxide Synthesis in Rat. Digestion 2018, 97, 205–211. [Google Scholar] [CrossRef]
- Alp, B.F.; Malkoc, E.; Demirer, Z.; Guragac, A.; Turker, T.; Altayli, E.; Ozcan, A.; Uysal, B.; Topal, T.; Akgul, E.O. Inhibition of inducible nitric oxide synthase prevents shock wave therapy induced renal injury. Ren. Fail. 2014, 36, 774–780. [Google Scholar] [CrossRef]
- Youn, J.; Lee, J.-S.; Na, H.-K.; Kundu, J.K.; Surh, Y.-J. Resveratrol and piceatannol inhibit iNOS expression and NF-κ B activation in dextran sulfate sodium-induced mouse colitis. Nutr. Cancer 2009, 61, 847–854. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; El-Adawy, S.A. Roflumilast, type 4 phosphodiesterase inhibitor, attenuates inflammation in rats with ulcerative colitis via down-regulation of iNOS and elevation of cAMP. Int. Immunopharmacol. 2018, 56, 36–42. [Google Scholar] [CrossRef]
- Rivera-Nieves, J.; Bamias, G.; Vidrich, A.; Marini, M.; Pizarro, T.T.; McDuffie, M.J.; Moskaluk, C.A.; Cohn, S.M.; Cominelli, F. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology 2003, 124, 972–982. [Google Scholar] [CrossRef]
- Pizarro, T.T.; Pastorelli, L.; Bamias, G.; Garg, R.R.; Reuter, B.K.; Mercado, J.R.; Chieppa, M.; Arseneau, K.O.; Ley, K.; Cominelli, F. SAMP1/YitFc mouse strain: A spontaneous model of Crohn’s disease-like ileitis. Inflamm. Bowel Dis. 2010, 17, 2566–2584. [Google Scholar] [CrossRef]
- Burns, R.C.; Rivera-Nieves, J.; Moskaluk, C.A.; Matsumoto, S.; Cominelli, F.; Ley, K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology 2001, 121, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, U.; Wisel, S.; Rajendren, V.M.; West, A.B. Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am. J. Physiol. 1997, 273, G913–G919. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, U.; Wisel, S.; Fromkes, J.J. Unique mechanism of inhibition of Na+-amino acid cotransport during chronic ileal inflammation. Am. J. Physiol. 1998, 275, G483–G489. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R.; Black, E.D. NHE3-dependent cytoplasmic alkalinization is triggered by Na(+)-glucose cotransport in intestinal epithelia. American journal of physiology. Cell Physiol. 2001, 281, C1533–C1541. [Google Scholar] [CrossRef]
- Forbush, B., 3rd. Assay of Na,K-ATPase in plasma membrane preparations: Increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal. Biochem. 1983, 128, 159–163. [Google Scholar] [CrossRef]
- Manoharan, P.; Coon, S.; Baseler, W.; Sundaram, S.; Kekuda, R.; Sundaram, U. Prostaglandins, not the leukotrienes, regulate Cl−/HCO3− exchange (DRA, SLC26A3) in villus cells in the chronically inflamed rabbit ileum. Biochim. Biophys. Acta Biomembr. 2013, 1828, 179–186. [Google Scholar] [CrossRef]
- Hardee, S.; Alper, A.; Pashankar, D.S.; Morotti, R.A. Histopathology of duodenal mucosal lesions in pediatric patients with inflammatory bowel disease: Statistical analysis to identify distinctive features. Pediatr. Dev. Pathol. 2014, 17, 450–454. [Google Scholar] [CrossRef]
- Conrad, M.A.; Carreon, C.K.; Dawany, N.; Russo, P.; Kelsen, J.R. Distinct histopathological features at diagnosis of very early onset inflammatory bowel disease. J. Crohn’s Colitis 2019, 13, 615–625. [Google Scholar] [CrossRef]
- Archampong, E.; Harris, J.; Clark, C. The absorption and secretion of water and electrolytes across the healthy and the diseased human colonic mucosa measured in vitro. Gut 1972, 13, 880–886. [Google Scholar] [CrossRef]
- Binder, H.J.; Ptak, T. Jejunal absorption of water and electrolytes in inflammatory bowel disease. J. Lab. Clin. Med. 1970, 76, 915–924. [Google Scholar] [PubMed]
- Powell, D. Immunophysiology of intestinal electrolyte transport. Compr. Physiol. 2010, 591–641. [Google Scholar] [CrossRef]
- Castro, G. Immunological regulation of electrolyte transport. In Textbook of Secretory Diarrhea; Lebenthal, E., Duffey, M., Eds.; Raven Press: New York, NY, USA, 1990; pp. 31–46. [Google Scholar]
- Sartor, R.B.; Powell, D.W. Mechanisms of diarrhea in intestinal inflammation and hypersensitivity: Immune system modulation of intestinal transport. In Diarrheal Diseases; Field, M., Ed.; Elsevier Science Publishing Co., Inc.: New York, NY, USA, 1991; pp. 75–114. [Google Scholar]
- Sundaram, U.; West, A.B. Effect of chronic inflammation on electrolyte transport in rabbit ileal villus and crypt cells. Am. J. Physiol. Gastrointest. 1997, 272, G732–G741. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.; Sundaram, U. Inducible nitric oxide regulates intestinal glutamine assimilation during chronic intestinal inflammation. Nitric Oxide 2015, 44, 98–104. [Google Scholar] [CrossRef]
- Singh, S.; Arthur, S.; Sundaram, U. Mechanisms of Regulation of Transporters of Amino Acid Absorption in Inflammatory Bowel Diseases. Compr. Physiol. 2020, 10, 673–686. [Google Scholar] [PubMed]
- Qian, J.; Fulton, D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front. Physiol. 2013, 4, 347. [Google Scholar] [CrossRef]
- Arthur, S.; Coon, S.; Kekuda, R.; Sundaram, U. Regulation of sodium glucose co-transporter SGLT1 through altered glycosylation in the intestinal epithelial cells. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1208–1214. [Google Scholar] [CrossRef]
- Manoharan, P.; Sundaram, S.; Singh, S.; Sundaram, U. Inducible nitric oxide regulates brush border membrane Na-glucose co-transport, but not Na: H exchange via p38 MAP kinase in intestinal epithelial cells. Cells 2018, 7, 111. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaniappan, B.; Sundaram, S.; Arthur, S.; Afroz, S.; Sundaram, U. Inducible Nitric Oxide Regulates Na-Glucose Co-Transport in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Ileitis. Nutrients 2020, 12, 3116. https://doi.org/10.3390/nu12103116
Palaniappan B, Sundaram S, Arthur S, Afroz S, Sundaram U. Inducible Nitric Oxide Regulates Na-Glucose Co-Transport in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Ileitis. Nutrients. 2020; 12(10):3116. https://doi.org/10.3390/nu12103116
Chicago/Turabian StylePalaniappan, Balasubramanian, Shanmuga Sundaram, Subha Arthur, Sheuli Afroz, and Uma Sundaram. 2020. "Inducible Nitric Oxide Regulates Na-Glucose Co-Transport in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Ileitis" Nutrients 12, no. 10: 3116. https://doi.org/10.3390/nu12103116
APA StylePalaniappan, B., Sundaram, S., Arthur, S., Afroz, S., & Sundaram, U. (2020). Inducible Nitric Oxide Regulates Na-Glucose Co-Transport in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Ileitis. Nutrients, 12(10), 3116. https://doi.org/10.3390/nu12103116




