The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain—Ex Vivo and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Solutions
2.2. Experimental Animals
2.3. Extra Virgin Olive Oil Extracts Preparation
2.4. Determination of Extra Virgin Olive Oil (EVOO) Extracts by Reverse-Phase Ultra Performance Liquid Chromatography-Photodiode Array (UPLC–PDA) Analysis
2.5. Uterine Preparations and Measurement of Uterine Contraction
2.6. Molecular Docking Site
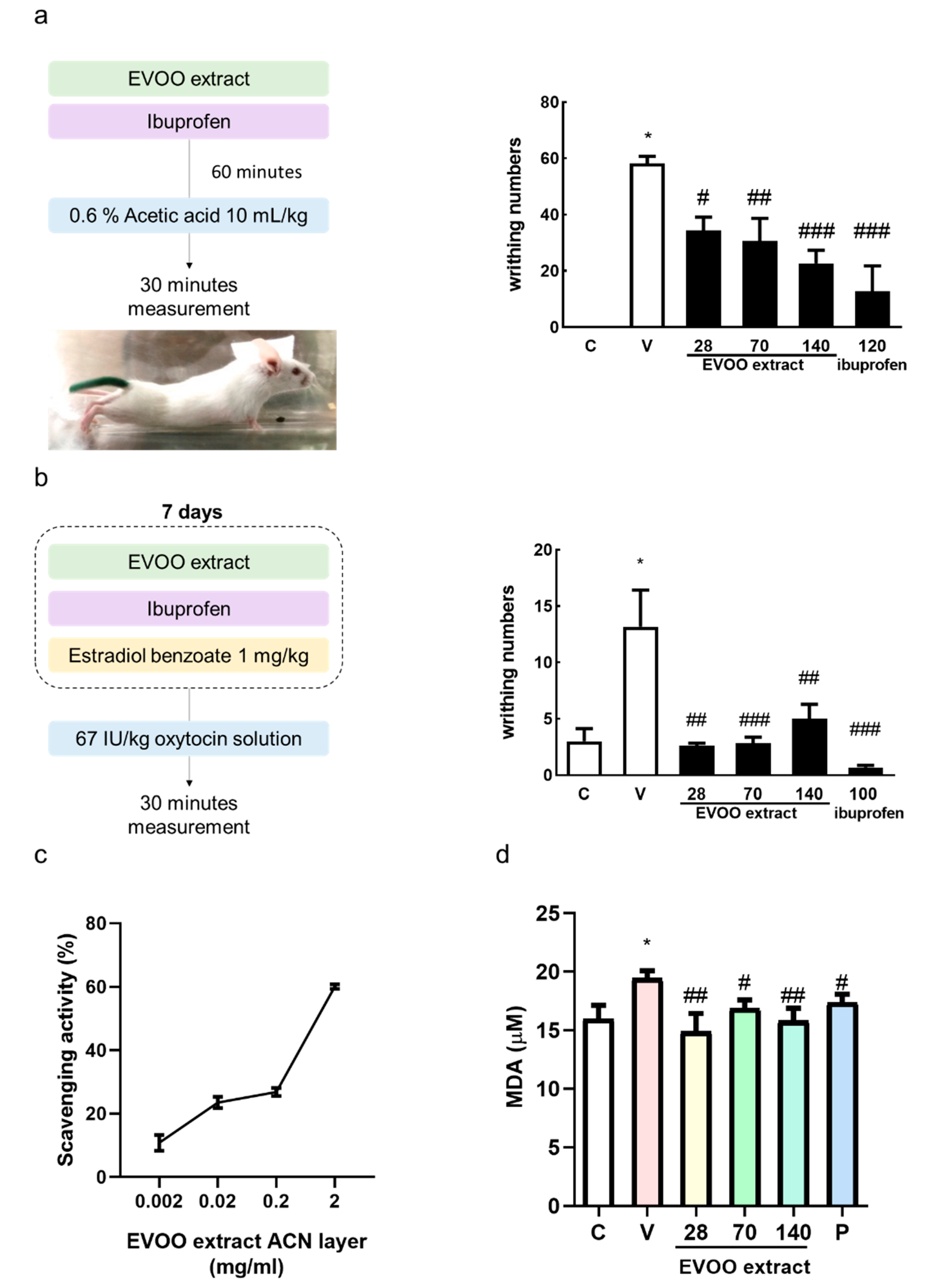
2.7. Acetic Acid-Induced Writhing Test
2.8. Oxytocin-Induced Writhing Test
2.9. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.10. Lipid Peroxidation: Determination of Malondialdehyde (MDA)
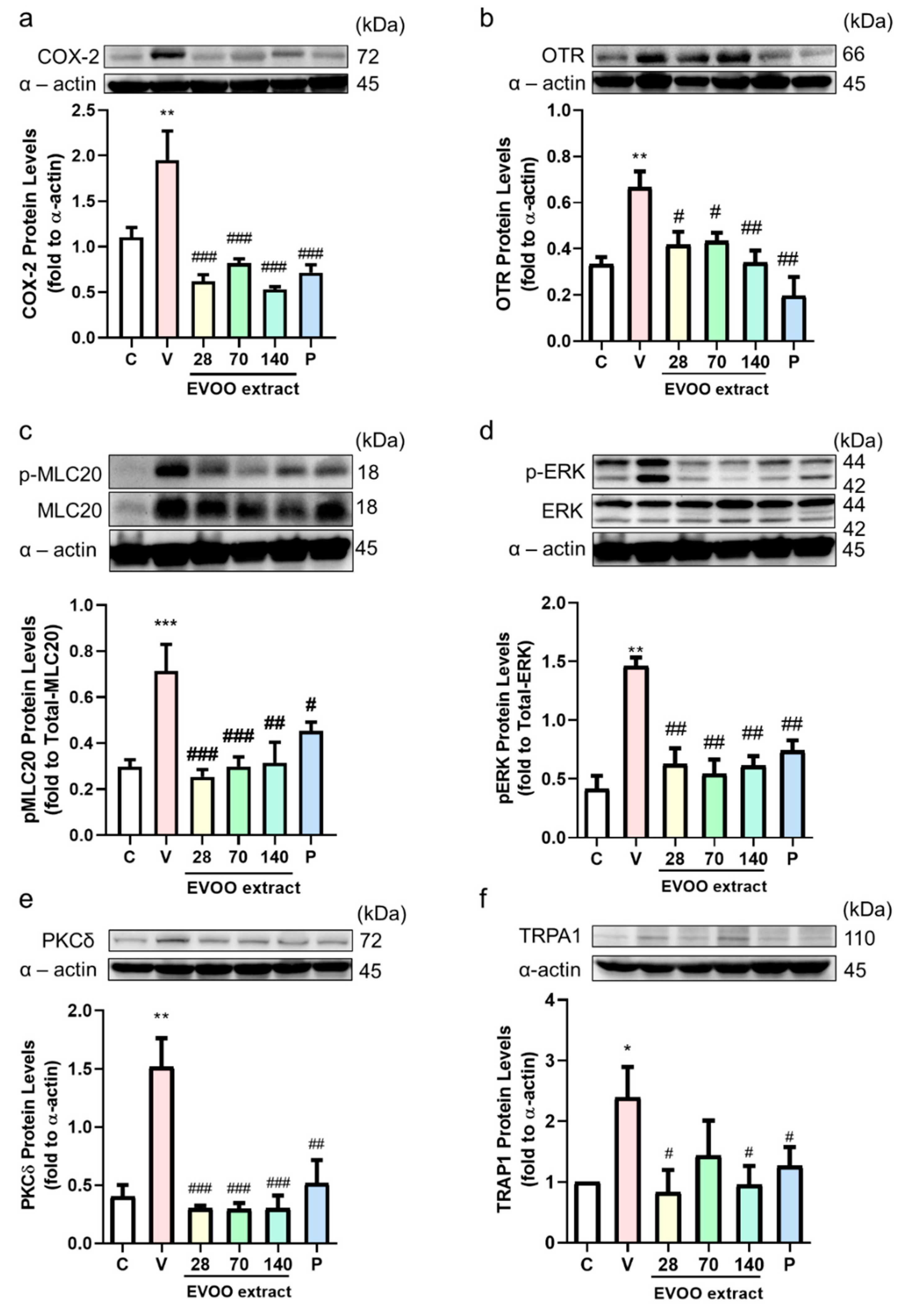
2.11. Protein Preparation and Western Blot Analysis
2.12. Statistical Analysis
3. Results
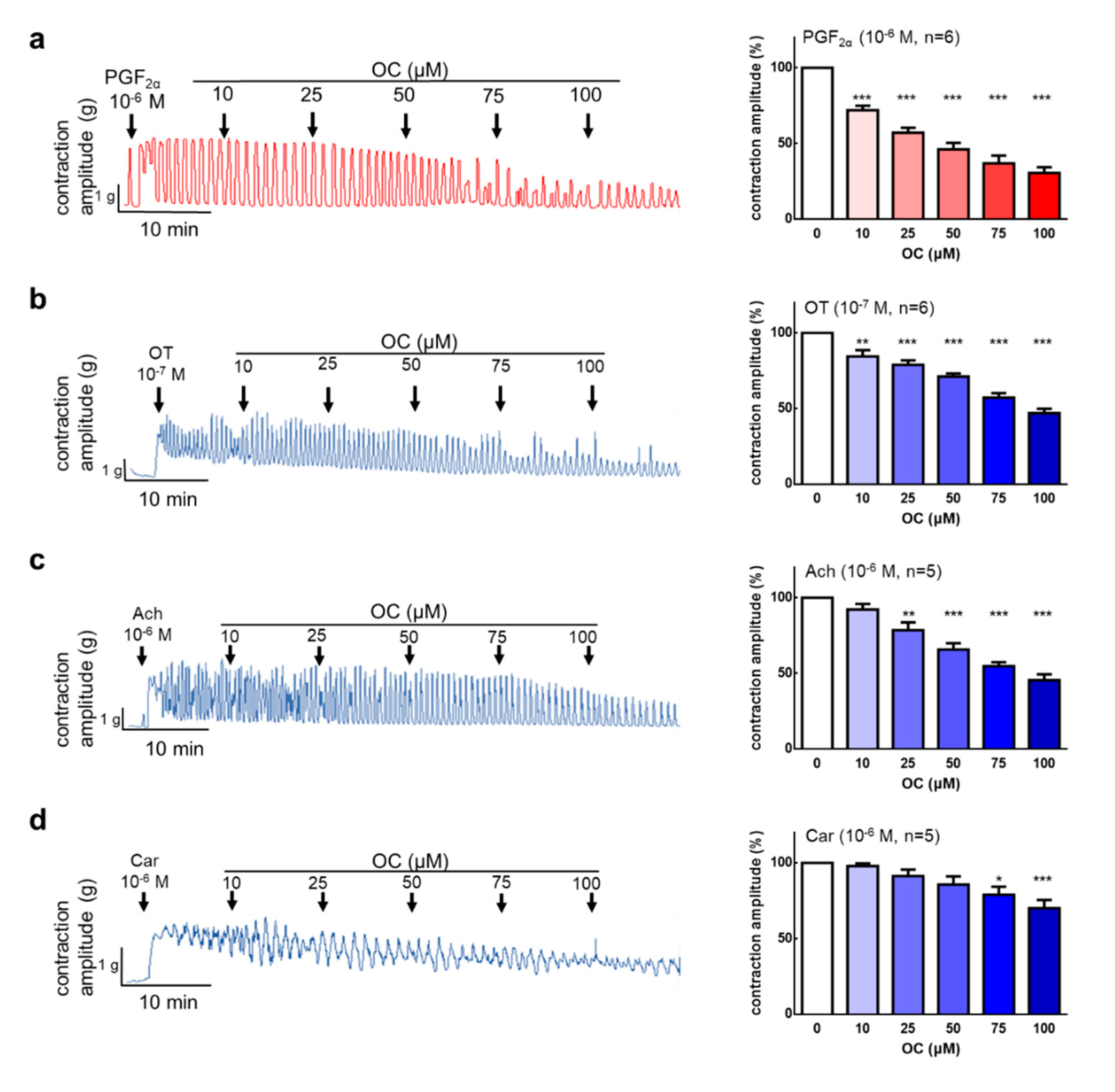
3.1. Oleocanthal Inhibits Prostaglandin, Oxytocin, Acetylcholine, and Carbachol-Induced Uterine Hypercontraction Ex Vivo
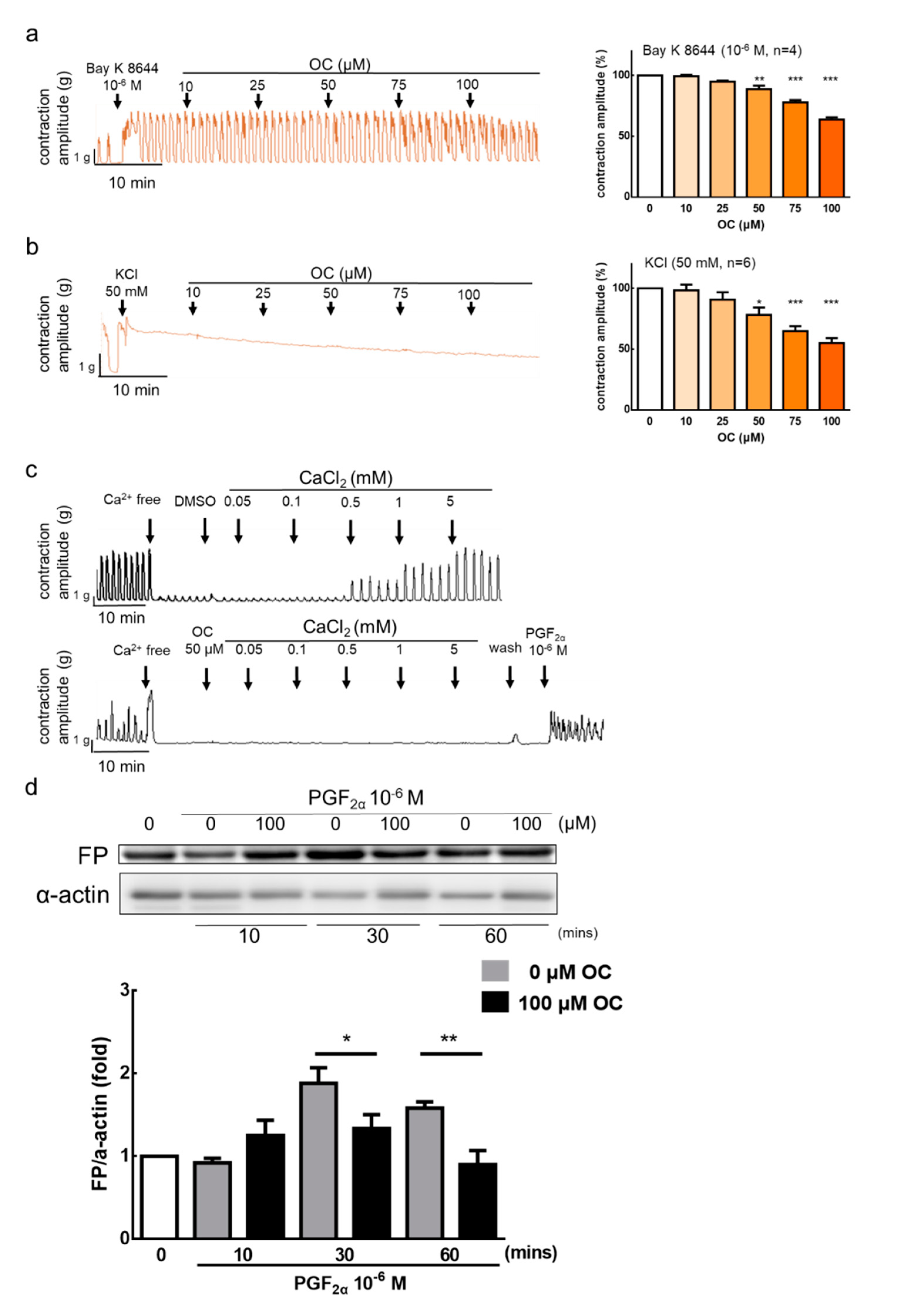
3.2. Effect of Oleocanthal on Ca2+-Dependent Contractions
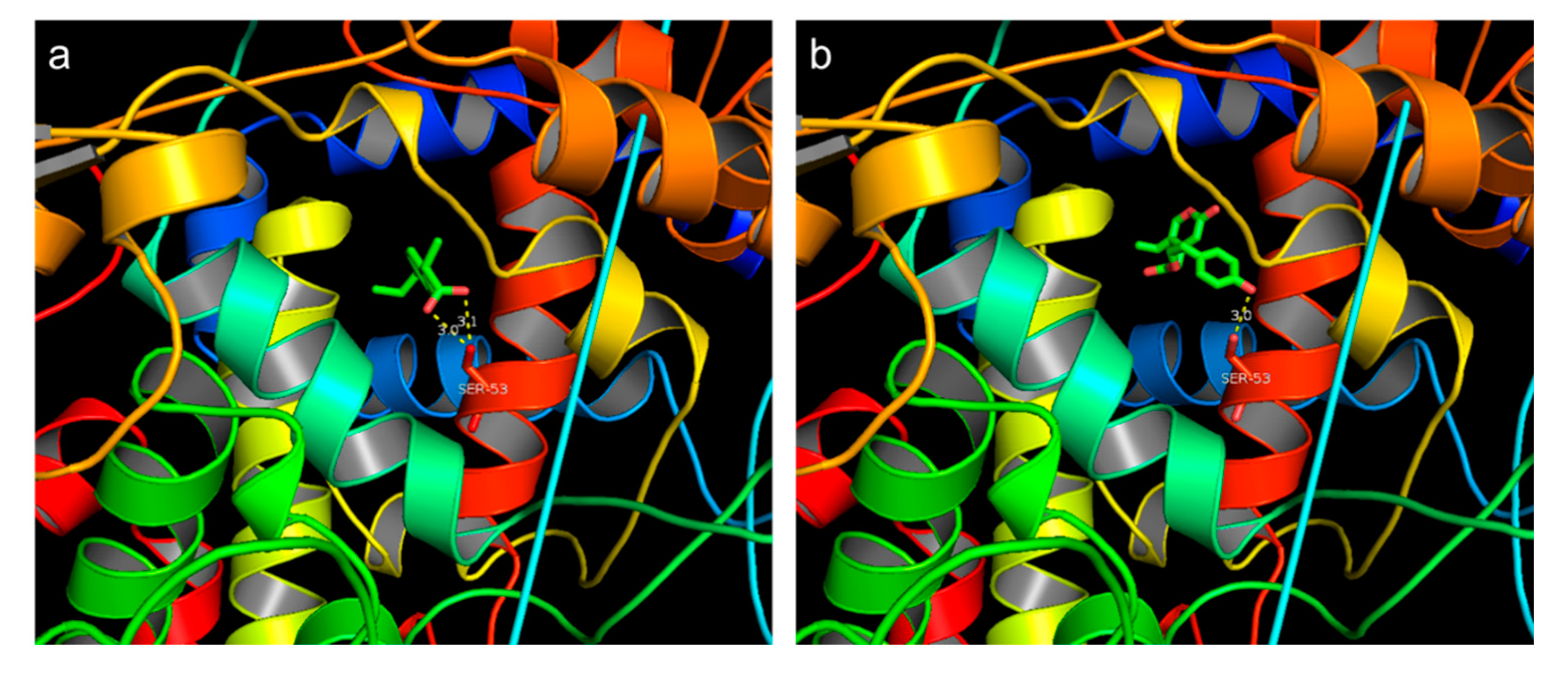
3.3. Molecular Docking Site
3.4. Extra Virgin Olive Oil Extraction Component and Inhibition Effect on Uterine Hypercontraction
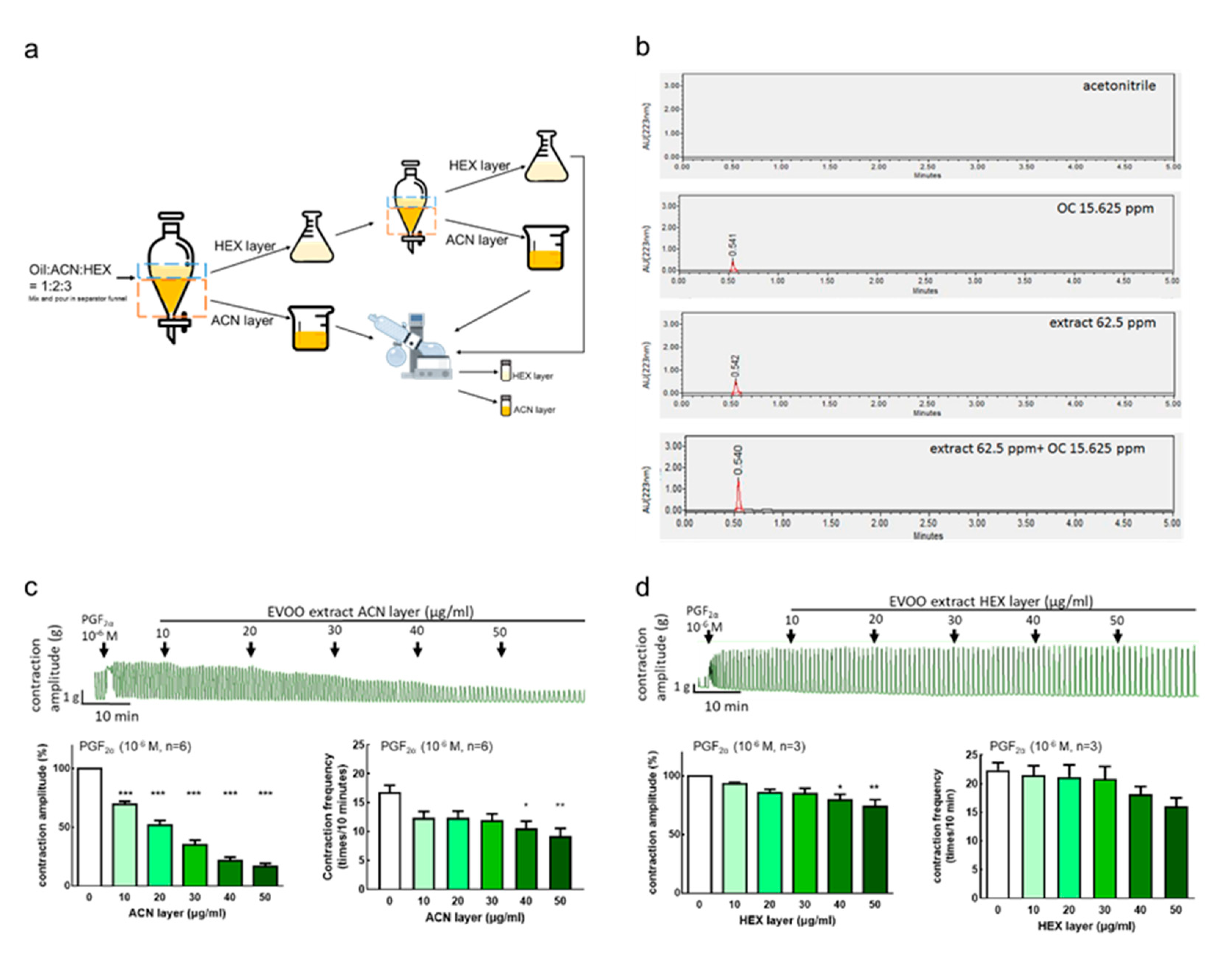
3.4.1. Extraction Rate of Extra Virgin Olive Oil
3.4.2. Extra Virgin Olive Oil Extraction and Oleocanthal Ultra Performance Liquid Chromatography (UPLC) Analysis Results
3.4.3. Inhibition Effect of Extra Virgin Olive Oil Extracts on Prostaglandin-Induced Uterine Hypercontraction Ex Vivo
3.5. Inhibition Effect of Extra Virgin Olive Oil Extracts on Writhing Response Induced by Acetic Acid/Oxytocin in Female ICR Mice
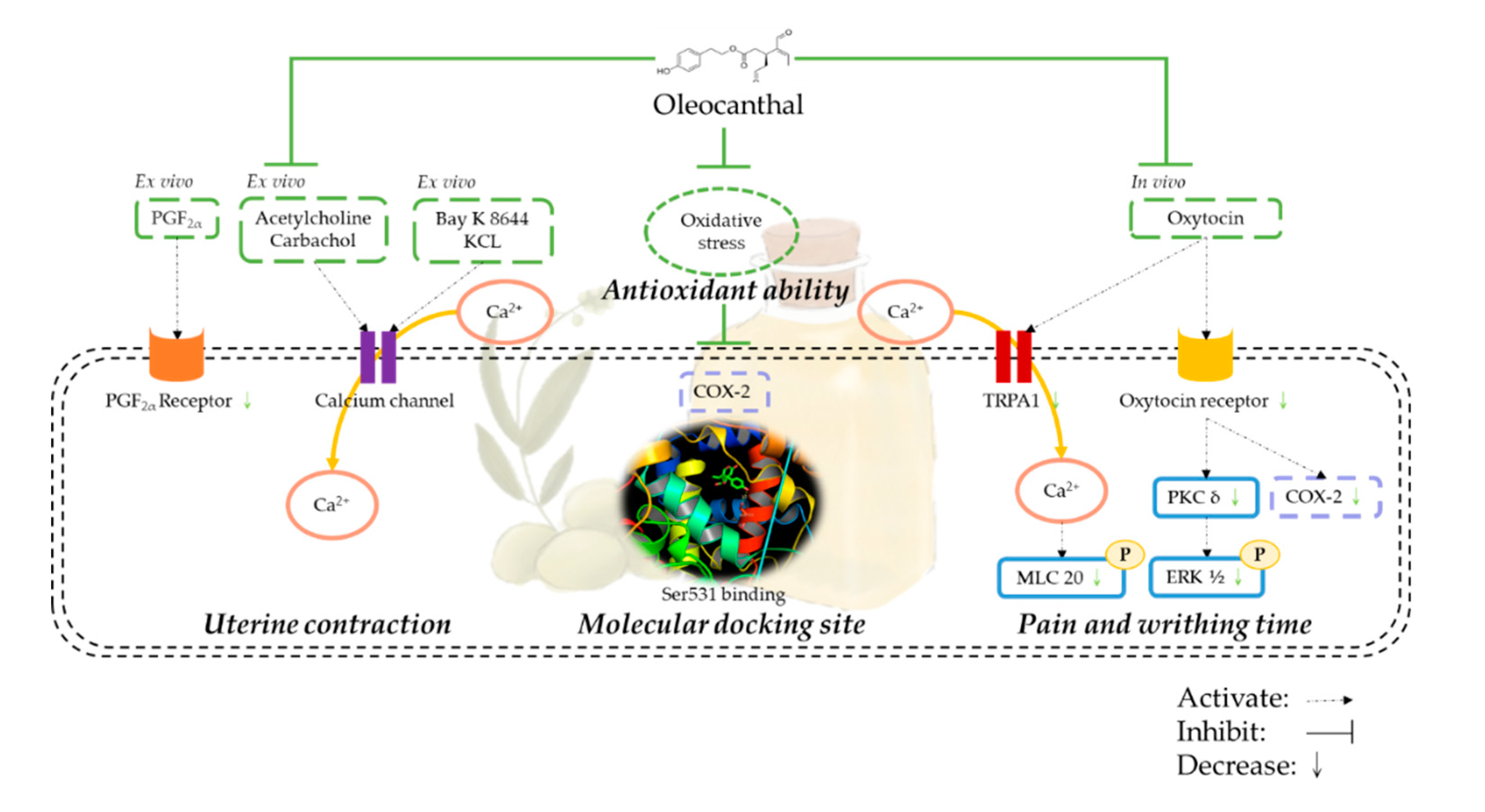
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Allen, S.S.; Allen, A.M.; Tosun, N.; Lunos, S.; al’Absi, M.; Hatsukami, D. Smoking- and menstrual-related symptomatology during short-term smoking abstinence by menstrual phase and depressive symptoms. Addict. Behav. 2014, 39, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Berkley, K.J. Primary dysmenorrhea: An urgent mandate. Pain 2013, 21, 1–8. [Google Scholar]
- Davis, A.R.; Westhoff, C.L. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J. Pediatric Adolesc. Gynecol. 2001, 14, 3–8. [Google Scholar] [CrossRef]
- Paul, J.; Maiti, K.; Read, M.; Hure, A.; Smith, J.; Chan, E.-C.; Smith, R. Phasic Phosphorylation of Caldesmon and ERK 1/2 during Contractions in Human Myometrium. PLoS ONE 2011, 6, e21542. [Google Scholar] [CrossRef]
- Ansari, H.R.; Husain, S.; Abdel-Latif, A.A. Activation of p42/p44 Mitogen-Activated Protein Kinase and Contraction by Prostaglandin F2alpha, Ionomycin, and Thapsigargin in Cat Iris Sphincter Smooth Muscle: Inhibition by PD98059, KN-93, and Isoproterenol. J. Pharmacol. Exp. Ther. 2001, 299, 178–186. [Google Scholar]
- Rosenwaks, Z.; Seegar-Jones, G. Menstrual pain: Its origin and pathogenesis. J. Reprod. Med. 1980, 25, 207–212. [Google Scholar]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Faramarzi, M.; Salmalian, H. Association of psychologic and nonpsychologic factors with primary dysmenorrhea. Iran. Red Crescent Med. J. 2014, 16, e16307. [Google Scholar] [CrossRef]
- Modaress Nejad, V.; Asadipour, M. Comparison of the effectiveness of fennel and mefenamic acid on pain intensity in dysmenorrhoea. East. Mediterr. Health J. 2006, 12, 423–427. [Google Scholar]
- Smith, C.A.; Zhu, X.; He, L.; Song, J. Acupuncture for primary dysmenorrhoea. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Ayeleke, R.O.; Farquhar, C.; Proctor, M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst. Rev. 2015, 2015, CD001751. [Google Scholar] [CrossRef] [PubMed]
- Hsia, S.-M.; Wang, K.-L.; Wang, P.S. Effects of Resveratrol, a Grape Polyphenol, on Uterine Contraction and Ca2+ Mobilization in Rats in Vivo and in Vitro. Endocrinology 2011, 152, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Hsia, S.M.; Kuo, Y.H.; Chiang, W.; Wang, P.S. Effects of adlay hull extracts on uterine contraction and Ca2+ mobilization in the rat. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E719–E726. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Grubić-Kezele, T. Olive Leaf Polyphenols Attenuate the Clinical Course of Experimental Autoimmune Encephalomyelitis and Provide Neuroprotection by Reducing Oxidative Stress, Regulating Microglia and SIRT1, and Preserving Myelin Integrity. Oxidative Med. Cell. Longev. 2020, 2020, 6125638. [Google Scholar] [CrossRef]
- Chung, D.; Caruso, R.L. Potential Role for Oxidative Stress in 2,2′-Dichlorobiphenyl–Induced Inhibition of Uterine Contractions but not Myometrial Gap Junctions. Toxicol. Sci. 2006, 93, 172–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Cao, Y.; Xie, Y.; Zhang, X.; Yang, Q.; Li, X.; Sun, J.; Qiu, P.; Cao, W.; Wang, S. Traditional Chinese medicine for the treatment of primary dysmenorrhea: How do Yuanhu painkillers effectively treat dysmenorrhea? Phytomedicine 2013, 20, 1095–1104. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Application of an advanced and wide scope non-target screening workflow with LC-ESI-QTOF-MS and chemometrics for the classification of the Greek olive oil varieties. Food Chem. 2018, 256, 53–61. [Google Scholar] [CrossRef]
- Rodríguez-López, P.; Lozano-Sanchez, J.; Borrás-Linares, I.; Emanuelli, T.; Menéndez, J.A.; Segura-Carretero, A. Structure-Biological Activity Relationships of Extra-Virgin Olive Oil Phenolic Compounds: Health Properties and Bioavailability. Antioxidants 2020, 9, 685. [Google Scholar] [CrossRef]
- Maiuri, M.C.; De Stefano, D.; Di Meglio, P.; Irace, C.; Savarese, M.; Sacchi, R.; Cinelli, M.P.; Carnuccio, R. Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 457–465. [Google Scholar] [CrossRef]
- Ferroni, F.; Maccaglia, A.; Pietraforte, D.; Turco, L.; Minetti, M. Phenolic antioxidants and the protection of low density lipoprotein from peroxynitrite-mediated oxidations at physiologic CO2. J. Agric. Food Chem. 2004, 52, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, F.; Morency, L.-P.; Najmanovich, R.J. NRGsuite: A PyMOL plugin to perform docking simulations in real time using FlexAID. Bioinformatics 2015, 31, 3856–3858. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Amran, M.; Hossain, A. Evaluation of Analgesic Activity by Acetic Acid Induced Writhing Method of Crude Extracts of Acacia nilotica. Sch. Acad. J. Pharm. 2017, 6, 126–138. [Google Scholar]
- Wei, Y.; Ma, T.; Wang, H.; Xing, J.; Wang, Y.; Gu, Z.; Mu, D.; Yin, Q.; Cheng, X.; Wang, C. Extracts of compound Muniziqi granule suppressed uterus contraction and ameliorated oxytocin-induced primary dysmenorrhea. J. Ethnopharmacol. 2018, 223, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wray, S.; Jones, K.; Kupittayanant, S.; Li, Y.; Matthew, A.; Monir-Bishty, E.; Noble, K.; Pierce, S.J.; Quenby, S.; Shmygol, A.V. Calcium signaling and uterine contractility. J. Soc. Gynecol. Investig. 2003, 10, 252–264. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid. Based Complement. Altern. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef]
- Dawood, M.Y.; Khan-Dawood, F.S. Differential suppression of menstrual fluid prostaglandin F2a, prostaglandin E2, 6-keto prostaglandin F1a and thromboxane B2 by suprofen in women with primary dysmenorrhea. Prostaglandins Other Lipid Mediat. 2007, 83, 146–153. [Google Scholar] [CrossRef]
- Sun, L.; Liu, L.-N.; Li, J.-C.; Lv, Y.-Z.; Zong, S.-B.; Zhou, J.; Wang, Z.-Z.; Kou, J.-P.; Xiao, W. The essential oil from the twigs of Cinnamomum cassia Presl inhibits oxytocin-induced uterine contraction in vitro and in vivo. J. Ethnopharmacol. 2017, 206, 107–114. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Z.; Yu, B.; Chai, C. An in vivo mouse model of primary dysmenorrhea. Exp. Anim. 2015, 64, 295–303. [Google Scholar] [CrossRef]
- Molnár, M.; Rigó, J., Jr.; Romero, R.; Hertelendy, F. Oxytocin activates mitogen-activated protein kinase and up-regulates cyclooxygenase-2 and prostaglandin production in human myometrial cells. Am. J. Obstet. Gynecol. 1999, 181, 42–49. [Google Scholar] [CrossRef]
- Wouters, E.; Hudson, C.A.; McArdle, C.A.; Bernal, A.L. Central role for protein kinase C in oxytocin and epidermal growth factor stimulated cyclooxygenase 2 expression in human myometrial cells. BMC Res. Notes 2014, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Alcaraz, M.J.; Sanchez-Hidalgo, M.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C.; Ferrandiz, M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Berrino, F. Mediterranean Diet and Its Association With Reduced Invasive Breast Cancer Risk. JAMA Oncol. 2016, 2, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B., 3rd; Beauchamp, G.K.; Breslin, P.A.S. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef]
- Bohonyi, N.; Pohóczky, K.; Szalontai, B.; Perkecz, A.; Kovács, K.; Kajtár, B.; Orbán, L.; Varga, T.; Szegedi, S.; Bódis, J.; et al. Local upregulation of transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1 ion channels in rectosigmoid deep infiltrating endometriosis. Mol. Pain 2017, 13. [Google Scholar] [CrossRef]
- Al Rihani, S.B.; Darakjian, L.I.; Kaddoumi, A. Oleocanthal-Rich Extra-Virgin Olive Oil Restores the Blood–Brain Barrier Function through NLRP3 Inflammasome Inhibition Simultaneously with Autophagy Induction in TgSwDI Mice. ACS Chem. Neurosci. 2019, 10, 3543–3554. [Google Scholar] [CrossRef]
- Berridge, M.J. Smooth muscle cell calcium activation mechanisms. J. Physiol. 2008, 586, 5047–5061. [Google Scholar] [CrossRef]
- Gawade, S.P. Acetic acid induced painful endogenous infliction in writhing test on mice. J. Pharmacol. Pharmacother. 2012, 3, 348. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, D.; Sun, Z.; Yang, J.; Chai, H.; Tang, L.; Guo, Y. Analgesic and uterine relaxant effects of isoliquiritigenin, a flavone from Glycyrrhiza glabra. Phytother. Res. PTR 2012, 26, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.Y. Dysmenorrhea. J. Reprod. Med. 1985, 30, 154–167. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.-F.; Hung, H.-C.; Chen, H.-Y.; Huang, K.-C.; Lin, P.-H.; Chang, J.-Y.; Huang, T.-C.; Hsia, S.-M. The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain—Ex Vivo and In Vivo Study. Nutrients 2020, 12, 3012. https://doi.org/10.3390/nu12103012
Chiang Y-F, Hung H-C, Chen H-Y, Huang K-C, Lin P-H, Chang J-Y, Huang T-C, Hsia S-M. The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain—Ex Vivo and In Vivo Study. Nutrients. 2020; 12(10):3012. https://doi.org/10.3390/nu12103012
Chicago/Turabian StyleChiang, Yi-Fen, Hui-Chih Hung, Hsin-Yuan Chen, Ko-Chieh Huang, Po-Han Lin, Jen-Yun Chang, Tsui-Chin Huang, and Shih-Min Hsia. 2020. "The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain—Ex Vivo and In Vivo Study" Nutrients 12, no. 10: 3012. https://doi.org/10.3390/nu12103012
APA StyleChiang, Y.-F., Hung, H.-C., Chen, H.-Y., Huang, K.-C., Lin, P.-H., Chang, J.-Y., Huang, T.-C., & Hsia, S.-M. (2020). The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain—Ex Vivo and In Vivo Study. Nutrients, 12(10), 3012. https://doi.org/10.3390/nu12103012





