Abstract
In cancer patients, loss of muscle mass is significantly associated with low tolerability of chemotherapy and poor survival. Despite the great strides in the treatment of cancer, targeted therapies such as tyrosine kinase inhibitors (TKIs) could exacerbate muscle wasting. Over recent years, the impact of skeletal muscle loss during TKI therapy on clinical outcomes has been in the spotlight. In this review, we focus on the different molecular pathways of TKIs potentially involved in muscle wasting. Then, we report the results of the studies assessing the effects of different TKI therapies—such as sorafenib, regorafenib, sunitinib, and lenvatinib—on muscle mass, and highlight their potential clinical implications. Finally, we discuss an integrative nutritional approach to be adopted during TKI treatment. The assessment of muscle mass from computerized tomography imaging could be helpful in predicting toxicity and prognosis in patients treated with TKI such as sorafenib. Early recognition of low muscle mass and effective personalized nutritional support could prevent or attenuate muscle mass wasting. However, the role of nutrition is still overlooked, and future clinical trials are needed to find the optimal nutritional support to countermeasure muscle mass depletion during TKI therapy.
Keywords:
muscle mass; sarcopenia; sorafenib; regorafenib; lenvatinib; sunitinib; toxicity; survival; nutrition; personalized medicine 1. Introduction
The discovery of the overexpression of kinases in various cancers has led to the development of tyrosine kinase inhibitors (TKIs). TKIs demonstrated to produce a significant improvement in survival rates in several cancers. Just to name a few examples, imatinib has revolutionized the treatment of chronic myelogenous leukemia as well as gastrointestinal stromal tumors (GISTs) [1]; sorafenib was the first therapy proven to prolong survival in patients with metastatic hepatocellular carcinoma (HCC) [2]; sunitinib provided for the first time a survival advantage over interferon to treat metastatic renal cell cancer (RCC) [3]. However, despite the great strides in the treatment of cancer, these agents can also induce frustrating dose-limiting toxicities (DLTs) partially due to the loss of body weight and muscle mass [4]. Indeed, the reduction in physical activity, the nutritional deficiencies resulting from cancer itself, and the side effects of oncologic treatment—such as nausea, vomiting, diarrhea, taste alteration, and early satiety—often lead to a loss of weight and lean tissue mass. Patients with poor nutritional status are often not able to tolerate chemotherapy and discontinue treatment. Low skeletal muscle mass was already shown to be a significant predictor of chemotherapy toxicity and survival in cancer patients [5,6,7]. Thus, research increasingly focuses on the importance of preserving skeletal muscle mass of patients receiving chemotherapy. In recent decades, a growing number of studies attempted to understand whether muscle wasting was exacerbated by TKI treatment and if such muscle loss was associated with toxicity and survival outcomes. This review aims to understand how TKI therapy could impact muscle mass in cancer patients and highlights potential clinical implications and nutritional opportunities. After focusing on the specific molecular pathways of TKI involved in muscle wasting, we review the impact of TKI therapy on muscle mass for several types of cancer and its implications in terms of clinical outcomes. Finally, we discuss the state of the art about nutritional strategies to counteract muscle wasting during these treatments.
2. Molecular Pathways of TKI Involved in Muscle Wasting
The maintenance of skeletal muscle mass is determined by a close balance between protein synthesis and protein degradation. Intracellular signaling cascades, regulating the mechanisms for muscle growth or muscle loss, initiate with a variety of chemical signals depending on nutritional and hormonal status—such as insulin or insulin-like growth factor-1 (IGF-1)—or energy state (AMP kinase) and physical activity, or other mediators of environmental stress (glucocorticoids, cytokines). A key point of integration in muscle protein synthesis is the phosphoinositide 3-kinase (PI3K)/thymoma viral proto-oncogene (AKT) kinase [8]. The insulin/IGF-1- Akt pathway increases skeletal muscle protein synthesis via inhibiting glycogen synthase kinase 3β and activating the mechanistic target of rapamycin complex 1 (mTORC1) signaling [9]. In turn, mTORC1 activates translation initiation and elongation, and ribosome biogenesis of proteins, and consequently muscle cell growth [10].
Tyrosine kinases are enzymes that target proteins involved in diverse normal cellular regulatory processes [11]. The receptors of tyrosine kinases are membrane-spanning cell surface proteins linking extracellular signals to the cytoplasm [12]. Ligand binding induces dimerization of these receptors, resulting in autophosphorylation of their cytoplasmic domains and activation of tyrosine kinase activity [11]. TKIs target different receptor tyrosine kinases such as vascular endothelial growth factor receptors (VEGFRs). VEGFRs 1/2/3 are located on vascular endothelial cells and play a key role in angiogenesis [13]. In healthy adults, angiogenesis is a complex multistep process that is tightly controlled by a balance between endogenous pro-angiogenic and anti-angiogenic factors. However, angiogenesis is a crucial prognostic factor in cancer and frequently correlates with tumor progression, disease severity, and metastatic potential [14]. TKIs such as sorafenib and sunitinib partly exert their anti-tumor activity by inhibiting the tyrosine kinase activity of VEGFR-2 [15]. Platelet-derived growth factor receptors (PDGFRs) are also TKIs’ targets involved in angiogenesis: mutational activation or upregulation of PDGFRs lead to uncontrolled blood vessel formation and cancer. Moreover, PDGFR-β emerged as a key regulator of cell growth and division and mediates a significant impact on malignant cells and tumor microenvironment [16]. Epidermal growth factor receptors (EGFRs) are transmembrane protein receptors for extracellular protein ligands belonging to the group of epidermal growth factor (EGF) [17]. The overexpression of the EGFR is associated with the development of a wide variety of tumors such as non-small cell lung cancer and breast cancer [18]. Finally, KIT, RET, and B-RAF are other receptor tyrosine kinases encoded by proto-oncogenes: the overexpression or mutations of these proteins can promote carcinogenesis in several tissues [19].
All these tyrosine kinase receptors have been associated with the signaling pathway of activation of the PI3K/AKT/mTOR, which promotes cell growth, survival, and proliferation. In many cancers, the PI3K/AKT/mTOR pathway is overactive, thus reducing apoptosis and allowing cell proliferation [20]. On the other hand, the activation of the AKT/mTOR pathway and its downstream targets is essential for regulating skeletal muscle fiber size [21]. In vivo, genetic activation of the AKT/mTOR pathway caused muscle hypertrophy and prevented atrophy, whereas blocking of this pathway blocked muscle hypertrophy [22]. By inhibiting receptor tyrosine kinase signaling, TKIs have shown to indirectly suppress AKT and mTOR. In particular, sorafenib blocks VEGFR, PDGFR, c-KIT, and RET and inhibits downstream Raf serine/threonine kinase activity to prevent tumor growth, as demonstrated in advanced HCC, RCC, and unresectable thyroid cancer [23]. Additionally, regorafenib is an oral multikinase inhibitor of VEGFR1-3, KIT, RET, B-RAF, and PDGFR [24]. As illustrated in HCC in Figure 1, several TKIs indirectly inhibit mTOR and consequently impair cell proliferation, protein synthesis, and muscle growth.
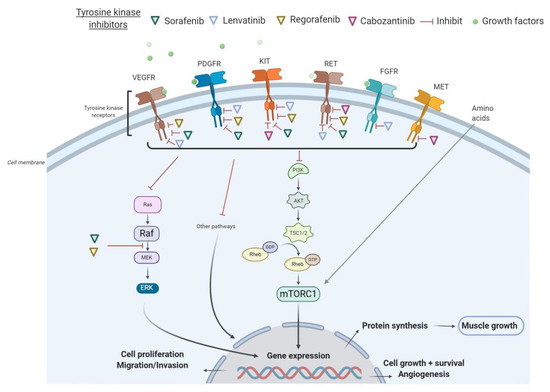
Figure 1.
Mechanisms of action of tyrosine kinase inhibitors (TKIs) in hepatocellular carcinoma (HCC). Abbreviations: FGFR, fibroblast growth factor receptors; GDP, guanosine diphosphate; GTP, guanosine triphosphate; mTORC1, mammalian target of rapamycin complex 1; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; TSC, tuberous sclerosis complex; VEGFR, vascular endothelial growth factor receptor.
Although the effects of mTOR inhibitors on muscle mass have still to be fully elucidated, a recent human study [25] demonstrated that the long-term use of mTOR inhibitors induces a marked loss of muscle mass. This confirms that TKIs may alter muscle mass, probably due to interferences with pathways of AKT/mTOR. As reported in the next paragraph, several studies assessed the impact of TKI treatment on muscle mass in cancer patients.
3. Impact of TKI Treatment on Muscle Wasting
An electronic search was conducted in PubMed and Web of Science databases, using different combinations of the search terms “(Afatinib OR Alectinib OR Axitinib OR Bosutinib OR Brigatinib OR Cabozantinib OR Ceritinib OR Crizotinib OR Dasatinib OR Erlotinib OR Gefitinib OR Ibrutinib OR Imatinib OR Lapatinib OR Lenvatinib OR Nilotinib OR Osimertinib OR Pazopanib OR Ponatinib OR Regorafenib OR Ruxolitinib OR Sorafenib OR Sunitinib OR Vandetanib) AND (sarcopenia OR (muscle OR muscular *) OR (body AND composition))”. The screening process was conducted independently by two reviewers. Any disagreements were solved through discussion until consensus. Our search was conducted until July 2020. Table 1 detailed the results of the eleven studies [26,27,28,29,30,31,32,33,34,35,36] assessing the effect of TKI treatment on skeletal muscle mass from computerized tomography (CT) scan at the third lumbar (L3) vertebra.

Table 1.
Studies assessing the effect of TKI treatment on skeletal muscle mass.
3.1. Sorafenib
Sorafenib is approved for the treatment of advanced HCC, metastatic RCC, and unresectable thyroid cancer [37]. Sorafenib blocks VEGFR, PDGFR, c-KIT, and RET, and inhibits downstream Raf serine/threonine kinase activity to prevent tumor growth [38].
Antoun et al. [31] studied muscle mass changes in 48 patients with metastatic RCC treated with sorafenib. They noted that 52% of patients had low skeletal muscle index (SMI) before initiation of treatment with sorafenib (for men < 52.4 cm2/m2 and for women < 38.5 cm2/m2), whereas after one year of treatment, 71% of patients had low SMI, with an average weight loss of 4.2 kg. Another study [34] evaluated 101 metastatic RCC patients including 45 patients treated with sorafenib and 30 with sunitinib. The mean SMI was reduced from 41.6 to 39.9 cm2/m2 after 3–4 months of targeted therapy. Furthermore, Uchikawa et al. evaluated 23 HCC patients treated with sorafenib and found a significant decrease in SMI between baseline and 1–3 months after starting treatment (p < 0.01) [33]. To better investigate the role of the treatment itself in muscle wasting, a post hoc analysis of the Phase III DECISION trial study [32] compared SMI changes among 365 patients with advanced thyroid cancer in sorafenib vs. placebo groups between baseline and 6 months after starting sorafenib therapy. At 6 months, the mean muscle mass of patients receiving sorafenib was lower than at baseline and significantly lower than for patients receiving placebo (p < 0.0001).
Although the mechanisms of sorafenib remain poorly understood, these findings could be partially explained by the inhibition of the Ras/Raf/MEK/extracellular signal-regulated kinase (ERK) signaling pathway that, in turn, inhibits muscle anabolism [39]. Moreover, the inhibition of VEGF expression by sorafenib may occur also via the ERK/nuclear factor (NF)-κB pathway to reduce metastasis and invasiveness of human HCC [40]. The NF-κB signaling pathway interestingly impacts both the muscle fibers and muscle stem cells [41]. These mechanisms of sorafenib—like other TKIs sharing the same molecular targets—may have a possible relationship with muscle wasting. However, these conjectures need to be confirmed with further experimental studies.
3.2. Lenvatinib
Lenvatinib is an oral multikinase inhibitor targeting VEGF1–3, fibroblast growth factor receptors 1–4 (FGFR1–4), RET, c-KIT, stem cell growth factor receptor (SCGFR), and PDGFRα [42]. It is approved as first-line treatment for advanced and metastatic differentiated thyroid carcinoma after radioactive iodine failure and for the treatment of advanced HCC in first-line therapy [43]. Recently, a brief report of two case reports of patients with advanced unresectable HCC treated with lenvatinib found a minimal impact of lenvatinib on muscle mass—detected with CT scan—with stable disease for over 24 months [27]. On the other hand, Hiraoka et al. reported a post hoc analysis of a multicentre study quantifying psoas muscle loss by CT scan in HCC patients treated with lenvatinib and demonstrated a decline in PI after 4 weeks of treatment (−0.210 ± 0.315 cm2/m2) in 41 patients and after 12 weeks of treatment (−0.275 ± 0.372 cm2/m2) in 25 patients [28]. However, Hiraoka et al. used psoas muscle assessment, which is less precise and suitable to assess total muscle mass than skeletal muscle indexes such as SMI and skeletal muscle area (SMA) (see above). Recently, a subgroup analysis of the study of Uchikawa et al. found in 8 patients (with advanced HCC and Child-Pugh grade A) treated with lenvatinib a significant decrease in SMI between baseline and 1–3 months after starting treatment (p = 0.025) [33], independently with progression disease. Further studies with a larger sample size are needed to clarify the impact of lenvatinib on muscle mass.
3.3. Sunitinib
Sunitinib is a multitargeted TKI, inhibiting PDGFRs, KIT, and VEGFR-1-2-3 [44]. Sunitinib is the reference standard of care for the first-line treatment of metastatic RCC or pancreatic neuroendocrine tumors (PNETs), and for the second-line treatment for patients affected by unresectable and/or metastatic GISTs who had a failure to imatinib. One study found a significant decrease in lean body mass (LBM) (p = 0.02) [35] and skeletal muscle area SMA (p = 0.02) in 18 metastatic RCC patients after starting sunitinib treatment (3–4 months) compared with baseline. Gu et al. [34] also found in 30 (out of 101) patients treated with sunitinib a reduction in the mean SMI from 41.6 to 39.9 cm2/m2 after 3–4 months of therapy.
3.4. Regorafenib
Regorafenib is an oral multikinase inhibitor, pharmacologically similar to sorafenib, that blocks kinases involved in angiogenesis (VEGFR-1-2-3), oncogenesis (c-KIT, Ret and wild-type, and V600-mutated BRAF), and the tumor microenvironment (PDGFR and FGFR) [38]. Regorafenib is, to date, approved for the second-line treatment of metastatic colorectal cancer (CRC), after the failure of fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy; moreover, it is effective in second-line therapy for the treatment of advanced HCC after sorafenib failure [45], and in patients affected by GISTs who had progression or intolerance to imatinib or sunitinib.
In an animal study, regorafenib has been shown to cause skeletal muscle loss through a possible mechanism including increasing levels of autophagy-dependent protein markers and abnormal mitochondrial homeostasis via ERK1/2 and glycogen synthase kinase 3 beta (GSK3β) pathways [46]. In humans, one study [29], evaluating 22 CRC patients treated with regorafenib, found a statistically significant skeletal muscle loss during treatment (median SMI change: −2.75 cm2/m2; p < 0.0001). Furthermore, a recent study assessed skeletal muscle mass (SMM) changes in 36 metastatic CRC patients, who received regorafenib or TAS-102 (a novel oral fluoropyrimidine) in third-line treatment [30]. The SMM change after regorafenib therapy was significantly worse compared with TAS-102 therapy (p = 0.001) [30].
3.5. Pazopanib
Pazopanib is a multikinase inhibitor approved for the treatment of patients with advanced RCC or advanced soft tissue sarcoma, in the second-line [47]. Pazopanib is a VEGFR inhibitor with activity against vascular EGFR-1, -2, and -3, and PDGFRs [48]. Kostek et al. [35] found, in 18 metastatic RCC patients, a non-significant decrease in LBM and SMA after starting pazopanib. Conversely, they noted that SMA and LBM were significantly reduced with sunitinib therapy compared with pazopanib therapy. The reason for these discrepancies is unclear, but it might be related to their different kinase selectivities [35]. Indeed, sunitinib was shown to inhibit more kinases than pazopanib [49]. However, further studies are warranted to analyze the effects of pazopanib on the muscle mass and the implications for clinical outcomes.
3.6. Axitinib
Axitinib mostly targets VEGFR-1-2-3 [50]. It is indicated for the treatment of advanced RCC after failure of one prior systemic therapy (sunitinib or cytokines) [51]. One study [26] evaluated the effect of axitinib on muscle mass in 24 patients with advanced non-metastatic RCC (n = 23), showing a significant decrease in SMI at 12 weeks after starting treatment compared to baseline (−2.9 cm2/m2; IQR 1.7–6.2; p < 0.001).
3.7. Vandetanib
Vandetanib selectively blocks EGFR and VEGFR-2. It is approved for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable or metastatic disease [52]. A randomized controlled trial of 33 patients with advanced medullary thyroid carcinoma compared SMI changes from baseline to month 3 after starting treatment in patients treated with vandetanib vs. patients treated with placebo [36]. Surprisingly, vandetanib was found to be significantly associated with a gain in muscle mass over time (p = 0.009). The authors hypothesized that vandetanib, by inhibiting the MAPK pathway, could reduce the burst of inflammatory cytokines—especially interleukin (IL) 6—involved in the stimulation of muscle growth and myogenesis through regulation of the proliferative capacity of muscle stem cells [53]. Another protective mechanism could be the reduction in diarrhea during vandetanib therapy. In any case, further studies are required to confirm these results.
Considering these preliminary findings and the negative prognostic role of low muscle mass in cancer, muscle wasting during multikinase inhibitor therapy may impact clinical outcomes. Several studies further assessed the associations between muscle mass changes during TKI treatment and toxicity and/or survival.
4. Implications of Muscle Wasting in Clinical Outcomes in Patients Treated with TKI
In this part, we highlight the impact of low muscle mass/muscle wasting on clinical outcomes during TKI treatment for several types of cancer.
4.1. RCC
A recent study found that RCC patients treated with axitinib with baseline low SMI tended to have a lower response rate to treatment [26]. Moreover, low SMI is predictive of treatment-related toxicity in patients with metastatic RCC receiving sunitinib [54] or sorafenib [31,55]. Particularly, Kostek et al. noted that loss of SMA and LBM with sunitinib was more substantial than with pazopanib [35]. Although treatment efficacies of both drugs were similar, DLTs were more frequent in RCC patients treated with sunitinib compared with pazopanib. Similarly, muscle wasting during TKI treatment could have a potential impact on survival outcomes. Patients with metastatic RCC and low SMI have been shown to have a worse overall survival (OS) after cytoreductive nephrectomy compared with patients with high SMI [56]. Fukushima et al. also showed that low SMI was significantly associated with worse OS in 92 patients with metastatic RCC [57].
4.2. HCC
In HCC patients receiving sorafenib, Mir et al. showed that the amount of DLTs was significantly correlated with low SMI [4]. Furthermore, a cohort study of European Caucasian cirrhotic patients with advanced HCC treated with sorafenib showed that low SMI was a predictor of reduced survival and cancer treatment toxicity [58]. Recently, Uojima et al. showed the number of severe adverse advents during the first two months of lenvatinib treatment was significantly associated with low SMI in 100 patients with HCC [59]. Low muscle mass has been already demonstrated to be a strong and independent risk factor for mortality in advanced HCC patients [60]. Consequently, we can infer that muscle wasting during treatment induced by sorafenib or lenvatinib therapy could negatively impact toxicity and survival in HCC patients. Moreover, the alterations of the nutritional status by TKI treatment and HCC itself could be also incremented by the underlying disease, such as liver cirrhosis [61]. Indeed, in cirrhosis, malnutrition may be due to several conditions: pancreatic insufficiency, cholestasis, portosystemic shunt, bile deficiency through inadequate absorption of long-chain fatty acids, metabolic alterations such as high protein catabolism, reduced glucose homeostasis due to alterations of gluconeogenesis, low glycogen stores, pro-inflammatory cytokines such as TNF alpha, interleukins [62]. Therefore, considering the high prevalence of sarcopenia in HCC patients and the effects of TKI shown on muscle mass, more attention on muscle mass changes during therapy is needed when TKIs are prescribed.
4.3. CRC
Low muscle mass is present in up to 40% of patients at the initial metastatic CRC diagnosis [63]. Moreover, several studies [64,65] found a loss of skeletal muscle during chemotherapy in patients with metastatic CRC and its association with treatment modifications, toxicity, and survival. Recently, Gokyer et al. assessed a significant association between low SMI and DLT in 36 patients with metastatic CRC who received regorafenib [66]. In this study, they determined more DLTs (p = 0.005) and grade 2 or 3 toxicities in low SMI patients compared with the high SMI group. However, there were no significant differences in terms of progression-free survival (PFS) and OS between sarcopenic and non-sarcopenic patients. More recently, Bekir et al. showed in a Cox regression analysis that loss of skeletal muscle mass was an independent prognostic indicator for OS (HR, 2.87; 95%CI: 1.07–7.42; p = 0.03) in 36 metastatic CRC patients [30]. These results confirmed that in clinical practice, CRC patients with low muscle mass should be detected and followed at the beginning of the treatment.
4.4. Thyroid Cancer
Despite a significant effect of sorafenib on muscle mass, the risk of early toxicity leading to dose modification was not significantly higher in thyroid cancer patients with low SMI compared with those with high SMI in a study of Huillard et al. [32]. These results contrast with those in HCC and RCC patients. Discrepancies between cancer types could be explained by the differences in underlying disease and the effects of prior treatments. Indeed, patients with RCC may have been treated also with interferon, a pro-inflammatory cytokine that has complex physiologic effects, including effects on skeletal muscle homeostasis and repair. In HCC, muscle mass depletion is a common feature of cirrhosis, commonly found in patients with HCC, representing an independent prognostic factor.
Although these findings need to be confirmed, given that muscle wasting could be exacerbated by TKI treatment and associated with worse clinical outcomes, monitoring muscle mass changes during TKI treatment and proposing adequate nutritional support could represent an opportunity to prevent or treat low muscle mass during TKI therapy, leading to better treatment tolerability and clinical outcomes.
5. Nutritional Challenges
5.1. Early Assessment and Monitoring of Nutritional Status During Treatment
As detailed in Table 1, many cancer patients undergoing TKI therapy are already sarcopenic before the initiation of treatment, with a percentage ranging from 30.4% to 54%. Sarcopenia is a condition characterized by a loss of skeletal muscle mass quantity, quality, and function. The European Working Group on Sarcopenia in Older People 2 (EWGSOP2) defined sarcopenia as primary when no specific cause is evident except for age. Indeed, aging is associated with hormone and cytokine imbalance, impaired muscle protein synthesis and regeneration, and increased splanchnic extraction (the retention of dietary amino acids by the gut and liver for their own needs) which lower the anabolic response to ingested proteins [67]. Sarcopenia is considered secondary when other causes are evident such as illness, physical inactivity, or inadequate intake of energy due to anorexia or malabsorption [68]. Cancer-related sarcopenia is recognized by international consensus as a negative prognostic factor for OS, complications after surgery, and toxicities in cancer patients. Cancer itself activates a systemic inflammation syndrome, with impaired protein turnover, anabolic resistance, loss of muscle mass, and acute-phase protein synthesis. Moreover, cancer patients often reduce food intake due to primary anorexia (sustained by the effect of cancer cytokines at the central nervous system level) and physical impairment of the digestive system (xerostomia, nausea, vomiting, diarrhea, intestinal obstruction, or malabsorption) [69]. Furthermore, hospitalization and bed-rest are associated with a significant decline in muscle mass and nutritional status [70,71].
As regards the studies reported in this review, the median age varied from 51 from 72 years: thus, muscle wasting could be also related to age-related anabolic resistance. However, studies assessed the effect on muscle changes from baseline to approximately 3–12 months of TKI treatment. Considering the short duration of treatment, the effect of age-related muscle loss could be reasonably overlooked as a confounding factor.
In this context, international guidelines [72] recommend the screening and assessment of the nutritional status of cancer patients at diagnosis with the evaluation of nutritional intake, weight changes, and body mass index (BMI). The most commonly used screening tools are Nutrition Risk Screening 2002 (NRS-2002), and Malnutrition Universal Screening Tool (MUST). Although such methods are standardized and easy to perform by healthcare staff—without specific nutritional skills—the assessment of nutritional status is still underused in oncology. In patients identified at high risk of malnutrition, objective and quantitative assessment of muscle mass, physical performance, and degree of systemic inflammation are strongly recommended by the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines [72]. Such evaluations are performed by clinical nutritionists and trained dietitians. Regarding muscle mass, a severe depletion is assessed by validated methods, such as
- −
- Appendicular skeletal muscle index (ASMI), determined by dual-energy x-ray absorptiometry (men < 7.26 kg/m2; women < 5.45 kg/m2);
- −
- SMI, determined from oncological CT imaging (men < 55 cm2/m2; women < 39 cm2/m2);
- −
- Whole body fat-free mass index without bone determined by bioelectrical impedance (men < 14.6 kg/m2; women < 11.4 kg/m2).
As we previously described, we systematically searched studies assessing muscle mass during TKI therapy. All studies assessing the effects of TKI treatment on skeletal muscle mass used computerized tomography (CT) image analysis at the third lumbar level (L3). CT scan is, to date, considered the gold standard non-invasive tool to assess muscle mass quantity and quality [73]. CT-scan images are always available for cancer patients since CT is routinely used at diagnosis for tumor staging and at follow-up visits to monitor response to treatments. Some studies [27,30,31] assessed SMA, evaluating all the cross-sectional skeletal muscle area at the third lumbar vertebra (bilateral rectus abdominis, external oblique, internal oblique, transverse abdominal, psoas, quadratus lumborum, and paraspinal muscle). Other studies calculated SMI by dividing SMA by the square of the height [26,29,33,34,36] or skeletal muscle density (SMD; in Hounsfield) [34] or LBM estimated from SMA as described by Mourtzakis et al. [73]. One study [28] used the bilateral psoas index (PI; in cm2/m2) by dividing psoas muscle area by the square of the height. However, the psoas is a minor muscle, and experts argue that it is not representative of overall sarcopenia [74,75].
Since the majority of patients experienced a loss of muscle mass during their disease course before initiation of TKI therapy, early assessment and reassessment of skeletal muscle mass during treatment should be performed to screen patients and to propose adequate nutritional support.
5.2. Personalized Nutritional Support
To date, as far as we are aware, no data are available on nutritional interventions and muscle mass in cancer patients treated with TKIs. Hence, we need to refer to standard guidelines for nutrition in cancer patients. The ESPEN guidelines strongly recommended a personalized nutritional support with specific energy (between 25 and 30 kcal/kg/day) and protein (above 1 g/kg/day and, if possible, up to 1.5 g/kg/day) requirements [72]. Accordingly, oral nutritional supplements (ONS) and artificial nutrition (enteral or parenteral) may be used if oral dietary intake is not sufficient. A recent large, multicenter randomized controlled clinical trial (EFFORT trial) [76] of 2088 medical inpatients at risk of malnutrition evaluated the effects of a protocol-guided individualized nutritional support—including ONS and artificial nutrition—to reach protein and caloric goals on the risk of adverse clinical outcomes. During the hospital stay, caloric and protein goals were, respectively, reached by 79%, and 76% of patients receiving nutritional support. After 30 days, these patients significantly experienced lower rates of adverse events and mortality compared with the control group. Even if cancer patients were only 20% of the entire study population, these findings are the testament of the effectiveness of individualized nutritional support on short-term outcomes in patients at risk of malnutrition [76]. A secondary analysis of the EFFORT trial further showed that there was no legacy effect on long-term outcomes such as mortality at 6 months [77]. This could be explained by the short duration of the length of hospital stay (only 10 days) and consequently too short a time for nutritional support. Thus, there could be a strong rationale to offer continued nutritional support after discharge to reduce high malnutrition-associated mortality. Other works conducted in cancer patients confirm the crucial role of personalized nutritional support. Particularly, in colon and liver cancer patients, the NutriCatt protocol—a nutritional prehabilitation to cancer surgery based on the ESPEN guidelines—ameliorated postoperative complications, length of stay, and hospital costs [78,79].
In cancer patients treated with TKIs, a recent observational study [80] showed that energy intake was lower than recommended by the ESPEN guidelines, and none of the patients covered the protein requirements during follow-up. These results highlighted the lack of nutritional assessment and support in multidisciplinary protocols, especially in cancer patients undergoing TKIs treatments.
Among other possible nutritional strategies, branched-chain amino acids (BCAAs) may reduce muscle mass loss in cancer patients. BCAAs are three amino acids with branched side chains (i.e., valine, leucine, and isoleucine). The ability of amino acids to stimulate protein synthesis is reduced in cancer patients. This anabolic resistance could be in a part counteracted by giving specific amino acids [81]. BCAAs have been shown to promote protein synthesis in muscle tissue in rats [82,83]. However, regulation of muscle protein synthesis in rats may differ in many respects compared with humans. Skeletal muscle comprises a much smaller percentage of the total body mass in rats as compared to humans [84]. Nevertheless, in humans, Nair et al. demonstrated that an intravenous infusion of leucine may decrease protein degradation, contributing to the decrease in plasma essential amino acids [85]. In patients with advanced abdominal metastatic adenocarcinoma [86,87], BCAA-enriched formulas may improve whole-body leucine kinetics and leucine balance, thereby favorably influencing protein metabolism in cancer cachexia. A study of Takeda et al. [88] enrolled 78 HCC patients treated with sorafenib into two groups: BCAA granules (Livact; Ajinomoto, Tokyo, Japan) containing 952 mg of L-isoleucine, 1904 mg of L-leucine, and 1144 mg of L-valine per sachet (intervention group) or only a regular diet (control group). In a multivariate analysis, the administration period of sorafenib, as well as the median survival time, were significantly longer in BCAA-treated patients than the control group [88]. This could be explained by the fact that plasma levels of BCAA decreased in patients with cirrhosis—a frequent underlying disease of HCC, leading to energy-protein malnutrition and impaired glycolysis and glycogenesis [89]. Many investigators reported the usefulness of BCAA supplementation in the treatment of cirrhosis and HCC [90,91,92]. However, the stimulation of protein synthesis by BCAAs—particularly leucine—could imply signaling pathways including mTOR [93]. Consequently, a large supplementation of BCAAs may potentially negatively impact tumor growth, as an undesirable side effect [94]. Hence, even if BCAA supplementation is probably useful for maintaining hepatic functional reserve in HCC patients [95], further studies are required to clarify the impact of BCAAs on protein balance and tumor growth to define the correct dosage of BCAAs supplementation.
β-hydroxy β-methyl butyrate (HMB) supplementation might be also a potential nutritional strategy to counteract the loss of muscle mass. A study [96] enrolled 472 patients with cancer which were supplemented by a mixture of HMB, glutamine (GLN), and arginine (ARG) or by an isonitrogenous, isocaloric control showing a strong trend towards higher fat-free mass in HMB/ARG/GLN-supplemented patients compared with controls. However, to date, studies assessing the impact of personalized nutritional strategies on muscle mass and clinical outcomes in cancer patients are very limited, and still lacking in patients treated with TKI therapy.
6. Conclusions
In sum, skeletal muscle changes have been observed during axitinib, lenvatinib, pazopanib, regorafenib, sorafenib, sunitinib, and vandetanib treatments. For all other TKIs, to our knowledge, no data are available, and studies are required to understand the impact of TKI therapy on muscle mass. Although the evidence remains to be confirmed, this review suggests that the loss of skeletal muscle mass may be exacerbated by different TKI treatments—such as axitinib, lenvatinib, regorafenib, sorafenib, or sunitinib—and could be associated with worse clinical outcomes in a wide range of cancers. Thus, the measurement of muscle mass from CT imaging could be helpful in predicting tolerance to TKI therapy and prognosis. In this context, every effort should be made to attenuate muscle wasting through early recognition of the loss of muscle mass and effective personalized nutritional support. Future clinical trials are needed to find the optimal nutritional support to countermeasure muscle mass depletion during TKI therapy.
Author Contributions
Conceptualization, E.R. and M.C.M.; data curation, P.R. and M.C.; formal analysis, P.R.; investigation, C.P. and E.B.; methodology, F.R.P. and M.P.; supervision, G.T. and A.G.; validation, A.S.; writing—original draft, P.R. and E.R.; writing—review and editing, E.R., M.C., M.C.M., C.P., E.B. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Dagher, R.; Cohen, M.; Williams, G.; Rothmann, M.; Gobburu, J.; Robbie, G.; Rahman, A.; Chen, G.; Staten, A.; Griebel, D.; et al. Approval summary: Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin. Cancer Res. 2002, 8, 3034–3038. [Google Scholar] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Coppin, C. Sunitinib for advanced renal cell cancer. Biology 2008, 2, 97–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2045–2054. [Google Scholar] [CrossRef]
- Rinninella, E.; Fagotti, A.; Cintoni, M.; Raoul, P.; Scaletta, G.; Scambia, G.; Gasbarrini, A.; Mele, M.C. Skeletal muscle mass as a prognostic indicator of outcomes in ovarian cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2020, 30, 654–663. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Glass, D.J. Pi3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010, 346, 267–278. [Google Scholar]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1- Akt/PKB pathway: Insights from genetic models. Skelet. Muscle. 2011, 1, 4. [Google Scholar] [CrossRef]
- Adegoke, O.A.; Abdullahi, A.; Tavajohi-Fini, P. mTORC1 and the regulation of skeletal muscle anabolism and mass. Appl. Physiol. Nutr. Metab. 2012, 37, 395–406. [Google Scholar] [CrossRef]
- Arora, A.; Scholar, E.M. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharm. Exp. 2005, 315, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Pawson, T. Regulation and targets of receptor tyrosine kinases. Eur. J. Cancer 2002, 38, S3–S10. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor (VEGFR) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Mendel, D.B.; Laird, A.D.; Smolich, B.D.; Blake, R.A.; Liang, C.; Hannah, A.L.; Shaheen, R.M.; Ellis, L.M.; Weitman, S.; Shawver, L.K.; et al. Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des. 2000, 15, 29–41. [Google Scholar] [PubMed]
- Gotink, K.J.; Verheul, H.M. Anti-angiogenic tyrosine kinase inhibitors: What is their mechanism of action? Angiogenesis 2010, 13, 1–14. [Google Scholar] [CrossRef]
- Nordby, Y.; Richardsen, E.; Rakaee, M.; Ness, N.; Donnem, T.; Patel, H.R.; Busund, L.T.; Bremnes, R.M.; Andersen, S. High expression of PDGFR-β in prostate cancer stroma is independently associated with clinical and biochemical prostate cancer recurrence. Sci. Rep. 2017, 7, 43378. [Google Scholar] [CrossRef] [PubMed]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 54. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Edinger, A.L.; Thompson, C.B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell. 2002, 13, 2276–2288. [Google Scholar] [CrossRef]
- Adnane, L.; Trail, P.A.; Taylor, I.; Wilhelm, S.M. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzym. 2006, 407, 597–612. [Google Scholar]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Shimokata, T.; Honda, K.; Kondoh, C.; Hayashi, N.; Yoshino, Y.; Sassa, N.; Nakano, Y.; Gotoh, M.; Ando, Y. Muscle wasting associated with the long-term use of mTOR inhibitors. Mol. Clin. Oncol. 2016, 5, 641–646. [Google Scholar] [CrossRef]
- Chéry, L.; Borregales, L.D.; Fellman, B.; Urbauer, D.L.; Garg, N.; Parker, N.; Katz, M.H.G.; Wood, C.G.; Karam, J.A. The Effects of Neoadjuvant Axitinib on Anthropometric Parameters in Patients with Locally Advanced Non-metastatic Renal Cell Carcinoma. Urology 2017, 108, 114–121. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Mele, M.C.; De Gaetano, A.M.; Marini, M.G.; Mora, V.; Gasbarrini, A. Minimal impact of lenvatinib (Lenvima®) on muscle mass in advanced hepatocellular carcinoma and implications for treatment duration. Two cases from the REFLECT study. Eur. Rev. Med. Pharm. Sci. 2019, 23, 10132–10138. [Google Scholar]
- Hiraoka, A.; Kumada, T.; Kariyama, K.; Takaguchi, K.; Atsukawa, M.; Itobayashi, E.; Tsuji, K.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Real-life Practice Experts for HCC (RELPEC) Study Group, HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med. 2019, 8, 137–146. [Google Scholar] [CrossRef]
- Huemer, F.; Schlintl, V.; Hecht, S.; Hackl, H.; Melchardt, T.; Rinnerthaler, G.; Greil, R.; Weiss, L. Regorafenib Is Associated With Increased Skeletal Muscle Loss Compared to TAS-102 in Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2019, 18, 159–166. [Google Scholar] [CrossRef]
- Hacioglu, M.B.; Kostek, O.; Kurt, N.; Kucukarda, A.; Gokyer, A.; Ustabasioglu, F.E.; Karatas, F.; Tuncbilek, N.; Uzunoglu, S.; Bilici, A.; et al. Comparison of skeletal muscle mass loss in patients with metastatic colorectal cancer treated with regorafenib or TAS-102. J. BUON 2019, 24, 2198–2204. [Google Scholar]
- Antoun, S.; Baracos, V.E.; Birdsell, L.; Escudier, B.; Sawyer, M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. 2010, 21, 1594–1598. [Google Scholar] [CrossRef]
- Huillard, O.; Jouinot, A.; Tlemsani, C.; Brose, M.S.; Arrondeau, J.; Meinhardt, G.; Fellous, M.; De Sanctis, Y.; Schlumberger, M.; Goldwasser, F. Body Composition in Patients with Radioactive Iodine-Refractory, Advanced Differentiated Thyroid Cancer Treated with Sorafenib or Placebo: A Retrospective Analysis of the Phase III DECISION Trial. Thyroid 2019, 29, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, S.; Kawaoka, T.; Namba, M.; Kodama, K.; Ohya, K.; Morio, K.; Nakahara, T.; Murakami, E.; Tsuge, M.; Hiramatsu, A.; et al. Skeletal muscle loss during tyrosine kinase inhibitor treatment for advanced hepatocellular carcinoma patients. Liver Cancer 2020, 9, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wu, J.; Liu, X.; Zhang, H.; Shi, G.; Zhu, Y.; Ye, D. Early skeletal muscle loss during target therapy is a prognostic biomarker in metastatic renal cell carcinoma patients. Sci. Rep. 2017, 7, 7587. [Google Scholar] [CrossRef] [PubMed]
- Köstek, O.; Yılmaz, E.; Hacıoğlu, M.B.; Demircan, N.C.; Gökyer, A.; Uzunoğlu, S.; Tunçbilek, N.; Çiçin, İ.; Erdoğan, B. Changes in skeletal muscle area and lean body mass during pazopanib vs sunitinib therapy for metastatic renal cancer. Cancer Chemother. Pharm. 2019, 83, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, M.H.; Borget, I.; Broutin, S.; Baracos, V.E.; Leboulleux, S.; Baudin, E.; Paci, A.; Deroussent, A.; Schlumberger, M.; Antoun, S. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: Results from a placebo-controlled study. J. Clin. Endocrinol. Metab. 2013, 98, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Allegato I Riassunto Delle Caratteristiche del Prodotto. Available online: https://www.ema.europa.eu/en/documents/product-information/nexavar-epar-product-information_it.pdf (accessed on 21 January 2020).
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef]
- Li, L.; Zhao, G.D.; Shi, Z.; Qi, L.L.; Zhou, L.Y.; Fu, Z.X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016, 12, 3045–3050. [Google Scholar] [CrossRef]
- Chiang, I.T.; Liu, Y.C.; Wang, W.H.; Hsu, F.T.; Chen, H.W.; Lin, W.J.; Chang, W.Y.; Hwang, J.J. Sorafenib inhibits TPA-induced MMP-9 and VEGF expression via suppression of ERK/NF-κB pathway in hepatocellular carcinoma cells. Vivo 2012, 26, 671–681. [Google Scholar]
- Damrauer, J.S.; Stadler, M.E.; Acharyya, S.; Baldwin, A.S.; Couch, M.E.; Guttridge, D.C. Chemotherapy-induced muscle wasting: Association with NF-κB and cancer cachexia. Eur. J. Transl. Myol. 2018, 28, 7590. [Google Scholar] [CrossRef]
- Zschäbitz, S.; Grüllich, C. Lenvatinib: A Tyrosine Kinase Inhibitor of VEGFR 1-3, FGFR 1-4, PDGFRα, KIT and RE. Recent Results Cancer Res. 2018, 211, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/product-information/lenvima-epar-productinformation_it.pdf (accessed on 21 January 2020).
- Motzer, R.J.; Rini, B.I.; Bukowski, R.M.; Curti, B.D.; George, D.J.; Hudes, G.R.; Redman, B.G.; Margolin, K.A.; Merchan, J.R.; Wilding, G.; et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006, 295, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Huot, J.R.; Essex, A.L.; Gutierrez, M.; Barreto, R.; Wang, M.; Waning, D.L.; Plotkin, L.I.; Bonetto, A. Chronic Treatment with Multi-Kinase Inhibitors Causes Differential Toxicities on Skeletal and Cardiac Muscles. Cancers 2019, 11, E571. [Google Scholar] [CrossRef] [PubMed]
- Allegato I Riassunto Delle Caratteristiche del Prodotto. Available online: https://www.ema.europa.eu/en/documents/product-information/votrient-epar-product-information_it.pdf (accessed on 6 October 2020).
- Schutz, F.A.; Choueiri, T.K.; Sternberg, C.N. Pazopanib: Clinicaldevelopment of a potent anti-angiogenic drug. Crit Rev. Oncol. Hematol. 2011, 77, 163–171. [Google Scholar] [CrossRef]
- Schmidinger, M.; Wittes, J. First-line treatment of metastatic renal cell carcinoma after COMPARZ and PISCES. Curr. Opin. Urol. 2015, 25, 395–401. [Google Scholar] [CrossRef]
- Rugo, H.S.; Herbst, R.S.; Liu, G.; Park, J.W.; Kies, M.S.; Steinfeldt, H.M.; Pithavala, Y.K.; Reich, S.D.; Freddo, J.L.; Wilding, G. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: Pharmacokinetic and clinical results. J. Clin. Oncol. 2005, 23, 5474–5483. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/product-information/inlyta-epar-productinformation_it.pdf (accessed on 21 January 2020).
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022465s-010S-012lbl.pdf (accessed on 21 January 2020).
- Belizário, J.E.; Fontes-Oliveira, C.C.; Borges, J.P.; Kashiabara, J.A.; Vannier, E. Skeletal muscle wasting and renewal: A pivotal role of myokine IL-6. Springerplus 2016, 5, 619. [Google Scholar] [CrossRef]
- Huillard, O.; Mir, O.; Peyromaure, M.; Tlemsani, C.; Giroux, J.; Boudou-Rouquette, P.; Ropert, S.; Delongchamps, N.B.; Zerbib, M.; Goldwasser, F. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br. J. Cancer. 2013, 108, 1034–1041. [Google Scholar] [CrossRef]
- Cushen, S.J.; Power, D.G.; Teo, M.Y.; MacEneaney, P.; Maher, M.M.; McDermott, R.; O’Sullivan, K.; Ryan, A.M. Body Composition by Computed Tomography as a Predictor of Toxicity in Patients With Renal Cell Carcinoma Treated With Sunitinib. Am. J. Clin. Oncol. 2017, 40, 47–52. [Google Scholar] [CrossRef]
- Sharma, P.; Zargar-Shoshtari, K.; Caracciolo, J.T.; Fishman, M.; Poch, M.A.; Pow-Sang, J.; Sexton, W.J.; Spiess, P.E. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol. Oncol. 2015, 33, 339.e17–e23. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Nakanishi, Y.; Kataoka, M.; Tobisu, K.; Koga, F. Prognostic Significance of Sarcopenia in Patients with Metastatic Renal Cell Carcinoma. J. Urol. 2016, 195, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, G.; Gigante, E.; Iavarone, M.; Begini, P.; Sangiovanni, A.; Iannicelli, E.; Biondetti, P.; Pellicelli, A.M.; Miglioresi, L.; Marchetti, P.; et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United Eur. Gastroenterol. J. 2018, 6, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Uojima, H.; Chuma, M.; Tanaka, Y.; Hidaka, H.; Nakazawa, T.; Iwabuchi, S.; Kobayashi, S.; Hattori, N.; Ogushi, K. Morimoto, M.; et al. Skeletal muscle mass influences tolerability and prognosis in hepatocellular carcinoma patients treated with lenvatinib. Liver Cancer 2019, 9, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Begini, P.; Gigante, E.; Antonelli, G.; Carbonetti, F.; Iannicelli, E.; Anania, G.; Imperatrice, B.; Pellicelli, A.M.; Fave, G.D.; Marignani, M. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann. Hepatol. 2017, 16, 107–114. [Google Scholar] [CrossRef]
- Molfino, A.; Johnson, S.; Medici, V. The Challenges of Nutritional Assessment in Cirrhosis. Curr. Nutr. Rep. 2017, 6, 274–280. [Google Scholar] [CrossRef]
- Silva, M.; Gomes, S.; Peixoto, A.; Torres-Ramalho, P.; Cardoso, H.; Azevedo, R.; Cunha, C.; Macedo, G. Nutrition in Chronic Liver Disease. GE Port. J. Gastroenterol. 2015, 22, 268–276. [Google Scholar] [CrossRef]
- Thoresen, L.; Frykholm, G.; Lydersen, S.; Ulveland, H.; Baracos, V.; Prado, C.M.; Birdsell, L.; Falkmer, U. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin. Nutr. 2013, 32, 65–72. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef]
- Kurk, S.; Peeters, P.; Stellato, R.; Dorresteijn, B.; de Jong, P.; Jourdan, M.; Creemers, G.J.; Erdkamp, F.; de Jongh, F.; Kint, P.; et al. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J. Cachexia Sarcopenia Muscle. 2019, 10, 803–813. [Google Scholar] [CrossRef]
- Gökyer, A.; Küçükarda, A.; Köstek, O.; Hacıoğlu, M.B.; Sunal, B.S.; Demircan, N.C.; Uzunoğlu, S.; Solak, S.; İşsever, K.; Çiçin, I.; et al. Relation between sarcopenia and dose-limiting toxicity in patients with metastatic colorectal cancer who received regorafenib. Clin. Transl. Oncol. 2019, 21, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Gachon, P.; Beaufrere, B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am. J. Clin. Nutr. 1997, 65, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; De Lorenzo, A.; Anselmi, G.; Gagliardi, L.; Addolorato, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. May nutritional status worsen during hospital stay? A sub-group analysis from a cross-sectional study. Intern. Emerg. Med. 2019, 14, 51–57. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Baracos, V.E. Psoas as a sentinel muscle for sarcopenia: A flawed premise. J. Cachexia Sarcopenia Muscle 2017, 8, 527–528. [Google Scholar] [CrossRef]
- Rutten, I.J.G.; Ubachs, J.; Kruitwagen, R.F.P.M.; Beets-Tan, R.G.H.; Olde Damink, S.W.M.; Van Gorp, T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 630–638. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Kutz, A.; Bregenzer, T.; Hoess, C.; et al. Six-month outcomes after individualized nutritional support during the hospital stay in medical patients at nutritional risk: Secondary analysis of a prospective randomized trial. Clin. Nutr. 2020. S0261-5614(20)30435-0. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Persiani, R.; D’Ugo, D.; Pennestrì, F.; Cicchetti, A.; Di Brino, E.; Cintoni, M.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. NutriCatt protocol in the Enhanced Recovery After Surgery (ERAS) program for colorectal surgery: The nutritional support improves clinical and cost-effectiveness outcomes. Nutrition 2018, 50, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Lai, Q.; Rinninella, E.; Mimmo, A.; Vellone, M.; Panettieri, E.; Adducci, E.; Cintoni, M.; Mele, M.C.; Gasbarrini, A.; et al. The impact of personalized nutritional support on postoperative outcome within the enhanced recovery after surgery (ERAS) program for liver resections: Results from the NutriCatt protocol. Updates Surg. 2020. [Google Scholar] [CrossRef]
- Higuera-Pulgar, I.; Ribed, A.; Carrascal-Fabian, M.L.; Romero-Jiménez, R.M.; Velasco-Gimeno, C.; Bretón-Lesmes, I.; Camblor-Álvarez, M.; Cuerda-Compes, C.; García-Peris, P. Evolution of nutritional status and survival in patients with cancer on tyrosine kinase inhibitors treatment. Endocrinol. Diabetes Nutr. 2019, 66, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Antoun, S.; Raynard, B. Muscle protein anabolism in advanced cancer patients: Response to protein and amino acids support, and to physical activity. Ann. Oncol. 2018, 29, ii10–ii17. [Google Scholar] [CrossRef]
- Buse, M.G. In vivo effects of branched chain amino acids on muscle protein synthesis in fasted rats. Horm. Metab. Res. 1981, 13, 502–505. [Google Scholar] [CrossRef]
- Garlick, P.J.; Grant, I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched chain amino acids. Biochem. J. 1988, 254, 579–584. [Google Scholar] [CrossRef]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, 30. [Google Scholar] [CrossRef]
- Nair, K.S.; Schwartz, R.G.; Welle, S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am. J. Physiol. 1992, 263, E928–E934. [Google Scholar] [CrossRef]
- Tayek, J.A.; Bistrian, B.R.; Hehir, D.J.; Martin, R.; Moldawer, L.L.; Blackburn, G.L. Improved protein kinetics and albumin synthesis by branched chain amino acid-enriched total parenteral nutrition in cancer cachexia. A prospective randomized crossover trial. Cancer 1986, 58, 147–157. [Google Scholar] [CrossRef]
- Hunter, D.C.; Weintraub, M.; Blackburn, G.L.; Bistrian, B.R. Branched chain amino acids as the protein component of parenteral nutrition in cancer cachexia. Br. J. Surg. 1989, 76, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Nishikawa, H.; Iguchi, E.; Ohara, Y.; Sakamoto, A.; Saito, S.; Nishijima, N.; Nasu, A.; Komekado, H.; Kita, R.; et al. Effect of treatment with branched-chain amino acids during sorafenib therapy for unresectable hepatocellular carcinoma. Hepatol. Res. 2014, 44, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.W.; Poon, R.T. Role of branched-chain amino acids in management of cirrhosis and hepatocellular carcinoma. Hepatol. Res. 2008, 38, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Ichikawa, T.; Nakao, K.; Miyaaki, H.; Shibata, H.; Matsuzaki, T.; Muraoka, T.; Honda, T.; Otani, M.; Akiyama, M.; et al. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr. Res. 2009, 29, 89–93. [Google Scholar] [CrossRef]
- Morihara, D.; Iwata, K.; Hanano, T.; Kunimoto, H.; Kuno, S.; Fukunaga, A.; Yotsumoto, K.; Takata, K.; Tanaka, T.; Sakurai, K.; et al. Late-evening snack with branched-chain amino acids improves liver function after radiofrequency ablation for hepatocellular carcinoma. Hepatol. Res. 2012, 42, 658–667. [Google Scholar] [CrossRef]
- Ishikawa, T.; Michitaka, I.; Kamimura, H.; Higuchi, K.; Kubota, T.; Seki, K.; Ohta, H.; Yoshida, T.; Kamimura, T. Oral branched-chain amino acids administration improves impaired liver dysfunction after radiofrequency ablation therapy for hepatocellular carcinoma. Hepatogastroenterology 2009, 56, 1491–1495. [Google Scholar]
- Kimball, S.R.; Jefferson, L.S. Amino acids as regulators of gene expression. Nutr. Metab. 2004, 17. [Google Scholar] [CrossRef]
- Nair, K.S.; Short, K.R. Hormonal and signaling role of branched-chain amino acids. J. Nutr. 2005, 135, 1547S–1552S. [Google Scholar] [CrossRef]
- Rinninella, E.; Cerrito, L.; Spinelli, I.; Cintoni, M.; Mele, M.C.; Pompili, M.; Gasbarrini, A. Chemotherapy for hepatocellular carcinoma: Current evidence and future perspectives. J. Clin. Transl. Hepatol. 2017, 5, 235–248. [Google Scholar] [CrossRef][Green Version]
- May, P.E.; Barber, A.; D’Olimpio, J.T.; Hourihane, A.; Abumrad, N.N. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am. J. Surg. 2002, 183, 471–479. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).