Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health
Abstract
:1. Introduction
2. Dietary Fibre Present in Cereals and Pseudo-Cereals
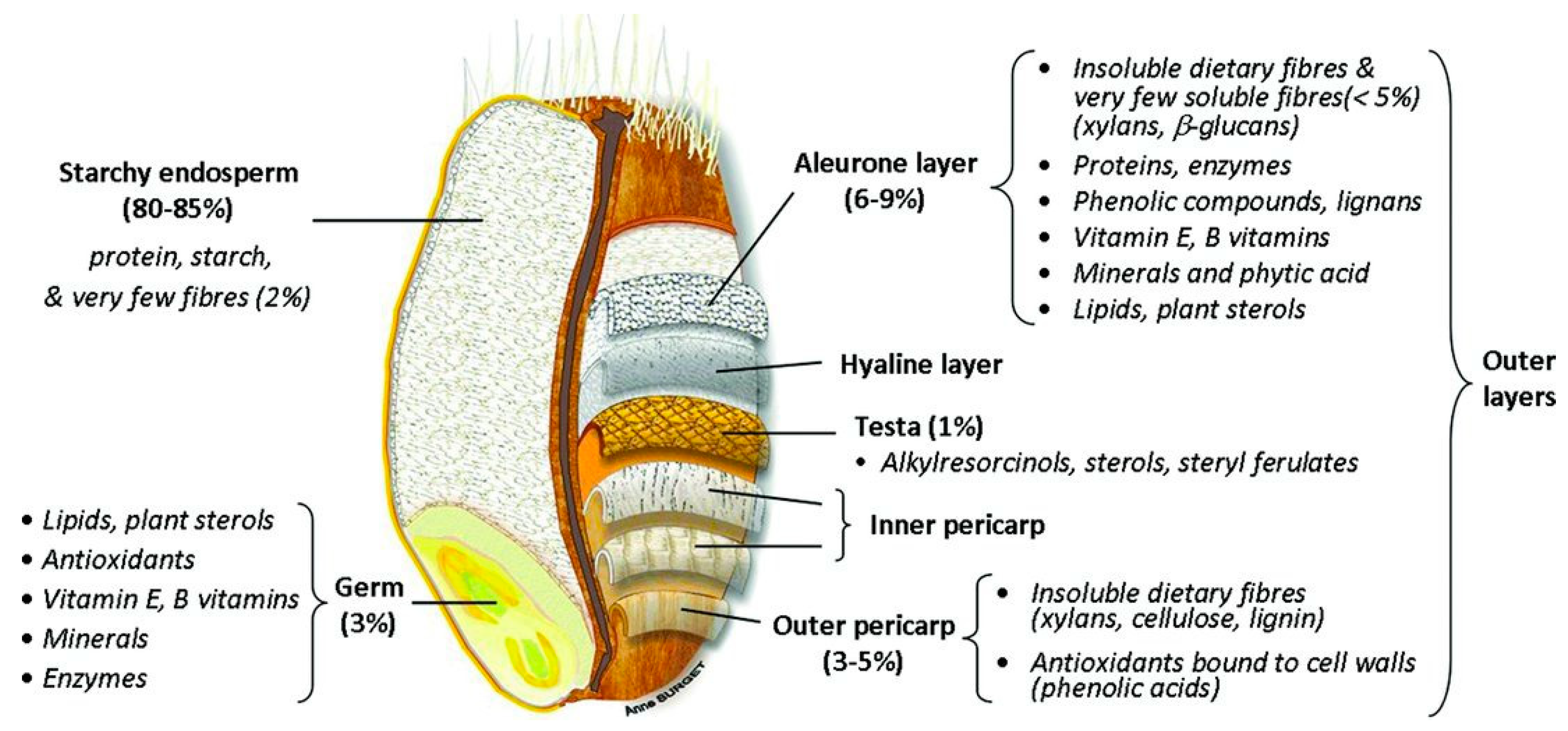
2.1. Structure of Cereal Grains
2.2. Dietary Fibre Composition of Different Cereals
2.2.1. Dietary Fibre Composition of Wheat
2.2.2. Dietary Fibre Composition of Barley and Oats
2.2.3. Dietary Fibre Composition of Rye
2.2.4. Dietary Fibre Composition of Other Grains
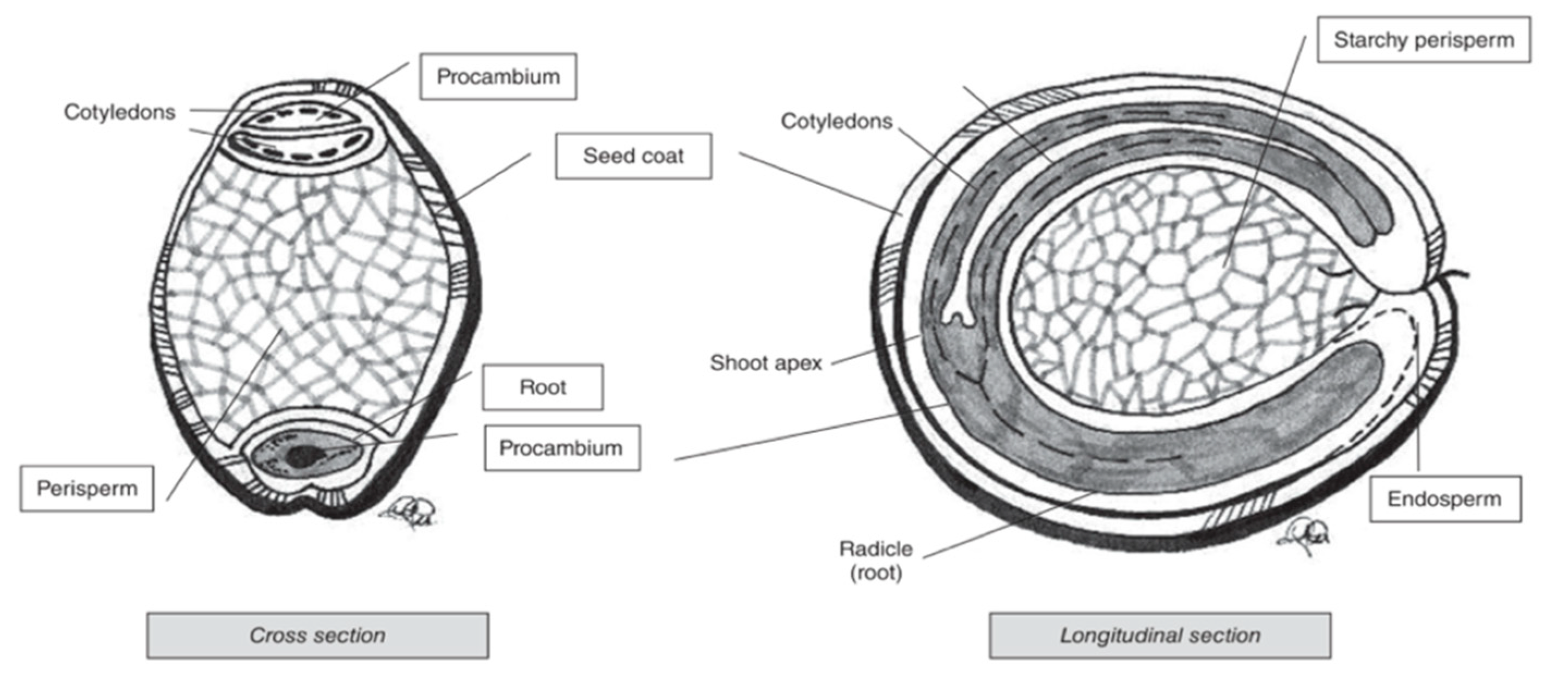
2.3. Structure of Pseudocereal Grains
2.4. Dietary Fibre Composition of Pseudocereals
3. Dietary Fibre from Whole Grains and Health Implications
3.1. Cardiovascular Health
3.2. Blood Glucose Levels
3.3. Gastrointestinal Health
3.4. Obesity and Weight Management
3.5. Undesirable Effects Associated with Consumption of Dietary Fibre
4. Conclusions
Funding
Conflicts of Interest
References
- Barrett, E.M.; Foster, S.I.; Beck, E.J. Whole grain and high-fibre grain foods: How do knowledge, perceptions and attitudes affect food choice? Appetite 2020, 149. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Van Dam, R.M.; Rimm, E.; Hu, F.B.; Qi, L. Response to letter regarding article, whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2011, 123. [Google Scholar] [CrossRef] [Green Version]
- Barrett, E.M.; Batterham, M.J.; Ray, S.; Beck, E.J. Whole grain, bran and cereal fibre consumption and CVD: A systematic review. Br. J. Nutr. 2019, 121, 914–937. [Google Scholar] [CrossRef]
- van der Kamp, J.W.; Poutanen, K.; Seal, C.J.; Richardson, D.P. Whole grain. In Definitions; Cereals & Grains Association, 2019; Available online: https://www.cerealsgrains.org/initiatives/definitions/Pages/WholeGrain.aspx (accessed on 17 September 2020).
- Definition of a Whole Grain. The Whole Grains Council. Available online: https://wholegrainscouncil.org/definition-whole-grain (accessed on 18 May 2020).
- Newman, C.W.; Newman, R.K.; Fastnaught, C.E. Barley. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 135–167. [Google Scholar]
- Delcour, J.A.; Poutanen, K. Fibre-Rich and Wholegrain Foods: Improving Quality; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Health Canada. Canada’s Food Guide; Health Canada: Ottawa, ON, Canada, 2011. [CrossRef]
- Slavin, J. Dietary guidelines: Are we on the right path? Nutr. Today 2012, 47, 245–251. [Google Scholar] [CrossRef]
- EU-Joint Research Centre. Health Promotion and Disease Prevention. Available online: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/whole-grain (accessed on 1 August 2020).
- Micha, R.; Khatibzadeh, S.; Shi, P.; Andrews, K.G.; Engell, R.E.; Mozaffarian, D. Global, regional and national consumption of major food groups in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evers, A.D.; Blakeney, A.B.; O’Brien, L. Cereal structure and composition. Aust. J. Agric. Res. 1999, 50, 629–650. [Google Scholar] [CrossRef] [Green Version]
- Liu, K. Physical properties of DDGS. In Distillers Grains: Production, Properties, and Utilization; Liu, K., Kurt, A.R., Eds.; AOCS Publishing: Urbana, IL, USA, 2016; pp. 121–142. [Google Scholar]
- Brouns, F.; Hemery, Y.; Price, R.; Anson, N.M. Wheat Aleurone: Separation, Composition, Health Aspects, and Potential Food Use. Crit. Rev. Food Sci. Nutr. 2012, 52, 553–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evers, T.; Millar, S. Cereal grain structure and development: Some implications for quality. J. Cereal Sci. 2002, 36, 261–284. [Google Scholar] [CrossRef]
- Dexter, J.E.; Wood, P.J. Recent applications of debranning of wheat before milling. Trends Food Sci. Technol. 1996, 7, 35–41. [Google Scholar] [CrossRef]
- Barron, C.; Surget, A.; Rouau, X. Relative amounts of tissues in mature wheat (Triticum aestivum L.) grain and their carbohydrate and phenolic acid composition. J. Cereal Sci. 2007, 45, 88–96. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Poutanen, K.; Micard, V. How does wheat grain, bran and aleurone structure impact their nutritional and technological properties? Trends Food Sci. Technol. 2015, 41, 118–134. [Google Scholar] [CrossRef]
- Liu, K. Structure and Grain Composition. In Distillers Grains: Production, Properties, and Utilization; Liu, K., Rosentrater, K.A., Eds.; AOCS Publishing: Urbana, IL, USA, 2016; pp. 45–69. [Google Scholar]
- Surget, A.; Barron, C. Histologie du grain de bl´e. Ind. des C´er´eales 2005, 145, 3–7. [Google Scholar]
- Health Canada. List of Dietary Fibres Reviewed and Accepted by Health Canada’s Food Directorate—Canada.ca. Available online: https://www.canada.ca/en/health-canada/services/publications/food-nutrition/list-reviewed-accepted-dietary-fibres.html (accessed on 12 August 2020).
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.O.; Komarek, A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017, 1, 47–59. [Google Scholar] [CrossRef]
- Dai, F.J.; Chau, C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42. [Google Scholar] [CrossRef] [Green Version]
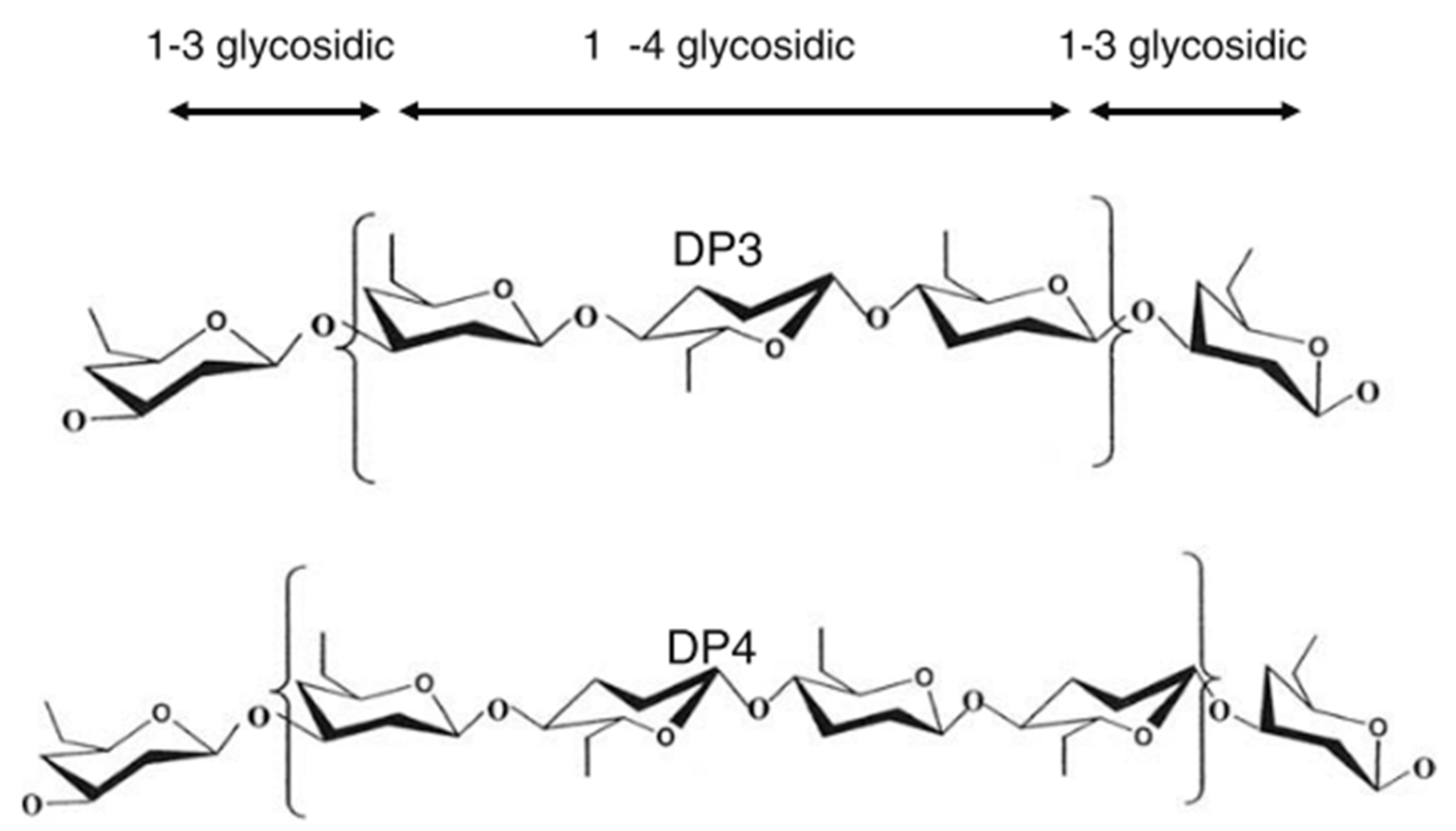
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52. [Google Scholar] [CrossRef]
- Wood, P.J. Cereal β-glucans in diet and health. J. Cereal Sci. 2007, 46, 230–238. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R. ProQuest Ebook Central. Charlest. Advis. 2017, 19, 39–43. [Google Scholar] [CrossRef]
- Esteban, R.M.; Mollá, E.; Benítez, V. Sources of Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease: Fiber’s Interaction between Gut Micoflora, Sugar Metabolism, Weight Control and Cardiovascular Health; Academic Press: London, UK, 2017; pp. 121–146. [Google Scholar]
- Gartaula, G.; Dhital, S.; Netzel, G.; Flanagan, B.M.; Yakubov, G.E.; Beahan, C.T.; Collins, H.M.; Burton, R.A.; Bacic, A.; Gidley, M.J. Quantitative structural organisation model for wheat endosperm cell walls: Cellulose as an important constituent. Carbohydr. Polym. 2018, 196, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M.C.; Morales, P. Dietary fiber sources and human benefits: The case study of cereal and pseudocereals. Adv. Food Nutr. Res. 2019, 90, 83–134. [Google Scholar] [PubMed]
- Sharma, P.; Bhandari, C.; Kumar, S.; Sharma, B.; Bhadwal, P.; Agnihotri, N. Dietary Fibers: A Way to a Healthy Microbiome. Diet Microbiome Health 2018, 299–345. [Google Scholar]
- Izydorczyk, M.S.; Dexter, J.E. Barley b-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products—A Review. Food Res. Int. 2008, 41, 850–868. [Google Scholar] [CrossRef]
- Aguedo, M.; Fougnies, C.; Dermience, M.; Richel, A. Extraction by three processes of arabinoxylans from wheat bran and characterization of the fractions obtained. Carbohydr. Polym. 2014, 105, 317–324. [Google Scholar] [CrossRef]
- De Santis, M.A.; Kosik, O.; Passmore, D.; Flagella, Z.; Shewry, P.R.; Lovegrove, A. Comparison of the dietary fibre composition of old and modern durum wheat (Triticum turgidum spp. durum) genotypes. Food Chem. 2018, 244, 304–310. [Google Scholar] [CrossRef]
- Rainakari, A.-I.; Rita, H.; Putkonen, T.; Pastell, H. New dietary fibre content results for cereals in the Nordic countries using AOAC 2011.25 method. J. Food Compos. Anal. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Dodevska, M.S.; Djordjevic, B.I.; Sobajic, S.S.; Miletic, I.D.; Djordjevic, P.B.; Dimitrijevic-Sreckovic, V.S. Characterisation of dietary fibre components in cereals and legumes used in Serbian diet. Food Chem. 2013, 141, 1624–1629. [Google Scholar] [CrossRef]
- Šterna, V.; Zute, S.; Jansone, I.; Kantane, I. Chemical Composition of Covered and Naked Spring Barley Varieties and Their Potential for Food Production. Polish J. Food Nutr. Sci. 2017, 67, 151–158. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Djurle, S.; Andersson, A.A.M.; Andersson, R. Milling and extrusion of six barley varieties, effects on dietary fibre and starch content and composition. J. Cereal Sci. 2016, 72, 146–152. [Google Scholar] [CrossRef]
- Messia, M.C.; Candigliota, T.; De Arcangelis, E.; Marconi, E. Arabinoxylans and β-glucans assessment in cereals. Ital. J. Food Sci. 2017, 29, 112–122. [Google Scholar]
- Hansen, H.B.; Rasmussen, C.V.; Bach Knudsen, K.E.; Hansen, A. Effects of genotype and harvest year on content and composition of dietary fibre in rye (Secale cereale L.) grain. J. Sci. Food Agric. 2003, 83, 76–85. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A. Influence of oxalate, phytate, tannin, dietary fiber, and cooking on calcium bioavailability of commonly consumed cereals and millets in India. Cereal Chem. 2015, 92, 389–394. [Google Scholar] [CrossRef]
- Prasad, V.S.S.; Hymavathi, A.; Babu, V.R.; Longvah, T. Nutritional composition in relation to glycemic potential of popular Indian rice varieties. Food Chem. 2018, 238, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, P.S.; Naveena, N.; Vishnuvardhana Rao, M.; Bhaskarachary, K. Compositional variability of nutrients and phytochemicals in corn after processing. J. Food Sci. Technol. 2017, 54, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Srichuwong, S.; Curti, D.; Austin, S.; King, R.; Lamothe, L.; Gloria-Hernandez, H. Physicochemical properties and starch digestibility of whole grain sorghums, millet, quinoa and amaranth flours, as affected by starch and non-starch constituents. Food Chem. 2017, 233, 1–10. [Google Scholar] [CrossRef]
- Robin, F.; Théoduloz, C.; Srichuwong, S. Properties of extruded whole grain cereals and pseudocereals flours. Int. J. Food Sci. Technol. 2015, 50, 2152–2159. [Google Scholar] [CrossRef]
- Pulvento, C.; Riccardi, M.; Lavini, A.; Iafelice, G.; Marconi, E.; D’Andria, R. Yield and Quality Characteristics of Quinoa Grown in Open Field Under Different Saline and Non-Saline Irrigation Regimes. J. Agron. Crop Sci. 2012, 198, 254–263. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Martínez, E.A.; López, J.; Marín, R.; Aranda, M.; Fuentes, F. Influence of contrasting environments on seed composition of two quinoa genotypes: Nutritional and functional properties. Chil. J. Agric. Res. 2013, 73, 6–7. [Google Scholar] [CrossRef]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Buckwheat seed milling fractions: Description, macronutrient composition and dietary fibre. J. Cereal Sci. 2001, 33, 271–278. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and food uses of teff (Eragrostis tef). Food Chem. 2018, 239, 402–415. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Munck, L. Dietary fibre contents and compositions of sorghum and sorghum-based foods. J. Cereal Sci. 1985, 3, 153–164. [Google Scholar] [CrossRef]
- Jayawardana, S.A.S.; Samarasekera, J.K.R.R.; Hettiarachchi, G.H.C.M.; Gooneratne, J.; Mazumdar, S.D.; Banerjee, R. Dietary fibers, starch fractions and nutritional composition of finger millet varieties cultivated in Sri Lanka. J. Food Compos. Anal. 2019, 82, 103249. [Google Scholar] [CrossRef] [Green Version]
- Marcotuli, I.; Hsieh, Y.S.Y.; Lahnstein, J.; Yap, K.; Burton, R.A.; Blanco, A.; Fincher, G.B.; Gadaleta, A. Structural Variation and Content of Arabinoxylans in Endosperm and Bran of Durum Wheat (Triticum turgidum L.). J. Agric. Food Chem. 2016, 64, 2883–2892. [Google Scholar] [CrossRef]
- Michelini, E.; Guardigli, M.; Magliulo, M.; Mirasoli, M.; Roda, A.; Simoni, P.; Baraldini, M. Bioluminescent biosensors based on genetically engineered living cells in environmental and food analysis. Anal. Lett. 2006, 39, 1503–1515. [Google Scholar] [CrossRef]
- Delcour, J.A.; Van Win, H.; Grobet, P.J. Distribution and structural variation of arabinoxylans in common wheat mill streams. J. Agric. Food Chem. 1999, 47, 271–275. [Google Scholar] [CrossRef] [PubMed]
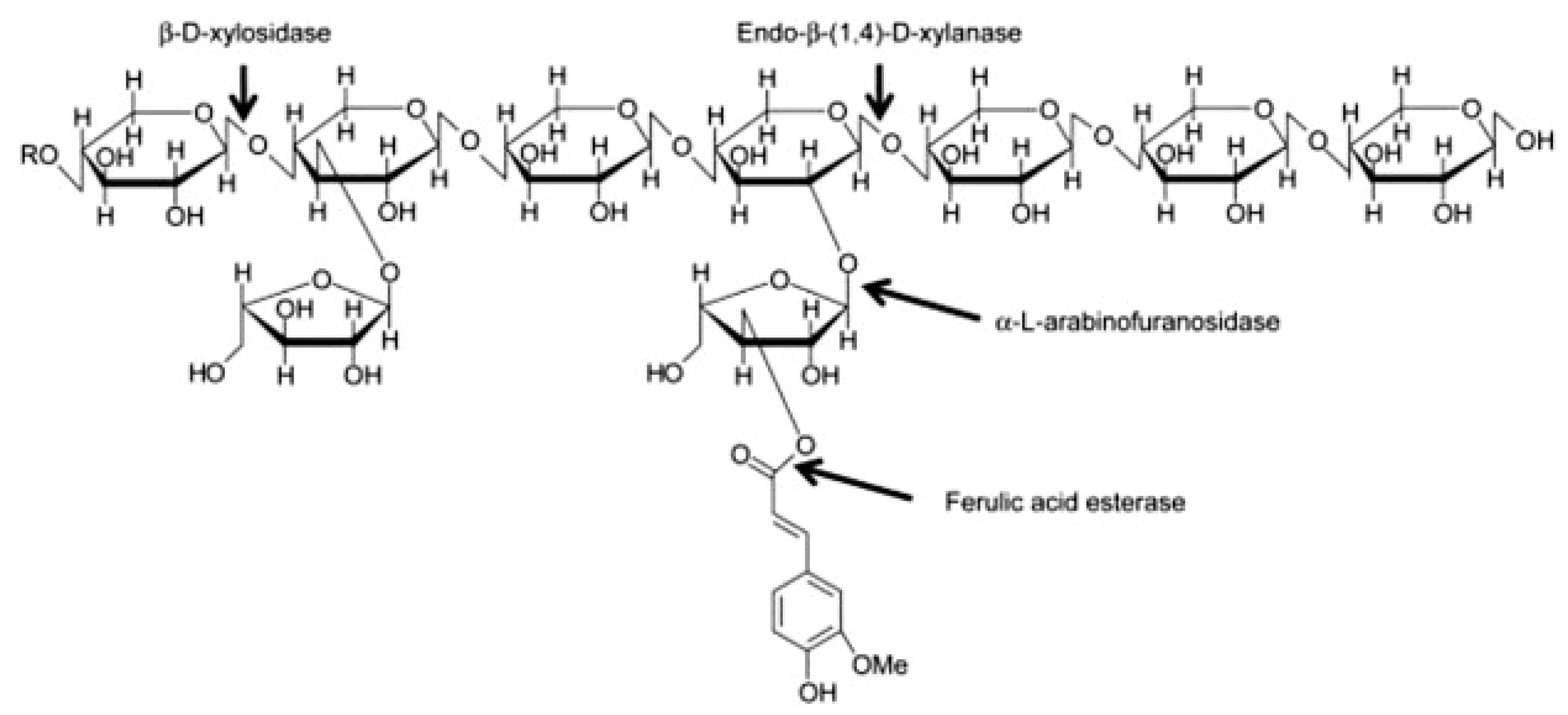
- Dornez, E.; Gebruers, K.; Delcour, J.A.; Courtin, C.M. Grain-associated xylanases: Occurrence, variability, and implications for cereal processing. Trends Food Sci. Technol. 2009, 20, 495–510. [Google Scholar] [CrossRef]
- Parker, M.L.; Ng, A.; Waldron, K.W. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum L. cv. Avalon) grains. J. Sci. Food Agric. 2005, 85, 2539–2547. [Google Scholar] [CrossRef]
- Saulnier, L.; Sado, P.E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: Exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281. [Google Scholar] [CrossRef]
- Barron, C.; Bar-L’Helgouac’h, C.; Champ, M.; Saulnier, L. Arabinoxylan content and grain tissue distribution are good predictors of the dietary fibre content and their nutritional properties in wheat products. Food Chem. 2020, 328, 8. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Lampi, A.M.; Nyström, L.; Piironen, V.; Li, L.; Ward, J.L.; Gebruers, K.; Courtin, C.M.; Delcour, J.A.; Boros, D.; et al. Phytochemical and dietary fiber components in barley varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9767–9776. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Vasanthan, T.; Temelli, F. Grain fractionation technologies for cereal beta-glucan concentration. Food Res. Int. 2008, 41, 876–881. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Di Criscio, T.; Marconi, E. Tocol and β-glucan levels in barley varieties and in pearling by-products. Food Chem. 2008, 107, 84–91. [Google Scholar] [CrossRef]
- Andersson, R.; Fransson, G.; Tietjen, M.; Åman, P. Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. J. Agric. Food Chem. 2009, 57, 2004–2008. [Google Scholar] [CrossRef]
- Meija, L.; Krams, I. Rye. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 169–208. [Google Scholar]
- Andersson, A.A.M.; Dimberg, L.; Åman, P.; Landberg, R. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311. [Google Scholar] [CrossRef]
- Rakha, A.; Åman, P.; Andersson, R. Characterisation of dietary fibre components in rye products. Food Chem. 2010, 119, 859–867. [Google Scholar] [CrossRef]
- Ji, C.M.; Shin, J.A.; Cho, J.W.; Lee, K.T. Nutritional evaluation of immature grains in two Korean rice cultivars during maturation. Food Sci. Biotechnol. 2013, 22, 903–908. [Google Scholar] [CrossRef]
- Fernando, B. Rice as a Source of Fibre. Rice Res. Open Access 2013, 1. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. Cereal Grains for the Food and Beverage Industries; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Fletcher, R.J. Pseudocereals: Overview. In Encyclopedia of Food Grains, 2nd ed.; Academic Press: London, UK, 2013; pp. 488–493. [Google Scholar]
- Velarde-Salcedo, A.J.; Bojórquez-Velázquez, E.; de la Rosa, A.P.B. Amaranth. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 209–250. [Google Scholar]
- Reguera, M.; Haros, C.M. Structure and Composition of Kernels. In Pseudocereals: Chemistry and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 28–48. [Google Scholar]
- Prego, I.; Maldonado, S.; Otegui, M. Seed structure and localization of reserves in Chenopodium quinoa. Ann. Bot. 1998, 82, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, M.S.; McMillan, T.; Bazin, S.; Kletke, J.; Dushnicky, L.; Dexter, J. Canadian buckwheat: A unique, useful and under-utilized crop. Can. J. Plant Sci. 2014, 94, 509–524. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Head, D. Characterization and Potential Uses of Functional Buckwheat Fractions Obtained by Roller Milling of New Canadian Buckwheat Genotypes. Eur. J. Plant Sci. Biotechnol. 2010, 4, 71–81. [Google Scholar]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 2009, 60, 240–257. [Google Scholar] [CrossRef]
- Mustafa, A.F.; Seguin, P.; Gélinas, B.; Gé Linas, B. Chemical composition, dietary fibre, tannins and minerals of grain amaranth genotypes. Int. J. Food Sci. Nutr. 2011, 62, 750–754. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Srichuwong, S.; Reuhs, B.L.; Hamaker, B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015, 167, 490–496. [Google Scholar] [CrossRef]
- Haros, C.M.; Schonlechner, R. Pseudocereals: Chemistry and Technology; Schonlechner, C.M.H.R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Huang, T.; Xu, M.; Lee, A.; Cho, S.; Qi, L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: Prospective analysis of 367,442 individuals. BMC Med. 2015, 13. [Google Scholar] [CrossRef] [Green Version]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical update: Cardiovascular disease in diabetes mellitus. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sikand, G.; Wong, N.D. Nutrition, Diet Quality, and Cardiovascular Health. In Molecular Basis of Nutrition and Aging: A Volume in the Molecular Nutrition Series; Academic Press: London, UK, 2016; pp. 315–330. [Google Scholar]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Martini, M.C.; Jacobs, D.R.; Marquart, L. Plausible mechanisms for the protectiveness of whole grains. Am. J. Clin. Nutr. 1999, 70, 459s–463s. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Bondy, S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioact. Carbohydrates Diet. Fibre 2019, 17. [Google Scholar] [CrossRef]
- CFR. CFR—Code of Federal Regulations Title 21. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.25 (accessed on 21 June 2020).
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- Tong, L.T.; Zhong, K.; Liu, L.; Qiu, J.; Guo, L.; Zhou, X.; Cao, L.; Zhou, S. Effects of dietary wheat bran arabinoxylans on cholesterol metabolism of hypercholesterolemic hamsters. Carbohydr. Polym. 2014, 112, 1–5. [Google Scholar] [CrossRef]
- Kieffer, D.A.; Piccolo, B.D.; Marco, M.L.; Kim, E.B.; Goodson, M.L.; Keenan, M.J.; Dunn, T.N.; Knudsen, K.E.B.; Adams, S.H.; Martin, R.J. Obese Mice Fed a Diet Supplemented with Enzyme-Treated Wheat Bran Display Marked Shifts in the Liver Metabolome Concurrent with Altered Gut Bacteria. J. Nutr. 2016, 146, 2445–2460. [Google Scholar] [CrossRef]
- Adam, A.; Levrat-Verny, M.-A.; Lopez, H.W.; Leuillet, M.; Demigné, C.; Rémésy, C. Whole Wheat and Triticale Flours with Differing Viscosities Stimulate Cecal Fermentations and Lower Plasma and Hepatic Lipids in Rats. J. Nutr. 2001, 131, 1770–1776. [Google Scholar] [CrossRef] [Green Version]
- Konishi, Y.; Arai, N.; Umeda, J.; Gunji, N.; Saeki, S.; Takao, T.; Minoguchi, R.; Kensho, G. Cholesterol lowering effect of the methanol insoluble materials from the quinoa seed pericarp. In Hydrocolloids; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 417–422. [Google Scholar]
- Plate, A.Y.; Aréas, J.A.G. Cholesterol-lowering effect of extruded amaranth (Amaranthus caudatus L.) in hypercholesterolemic rabbits. Food Chem. 2002, 76, 1–6. [Google Scholar] [CrossRef]
- Liu, S.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Rimm, E.; Manson, J.A.E.; Hennekens, C.H.; Willett, W.C. Whole-grain consumption and risk of coronary heart disease: Results from the Nurses’ Health Study. Am. J. Clin. Nutr. 1999, 70, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, B.M.; Latre, M.L.; Esteban, E.M.A.; Ordov ás, J.M.; Casasnovas, J.A.; Peñalvo, J.L. Ingesta de fibra soluble e insoluble y factores de riesgo de síndrome metabólico y enfermedad cardiovascular en adultos de mediana edad: La cohorte AWHS. Nutr. Hosp. 2014, 30, 1279–1288. [Google Scholar]
- Keenan, J.M.; Goulson, M.; Shamliyan, T.; Knutson, N.; Kolberg, L.; Curry, L. The effects of concentrated barley β-glucan on blood lipids in a population of hypercholesterolaemic men and women. Br. J. Nutr. 2007, 97, 1162–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Diets Containing Barley Significantly Reduce Lipids in Mildly Hypercholesterolemic Men and Women. Am. J. Clin. Nutr. 2004, 80, 1185–1193. [Google Scholar] [CrossRef]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-lowering effects of oat β-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Goverment of Canada. Hypertension Facts and Figures—Canada.ca. Available online: https://www.canada.ca/en/public-health/services/chronic-diseases/cardiovascular-disease/hypertension-facts-figures.html (accessed on 21 September 2020).
- Aljuraiban, G.S.; Griep, L.M.O.; Griep, L.M.O.; Chan, Q.; Daviglus, M.L.; Stamler, J.; Van Horn, L.; Elliott, P.; Frost, G.S. Total, insoluble and soluble dietary fibre intake in relation to blood pressure: The INTERMAP Study. Br. J. Nutr. 2015, 114, 1480–1486. [Google Scholar] [CrossRef] [Green Version]
- Streppel, M.T.; Arends, L.R.; Van’t Veer, P.; Grobbee, D.E.; Geleijnse, J.M. Dietary fiber and blood pressure: A meta-analysis of randomized placebo-controlled trials. Arch. Intern. Med. 2005, 165, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Razavi, A.C.; Bazzano, L.A.; He, J.; Whelton, S.P.; Fernandez, C.; Ley, S.; Qi, L.; Krousel-Wood, M.; Harlan, T.S.; Kelly, T.N. Consumption of animal and plant foods and risk of left ventricular diastolic dysfunction: The Bogalusa Heart Study. ESC Hear. Fail. 2020, 7, 2700–2710. [Google Scholar] [CrossRef]
- Alonso, A.; Beunza, J.J.; Bes-Rastrollo, M.; Pajares, R.M.; Martínez-González, M.Á. Vegetable Protein and Fiber from Cereal Are Inversely Associated with the Risk of Hypertension in a Spanish Cohort. Arch. Med. Res. 2006, 37, 778–786. [Google Scholar] [CrossRef]
- Keenan, J.M.; Pins, J.J.; Frazel, C.; Moran, A.; Turnquist, L. Oat ingestion reduces systolic and diastolic blood pressure in patients with mild or borderline hypertension: A pilot trial. J. Fam. Pract. 2002, 51, 369. [Google Scholar]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Government of Canada. Diabetes in Canada—Canada.ca. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/diabetes-canada-highlights-chronic-disease-surveillance-system.html (accessed on 22 June 2020).
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Otto, B.; Reich, S.C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Möhlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrion, M.; Francey, C.; Lê, K.A.; Lamothe, L. Cereal B-glucans: The impact of processing and how it affects physiological responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mälkki, Y.; Virtanen, E. Gastrointestinal Effects of Oat Bran and Oat Gum: A Review. LWT Food Sci. Technol. 2001, 34, 337–347. [Google Scholar] [CrossRef]
- Li, L.; Pan, M.; Pan, S.; Li, W.; Zhong, Y.; Hu, J.; Nie, S. Effects of insoluble and soluble fibers isolated from barley on blood glucose, serum lipids, liver function and caecal short-chain fatty acids in type 2 diabetic and normal rats. Food Chem. Toxicol. 2020, 135, 110937. [Google Scholar] [CrossRef]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Davison, K.M.; Temple, N.J. Cereal fiber, fruit fiber, and type 2 diabetes: Explaining the paradox. J. Diabetes Complicat. 2018, 32, 240–245. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, M.J.; Cho, H.Y.; Kim, E.K.; Shin, D.H. Antioxidative and anti-diabetic effects of amaranth (Amaranthus esculantus) in streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2006, 24, 195–199. [Google Scholar] [CrossRef]
- Capriles, V.D.; Coelho, K.D.; Guerra-Matias, A.C.; Arêas, J.A.G. Effects of Processing Methods on Amaranth Starch Digestibility and Predicted Glycemic Index. J. Food Sci. 2008, 73, 160–164. [Google Scholar] [CrossRef]
- The InterAct Consortium Dietary fibre and incidence of type 2 diabetes in eight European countries: The EPIC-InterAct Study and a meta-analysis of prospective studies. Epic. Study Meta Anal. Prospect. Stud. 2015, 58, 1394–1408.
- Braaten, J.T.; Scott, F.W.; Wood, P.J.; Riedel, K.D.; Wolynetz, M.S.; Brulé, D.; Collins, M.W. High beta-glucan oat bran and oat gum reduce postprandial blood glucose and insulin in subjects with and without type 2 diabetes. Diabet. Med. 1994, 11, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhao, T.; Zhou, Y.; Shi, X.; Zou, Y.; Zhao, G. Effect of Oat β-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Li, S.; Fu, Y.; Li, C.; Chen, D.; Chen, H. Arabinoxylan structural characteristics, interaction with gut microbiota and potential health functions. J. Funct. Foods 2019, 54, 536–551. [Google Scholar] [CrossRef]
- Giulia Falchi, A.; Grecchi, I.; Muggia, C.; Palladini, G.; Perlini, S. Effects of a Bioavailable Arabinoxylan-enriched White Bread Flour on Postprandial Glucose Response in Normoglycemic Subjects. J. Diet. Suppl. 2016, 13, 626–633. [Google Scholar] [CrossRef]
- Juvonen, K.R.; Purhonen, A.-K.; Salmenkallio-Marttila, M.; Lähteenmäki, L.; Laaksonen, D.E.; Herzig, K.-H.; Uusitupa, M.I.J.; Poutanen, K.S.; Karhunen, L.J. Viscosity of Oat Bran-Enriched Beverages Influences Gastrointestinal Hormonal Responses in Healthy Humans. J. Nutr. 2009, 139, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Kellow, N.J.; Walker, K.Z. Authorised EU health claim for arabinoxylan. In Foods, Nutrients and Food Ingredients with Authorised EU Health Claims; Woodhead Publishing: Cambridge, UK, 2018; Volume 3, pp. 201–218. [Google Scholar]
- Weickert, M.O.; Pfeiffer, A.F.H. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef]
- Feldman, A.L.; Long, G.H.; Johansson, I.; Weinehall, L.; Fhärm, E.; Wennberg, P.; Norberg, M.; Griffin, S.J.; Rolandsson, O. Change in lifestyle behaviors and diabetes risk: Evidence from a population-based cohort study with 10 year follow-up. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 39. [Google Scholar] [CrossRef] [Green Version]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Colorectal Cancer Statistics—Canadian Cancer Society. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/colorectal/statistics/region=on (accessed on 22 June 2020).
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.; Kim, H.; Lee, D.H.; Lee, A.; Giovannucci, E.L.; Kang, S.S.; Keum, N. Different dietary fibre sources and risks of colorectal cancer and adenoma: A dose-response meta-analysis of prospective studies. Br. J. Nutr. 2019, 122, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Zyła, E.; Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Harasym, J.; Gromadzka-Ostrowska, J. Beneficial effects of oat beta-glucan dietary supplementation in colitis depend on its molecular weight. Molecules 2019, 24, 3591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Canada. Inflammatory Bowel Disease (IBD)—Canada.ca. Available online: https://www.canada.ca/en/public-health/services/chronic-diseases/inflammatory-bowel-disease.html (accessed on 15 July 2020).
- Suchecka, D.; Harasym, J.P.; Wilczak, J.; Gajewska, M.; Oczkowski, M.; Gudej, S.; Błaszczyk, K.; Kamola, D.; Filip, R.; Gromadzka-Ostrowska, J. Antioxidative and anti-inflammatory effects of high beta-glucan concentration purified aqueous extract from oat in experimental model of LPS-induced chronic enteritis. J. Funct. Foods 2015, 14, 244–254. [Google Scholar] [CrossRef]
- Andersen, V.; Chan, S.; Luben, R.; Khaw, K.-T.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Grip, O.; Bergmann, M.M.; Boeing, H.; et al. Fibre intake and the development of inflammatory bowel disease: A European prospective multi-centre cohort study (EPIC-IBD). J. Crohn’s Colitis 2018, 12, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; De Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [Green Version]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review article: Dietary fibre in the era of microbiome science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Peters, B.A.; Friedlander, C.; Freiman, H.J.; Goedert, J.J.; Sinha, R.; Miller, G.; Bernstein, M.A.; Hayes, R.B.; Ahn, J. Association of dietary fibre intake and gut microbiota in adults. Br. J. Nutr. 2018, 120, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Martens, E.C. Microbiome: Fibre for the future. Nature 2016, 529, 158–159. [Google Scholar] [CrossRef] [Green Version]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [Green Version]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.H.; Tan, M.; Seow-Choen, F. Micronized purified flavonidic fraction compared favorably with rubber band ligation and fiber alone in the management of bleeding hemorrhoids: Randomized controlled trial. Dis. Colon Rectum 2000, 43, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H. The effect of dietary fiber on fecal weight and composition. In CRC Handbook of Dietary Fiber in Human Nutrition, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 183–252. [Google Scholar]
- Smith, C.E.; Tucker, K.L. Health benefits of cereal fibre: A review of clinical trials. Nutr. Res. Rev. 2011, 24, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Vijver, L.P.L.; van den Bosch, L.M.C.; van den Brandt, P.A.; Goldbohm, R.A. Whole-grain consumption, dietary fibre intake and body mass index in the Netherlands cohort study. Eur. J. Clin. Nutr. 2009, 63, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Kromhout, D.; Bloemberg, B.; Seidell, J.C.; Nissinen, A.; Menotti, A. Physical activity and dietary fiber determine population body fat levels: The Seven Countries Study. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Vitaglione, P.; Lumaga, R.B.; Stanzione, A.; Scalfi, L.; Fogliano, V. β-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite 2009, 53, 338–344. [Google Scholar] [CrossRef]
- Aoe, S.; Ikenaga, T.; Noguchi, H.; Kohashi, C.; Kakumoto, K.; Kohda, N. Effect of Cooked White Rice with High β-glucan Barley on Appetite and Energy Intake in Healthy Japanese Subjects: A Randomized Controlled Trial. Plant Foods Hum. Nutr. 2014, 69, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, J.; Miri, A.; Černevičiūtė, R.; Thompson, J.; de Souza, N.N.; Sultana, R.; Kord Varkaneh, H.; Mousavi, S.M.; Hekmatdoost, A. Effects of cereal beta-glucan consumption on body weight, body mass index, waist circumference and total energy intake: A meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 43, 131–139. [Google Scholar] [CrossRef]
- Hoad, C.L.; Rayment, P.; Spiller, R.C.; Marciani, L.; de Celis, A.B.; Traynor, C.; Mela, D.J.; Peters, H.P.F.; Gowland, P.A. In Vivo Imaging of Intragastric Gelation and Its Effect on Satiety in Humans. J. Nutr. 2004, 134, 2293–2300. [Google Scholar] [CrossRef] [Green Version]
- Beck, E.J.; Tosh, S.M.; Batterham, M.J.; Tapsell, L.C.; Huang, X.F. Oat β-glucan increases postprandial cholecystokinin levels, decreases insulin response and extends subjective satiety in overweight subjects. Mol. Nutr. Food Res. 2009, 53, 1343–1351. [Google Scholar] [CrossRef]
- Baye, K.; Guyot, J.P.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Miyada, T.; Nakajima, A.; Ebihara, K. Iron bound to pectin is utilised by rats. Br. J. Nutr. 2011, 106, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokhar, S.; Kapoor, A.C. Effect of dietary fibres on bioavailability of vitamin A and thiamine. Plant Foods Hum. Nutr. 1990, 40, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.A.; Wyrwisz, J.; Karp, S.; Wierzbicka, A. Particle size of dietary fiber preparation affects the bioaccessibility of selected vitamin B in fortified wheat bread. J. Cereal Sci. 2017, 77, 166–171. [Google Scholar] [CrossRef]
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176. [Google Scholar] [CrossRef] [Green Version]
| TDF | IDF | SDF | Reference | |
|---|---|---|---|---|
| Wheat (Triticum aestivum L., Triticum durum Desf.) | 11.6–17.0 | 10.2–14.7 | 1.4–2.3 | [38] |
| 10.2–15.7 | 7.2–11.4 | 1.9–2.9 | [39] | |
| 9.2 | - | - | [40] | |
| Oat (Avena sativa L.) | 13.7–30.1 | - | 11.5–20.0 | [41] |
| 10.3 | 6.5 | 3.8 | [23] | |
| 11.5–37.7 | 8.6–33.9 | 2.9–3.8 | [42] | |
| Barley (Hordeum vulgare L.) | 14.6–27.1 | 12.0–22.1 | 2.6–5.0 | [42] |
| 16.8–27.9 | - | - | [43] | |
| 10.1 | - | - | [44] | |
| Rye (Secalecereale L.) | 15.2–20.9 | 11.1–15.9 | 3.7–4.5 | [32] |
| 14.7–20.9 | 10.8–15.9 | 3.4–6.6 | [45] | |
| Rice (Oryza sativa L.) | 9.9 | 5.4 | 4.4 | [46] |
| 2.7–4.9 | 1.9–4.2 | 0.6–1.1 | [47] | |
| Corn (Zea mays L.) | 3.7–8.6 | 3.1–6.1 | 0.5–2.5 | [48] |
| 13.1–19.6 | 11.6–16.0 | 1.5–3.6 | [42] | |
| Amaranth (Amaranthus spp.) | 8.9–20.6 | - | - | [49] |
| 11.4 | 7.7 | 3.7 | [50] | |
| 11.8 | 9.1 | 2.7 | [51] | |
| Quinoa (Chenopodium quinoa Willd.) | 7–9.5 | 4.9–5.6 | 2.1–3.9 | [50] |
| 16.2–21.6 | - | - | [52] | |
| 11.6–15.1 | 9.9–12.2 | 0.4–2.9 | [53] | |
| Buckwheat (Fagopyrumesculentum Moench.) | 7.0 | 2.2 | 4.8 | [54] |
| 11.9 | 5.8 | 6.1 | [55] | |
| Teff [Eragrostis tef (Zucc.) Trotter] | 4.54 | - | 0.85 | [56] |
| Sorghum (Sorghum bicolor) | 7.55–12.3 | 6.52–7.90 | 1.05–1.23 | [57] |
| Millets (Eleusine coracana (L.) Gaertn.) | 13.0–13.8 | 12.5–13.2 | 0.52–0.59 | [58] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
P., N.P.V.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. https://doi.org/10.3390/nu12103045
P. NPV, Joye IJ. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients. 2020; 12(10):3045. https://doi.org/10.3390/nu12103045
Chicago/Turabian StyleP., Nirmala Prasadi V., and Iris J. Joye. 2020. "Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health" Nutrients 12, no. 10: 3045. https://doi.org/10.3390/nu12103045




