Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
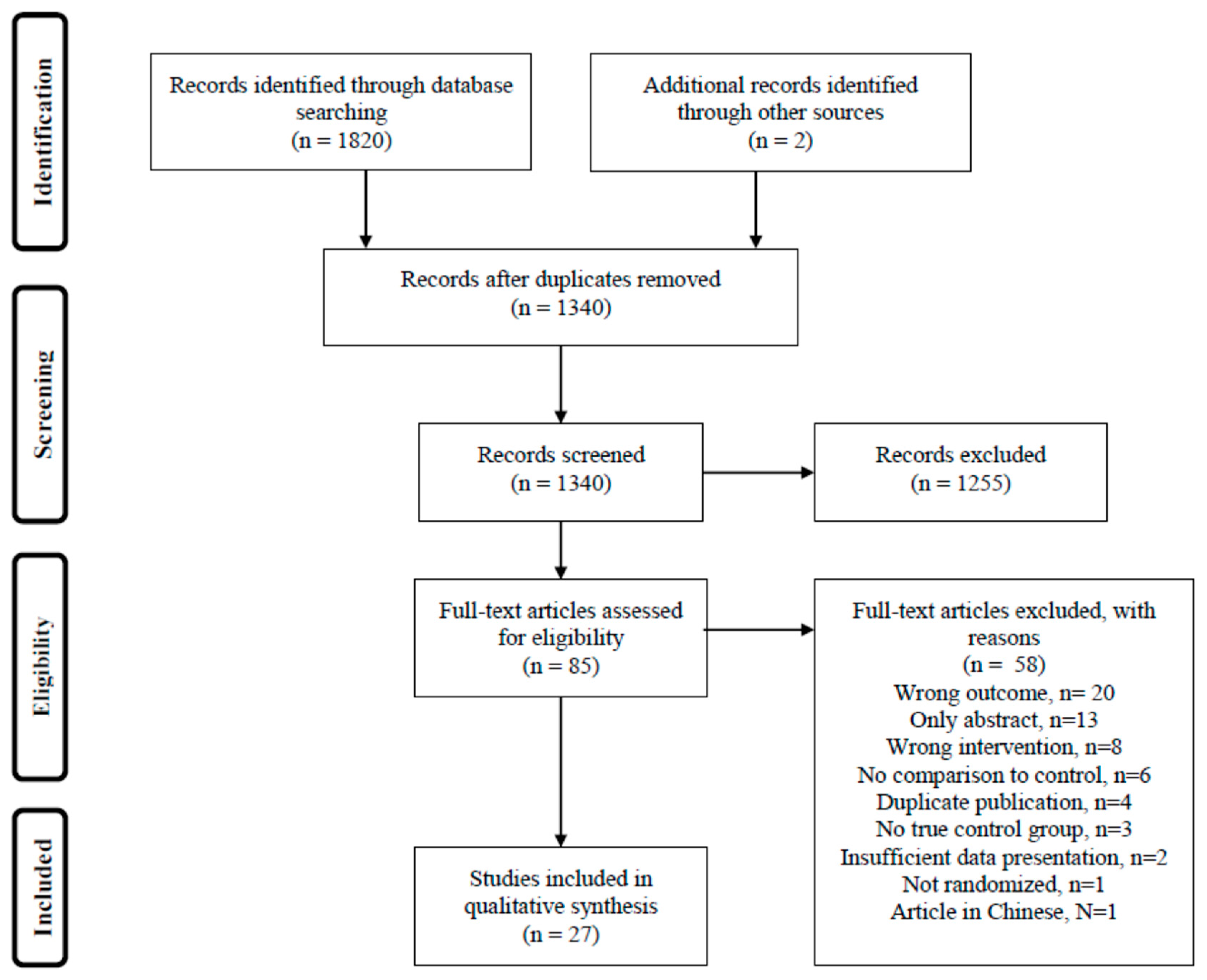
2. Materials and Methods
- Adults (≥18 years) with RA;
- Randomized controlled trials—either parallel or crossover designs;
- Both or either diet or dietary supplements, including whole diet, specific foods, spices, herbs, nutrients, dietary antioxidants and pre-, pro- or synbiotics;
- Disease activity measured by DAS28 or DAS (see below) as an outcome of the intervention.
- Animal models;
- Observational studies;
- Non-randomized trials;
- Trials that lack a control group or regimen, including those comparing high dose to low dose;
- Results that were not peer reviewed, such as conference abstracts or publications in journals with no peer review process;
- Studies on natural remedies of a traditional medicinal type or herbal remedies, or food extracts;
- Studies that include other measures of disease activity than DAS/DAS28;
- Studies of other types of rheumatic diseases, including juvenile idiopathic arthritis.
Data Collection and Processing
3. Results
3.1. Studies on Whole Diets, Foods or Meals
3.2. Studies on Spices in High Doses
3.3. Studies on n-3 Fatty Acid Supplements
3.4. Studies on Vitamin D Supplements
3.5. Studies on Other Micronutrients
3.6. Studies on Single Antioxidants
3.7. Studies on Pre-, Pro- and Synbiotics
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nam, J.L.; Winthrop, K.L.; van Vollenhoven, R.F.; Pavelka, K.; Valesini, G.; Hensor, E.M.; Worthy, G.; Landewe, R.; Smolen, J.S.; Emery, P.; et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: A systematic literature review informing the EULAR recommendations for the management of RA. Ann. Rheum. Dis. 2010, 69, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Steer, S. The course of established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2007, 21, 943–967. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, S.K.; Frits, M.; Cui, J.; Zhang, Z.Z.; Mahmoud, T.; Iannaccone, C.; Lin, T.C.; Yoshida, K.; Weinblatt, M.E.; Shadick, N.A.; et al. Diet and Rheumatoid Arthritis Symptoms: Survey Results From a Rheumatoid Arthritis Registry. Arthritis Care Res. 2017, 69, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Badsha, H. Role of Diet in Influencing Rheumatoid Arthritis Disease Activity. Open Rheumatol. J. 2018, 12, 19–28. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.P.I.; Bagley, P.J.; Selhub, J.; Nadeau, M.; Roubenoff, R. Abnormal vitamin B6 status is associated with severity of symptoms in patients with rheumatoid arthritis. Am. J. Med. 2003, 114, 283–287. [Google Scholar] [CrossRef]
- Sahebari, M.; Ayati, R.; Mirzaei, H.; Sahebkar, A.; Hejazi, S.; Saghafi, M.; Saadati, N.; Ferns, G.A.; Ghayour-Mobarhan, M. Serum Trace Element Concentrations in Rheumatoid Arthritis. Biol. Trace Elem. Res. 2016, 171, 237–245. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Torab, R.; Ghalibaf, M.; Norouzi, S.; Mehrabany, E.V. Might patients with immune-related diseases benefit from probiotics? Nutrition 2013, 29, 583–586. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the mediterranean diet: A literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Ramezani-Jolfaie, N.; Mohammadi, M.; Salehi-Abargouei, A. The effect of healthy Nordic diet on cardio-metabolic markers: A systematic review and meta-analysis of randomized controlled clinical trials. Eur. J. Nutr. 2018, 58, 2159–2174. [Google Scholar] [CrossRef] [PubMed]
- Koebnick, C.; Garcia, A.L.; Dagnelie, P.C.; Strassner, C.; Lindemans, J.; Katz, N.; Leitzmann, C.; Hoffmann, I. Long-term consumption of a raw food diet is associated with favorable serum LDL cholesterol and triglycerides but also with elevated plasma homocysteine and low serum HDL cholesterol in humans. J. Nutr. 2005, 135, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC-Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–269. [Google Scholar] [CrossRef]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, 21, CD006400. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Fransen, J.; Stucki, G.; van Riel, P.L.C.M. Rheumatoid arthritis measures: Disease Activity Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI). Arthritis Care Res. 2003, 49, S214–S224. [Google Scholar] [CrossRef]
- NNR5 Working Group. A Guide for Conducting Systematic Literature Reviews for the 5th Edition of the Nordic Nutrition Recommendations, Revised ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2011; Available online: http://www.slv.se/upload/NNR5/A%20guide%20for%20conducting%20SLR%20for%20NNR5%20FINAL.pdf (accessed on 5 July 2019).
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar]
- Skoldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef]
- Nenonen, M.T.; Helve, T.A.; Rauma, A.L.; Hanninen, O.O. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Br. J. Rheumatol. 1998, 37, 274–281. [Google Scholar] [CrossRef]
- Vadell, A.K.E.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)-a randomized, controlled crossover trial indicating effects on disease activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Thimóteo, N.S.B.; Iryioda, T.M.V.; Alfieri, D.F.; Rego, B.E.F.; Scavuzzi, B.M.; Fatel, E.; Lozovoy, M.A.B.; Simão, A.N.C.; Dichi, I. Cranberry juice decreases disease activity in women with rheumatoid arthritis. Nutrition 2019, 60, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, H.M.; Gjertsson, I.; Eneljung, T.; Winkvist, A. Influence of Blue Mussel (Mytilus edulis) Intake on Disease Activity in Female Patients with Rheumatoid Arthritis: The MIRA Randomized Cross-Over Dietary Intervention. Nutrients 2018, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Shahram, F.; Mahmoudi, M.; Tavakoli, H.; Yousefi, B.; Arablou, T.; Jafari Karegar, S. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene 2019, 698, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Shishehbor, F.; Rezaeyan Safar, M.; Rajaei, E.; Haghighizadeh, M.H. Cinnamon Consumption Improves Clinical Symptoms and Inflammatory Markers in Women With Rheumatoid Arthritis. J. Am. Coll. Nutr. 2018, 37, 685–690. [Google Scholar] [CrossRef]
- Hamidi, Z.; Aryaeian, N.; Abolghasemi, J.; Shirani, F.; Hadidi, M.; Fallah, S.; Moradi, N. The effect of saffron supplement on clinical outcomes and metabolic profiles in patients with active rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2020, 34, 1650–1658. [Google Scholar] [CrossRef]
- Galarraga, B.; Ho, M.; Youssef, H.M.; Hill, A.; McMahon, H.; Hall, C.; Ogston, S.; Nuki, G.; Belch, J.J. Cod liver oil (n-3 fatty acids) as an non-steroidal anti-inflammatory drug sparing agent in rheumatoid arthritis. Rheumatology 2008, 47, 665–669. [Google Scholar] [CrossRef]
- Das Gupta, A.B.; Hossain, A.K.; Islam, M.H.; Dey, S.R.; Khan, A.L. Role of omega-3 fatty acid supplementation with indomethacin in suppression of disease activity in rheumatoid arthritis. Bangladesh Med. Res. Counc. Bull. 2009, 35, 63–68. [Google Scholar] [CrossRef]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.M.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Hein, G.; Müller, A.; Eidner, T.; Vogelsang, H.; Basu, S.; Jahreis, G. Long-term moderate intervention with n-3 long-chain PUFA-supplemented dairy products: Effects on pathophysiological biomarkers in patients with rheumatoid arthritis. Br. J. Nutr. 2009, 101, 1517–1526. [Google Scholar] [CrossRef]
- Remans, P.H.; Sont, J.K.; Wagenaar, L.W.; Wouters-Wesseling, W.; Zuijderduin, W.M.; Jongma, A.; Breedveld, F.C.; Van Laar, J.M. Nutrient supplementation with polyunsaturated fatty acids and micronutrients in rheumatoid arthritis: Clinical and biochemical effects. Eur. J. Clin. Nutr. 2004, 58, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Buondonno, I.; Rovera, G.; Sassi, F.; Rigoni, M.M.; Lomater, C.; Parisi, S.; Pellerito, R.; Isaia, G.C.; D’Amelio, P. Vitamin D and immunomodulation in early rheumatoid arthritis: A randomized double-blind placebo-controlled study. PLoS ONE 2017, 12, e0178463. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.E.; Bartels, C.M.; Gangnon, R.E.; Jones, A.N.; Gogineni, J. An evaluation of high-dose vitamin D for rheumatoid arthritis. J. Clin. Rheumatol. 2014, 20, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Rastmanesh, R.; Abargouei, A.S.; Shadman, Z.; Ebrahimi, A.A.; Weber, C.E. A pilot study of potassium supplementation in the treatment of hypokalemic patients with rheumatoid arthritis: A randomized, double-blinded, placebo-controlled trial. J. Pain 2008, 9, 722–731. [Google Scholar] [CrossRef]
- Shishavan, N.G.; Gargari, B.P.; Jafarabadi, M.A.; Kolahi, S.; Haggifar, S.; Noroozi, S. Vitamin K(1) Supplementation Did Not Alter Inflammatory Markers and Clinical Status in Patients with Rheumatoid Arthritis. Int. J. Vitam. Nutr. Res. 2018, 88, 251–257. [Google Scholar] [CrossRef]
- van Ede, A.E.; Laan, R.F.; Rood, M.J.; Huizinga, T.W.; van de Laar, M.A.; van Denderen, C.J.; Westgeest, T.A.; Romme, T.C.; de Rooij, D.J.; Jacobs, M.J.; et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: A forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2001, 44, 1515–1524. [Google Scholar] [CrossRef]
- Gargari, B.P.; Kolahi, S.; Dehghan, P.; Khabbazi, A.; Mirtaheri, E. Effects of alpha-lipoic acid supplementation on clinical status and anthropometric indices in women with rheumatoid arthritis. Curr. Top. Nutraceutical Res. 2015, 13, 33–40. [Google Scholar]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef]
- Nachvak, S.M.; Alipour, B.; Mahdavi, A.M.; Aghdashi, M.A.; Abdollahzad, H.; Pasdar, Y.; Samadi, M.; Mostafai, R. Effects of coenzyme Q10 supplementation on matrix metalloproteinases and DAS-28 in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Clin. Rheumatol. 2019, 38, 3367–3374. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Alavi, A.; Goodfellow, L.; Fraser, O.; Tarelli, E.; Bland, M.; Axford, J. A double-blind, randomized, placebo-controlled study to explore the efficacy of a dietary plant-derived polysaccharide supplement in patients with rheumatoid arthritis. Rheumatology 2011, 50, 1111–1119. [Google Scholar] [CrossRef][Green Version]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akhavan, R.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Pineda Mde, L.; Thompson, S.F.; Summers, K.; de Leon, F.; Pope, J.; Reid, G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Monit. 2011, 17, CR347–CR354. [Google Scholar]
- Zamani, B.; Farshbaf, S.; Golkar, H.R.; Bahmani, F.; Asemi, Z. Synbiotic supplementation and the effects on clinical and metabolic responses in patients with rheumatoid arthritis: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2017, 117, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Nasir, B.; Haq, I.U.; Kim, S.J. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef]
- Guerreiro, C.S.; Calado, Â.; Sousa, J.; Fonseca, J.E. Diet, Microbiota, and Gut Permeability-The Unknown Triad in Rheumatoid Arthritis. Frontiers Med. 2018, 5, 349. [Google Scholar] [CrossRef]
- Baker, J.F.; England, B.R.; Mikuls, T.R.; Sayles, H.; Cannon, G.W.; Sauer, B.C.; George, M.D.; Caplan, L.; Michaud, K. Obesity, Weight Loss, and Progression of Disability in Rheumatoid Arthritis. Arthritis Care Res. 2018, 70, 1740–1747. [Google Scholar] [CrossRef]
- Smedslund, G.; Byfuglien, M.G.; Olsen, S.U.; Hagen, K.B. Effectiveness and safety of dietary interventions for rheumatoid arthritis: A systematic review of randomized controlled trials. J. Am. Diet. Assoc. 2010, 110, 727–735. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.T.; Alex, S.; Mota, M.R.; Sales, N.B.; Brown, L.E.; Bottaro, M. Effect of strength training combined with antioxidant supplementation on muscular performance. Appl. Physiol. Nutr. Metab. 2018, 43, 775–781. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Tolerable Upper Intake Level of vitamin D. EFSA J. 2012, 10, 2813. [Google Scholar]
- European Food Safety Authority (EFSA). Coumarin in flavourings and other food ingredients with flavouring properties. EFSA J. 2008, 793, 1–15. [Google Scholar]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124 e4. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Müller, H.; de Toledo, F.W.; Resch, K.L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar]
- Cramp, F.; Hewlett, S.; Almeida, C.; Kirwan, J.R.; Choy, E.H.; Chalder, T.; Pollock, J.; Christensen, R. Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst. Rev. 2013, 23, CD008322. [Google Scholar] [CrossRef]
- Mohammed, A.T.; Khattab, M.; Ahmed, A.M.; Turk, T.; Sakr, N.; Khalil, A.M.; Abdelhalim, M.; Sawaf, B.; Hirayama, K.; Huy, N.T. The therapeutic effect of probiotics on rheumatoid arthritis: A systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 2017, 36, 2697–2707. [Google Scholar] [CrossRef]
| Author, Year, Country [Ref] | Participants | Population | Study Duration | Intervention | Control | DAS28 Main Results | Adjusted Analysis | Study Quality | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Sköldstam 2012 Sweden [20] RCT UB | N randomized: 56 N control: 27 N intervention: 29 Total drop out: 9% | 80% women 49% MTX use Disease activity: Intermediate | 12 weeks | Mediterranean diet Foods provided: Some meals in the initial 3 weeks. Margarine, olive oil, canola oil, frozen vegetables, tea. | Usual diet Foods provided: Some meals in the initial 3 weeks | DAS28 ESR I: 4.4 → 3.9 C: 4.3 → 4.3 P = 0.047 | Unadjusted | B | No relevant power calculation Weight loss in intervention group Study violations not excluded |
| Nenonen 1998Finland [21] RCT UB | N randomized: 43 N control: 21N intervention: 22 Total drop out: 5% | 86% women 35% MTX use Disease activity: Intermediate | 3 months | Uncooked vegan diet Foods provided: All foods | Usual omnivore diet Foods provided: None | DAS28: I: 3.26 → 3.01 C: 3.44 → 3.46 P = 0.7 | Adjusted for weight change | C | No relevant power calculation Weight loss in intervention group Calculation of DAS28 unclear |
| Vadell 2019Sweden [22] RCOT SB (assessors) | N randomized: 50 N control: N intervention: Total drop out: 12% | 77% women 66% MTX use Disease activity: Intermediate | 10 weeks | Anti-inflammatory, Mediterranean style diet, rich in fruit, berries, vegetable, nuts, whole grain, low fat dairy, fish and vegetable oil Foods provided: Breakfast, main meal, one snack per day for 5 d/w | Average Swedish diet, high in red meat, refined grains, butter, quark, protein bars Foods provided: Breakfast, main meal, one snack per day for 5 d/w | DAS28 ESR: I: 3.39 → 3.05 C: 3.42 → 3.27 P = NS | Adjusted for baseline, diet sequence, batch and starting diet | A | No drop out analysis but few dropouts Weight stable during both diets Adjusted for baseline and carry over effects |
| Thimoteo 2019Brazil [23] RCT UB | N randomized: 41N intervention: 23 N control:18 Total drop out: 7% | 100% women 63% MTX use Disease activity: Intermediate | 3 months | Usual diet plus 500 mL/d of reduced calorie cranberry juice | Usual diet | DAS28 ESR: I: 3.48 → 2.99 C: 3.59 → 3.52 P = NS | Unadjusted | B | No drop out analysis but few drop outs Excluded those with poor compliance |
| Lindqvist 2018Sweden [24] RCOT SB (assessors) | N randomized: 39 N control: 19 N intervention: 20 Total drop out: 41% | 100% women 60% MTX use Disease activity: Intermediate | 11 weeks | 5 weekly meals with 75 g of blue mussels in addition to normal diet Foods provided: all 5 meals/w | 5 weekly meals with meat/chicken in addition to normal diet Foods provided: all 5 meals/w | DAS28 (ITT) I: 3.96 → 3.40 C: 3.95 → 3.88 P = 0.023 | Unadjusted | B | High drop out Did not consider carry over effects in analysis NS effects on DAS28 ESR in PP analysis DAS28 CRP also lower after intervention diet |
| Aryaeian 2019 Iran [25] RCT DB | N randomized: 70N intervention: 35 N control: 35 Total drop out: 10% | 89% women 97% MTX use Disease activity: Intermediate | 12 weeks | 1500 mg ginger powder in 2 capsules daily | Similar capsules with wheat flour | DAS28 ESR: I: 4.73 → 3.44 C: 4.51 → 4.30 P = 0.003 | Unadjusted | B | No relevant power calculation No drop out analysis but few drop outs |
| Shishehbor 2018 Iran [26] RCT DB | N randomized: 40 N intervention: 20 N control: 20 Total drop out: 10% | 100% women 78% MTX use Disease activity: High | 8 weeks | 2000 mg cinnamon powder in 4 capsules daily | Similar capsules with starch | DAS28: I: 6.04 → 3.92 C: 5.35 → 5.64 P < 0.001 | Adjusted for baseline and menopausal status | A | Almost worse by control |
| Hamidi 2020 Iran [27] RCT DB | N randomized: 66 N intervention: 33 N control: 33 Total drop out: 8% | 100% women % MTX use not specified Disease activity: Intermediate | 3 months | 100 mg/d saffron in 1 tablet | Placebo (100 mg hydroxyl-propylmethyl cellulose in 1 tablet) | DAS28 CRP: I: 5.09 → 4.33 C: 4.92 → 5.19 P < 0.001 | Unadjusted | B | Almost worse by control No drop out analysis but few drop outs Excluded uncompliant participants, though few Did not adjust for baseline, despite slight imbalance |
| Author, Year, Country (Ref) | Participants | Population | Study Duration | Intervention | Control | DAS28 Main Results | Adjusted Analysis | Study Quality | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Galarraga 2008UK (Scotland) [28] RCT DB | N randomized: 97N intervention: 49 N control: 48 Total drop out: 40% | 71% women 77% DMARD use Disease activity: Intermediate | 12 weeks | 10 g cod liver oil in capsules containing: 1500 mg EPA, 700 mg DHA, 800 µg vit A, 5 µg vit D, 20 IE vit E | Placebo (air filled capsules) | DAS28 CRP: I: 4.5 → 4.3 C: 4.5 → 4.3 P = 0.976 | Unadjusted | B | Study duration was 9 mo but after 12 w NSAID was reduced Amount of n3/d unclear No drop out analysis High drop out |
| Das Gupta 2009 Bangladesh [29] RCT UB | N randomized: 100N intervention: 50 N control: 50 Total drop out: 19% | % of women not specified % MTX use not specified Disease activity: High | 12 weeks | 75 mg/d indomethacin 3 g/d n3 FA in capsules | 75 mg/d indomethacin | DAS28: I: 7.2 → 4.2 C: 7.3 → 4.8 P < 0.05 | Unadjusted | B | No clear aim or hypothesis No clear description of participant characteristics Calculation of DAS28 unclear |
| Dawczynski 2018 Germany [30] RCOT DB | N randomized: 38N intervention: 38 N control: 38 Total drop out: 34% | 84% women % MTX use not specified Disease activity: Intermediate | 10 weeks | 8 g micro algae, enriched in 60 g sausage, 8 g tomato spread, 30 g milk powder Amount of n3/d: 2.36 g | 8 g sun flower oil, enriched in 60 g sausage, 8 g tomato spread, 30 g milk powder | DAS28: I: 4.5 → 3.88 C: 3.99 → 4.13 P = 0.085 | Adjusted for sequence and baseline value | B | Excluded those with poor compliance Calculation of DAS28 unclear |
| Dawczynski 2009 Germany [31] RCOT UB | N randomized: 45N intervention: 45 N control: 45 Total drop out: 13% | 96% women % MTX use not specified Disease activity: Intermediate | 12 weeks | 40 g fat in the form of 200 g yoghurt, 30 g cheese and butter. Milk fat was in part exchanged with oils high in EPA, DHA and alpha linoleic acid. Amount of n3/d: 2.4 g | Commercial dairy products with similar fat content | DAS28: I: 4.45 → 4.32 C: 4.18 → 4.24 P = NS | Adjusted for sequence and baseline value | B | Excluded those with poor compliance Adjusted for potential carry over effects in analysis Calculation of DAS28 unclear No relevant power calculation |
| Remans2004 Netherlands [32] RCT DB | N randomized: 66N intervention: 33 N control: 33 Total drop out: 17% | 82% women 62% MTX use Disease activity: High | 4 months | Liquid nutritional supplement containing PUFA (1400 mg EPA 211 mg DHA, 40 mg DPA, 16 mg ALA) and micronutrients | Placebo drink with the same taste, odor and color but with artificial sweetener | DAS28: I: 5.36 → 5.58 C: 5.14 → 5.35 P = NS | Unadjusted | B | Slight gain in body weight in intervention group Calculation of DAS28 unclear No drop out analysis |
| Buondonno 2017 Italy [33] RCT DB | N randomized: 39 N intervention: 21 N control: 18 Total drop out: 8% | 100% women 100% MTX use Disease activity: High | 3 months | 300 000 IU (7500 µg) of vitamin D3 administered once | Placebo | DAS28 ESR: I: ? → 5.6 C: ? →5.8 P = NS | Unadjusted | B | Unclear DAS28 at baseline No dropout analysis, but low drop out |
| Hansen 2014 USA [34] RCT DB | N randomized: 22 N intervention: 11 N control: 11 Total drop out: 0% | 46% women % MTX use not specified Disease activity: Low | 12 months | Month 1: 3*50 000 IU vitamin D2/week Month 2-11: 2*50 000 IU vitamin D2/month 1500 mg calcium daily | Placebo 1500 mg calcium daily | DAS28: I: 3.0 → 3.03 C: 2.54 → 2.96 P = NS | Unadjusted | C | Sparse methods and results No relevant power calculation Calculation of DAS28 not presented |
| Rastmanesh 2008 Iran [35] RCT DB | N randomized: 38N intervention: 18 N control: 18 Total drop out: 16% | 100% women 100% DMARD use Disease activity: High | 28 days | 6000 mg of potassium in the form of enriched white grape juice | Placebo grape juice | DAS28: I: 5.86 → −0.69 C: 5.80 → −0.1 P < 0.01 | Adjusted for baseline | B | Hypokalemic participants Short study duration Calculation of DAS28 unclear Compliance unclear Conflict of interest statement missing |
| Shishavan 2015 Iran [36] RCT DB | N randomized: 64N intervention: 32 N control: 32 Total drop out: 9% | 100% women 91% MTX use Disease activity: Remission | 8 weeks | 10 µg/day of vitamin K1 as a chewable tablet | Placebo | DAS28 CRP: I: 1.74 → 1.59 C: 2.26 → 1.85 P = NS | Adjusted (baseline, duration, folic acid intake, energy intake and weight) | A | No drop out analysis, but few drop outs Participants in remission at baseline |
| Van Ede 2001 Netherlands [37] RCT DB | N randomized: 434N intervention 1: 143 N intervention 2: 147 N control: 144 Total drop out unclear | 71% women 100% MTX use Disease activity: Intermediate | 12 months | Intervention 1: 1 mg/day of folic acid (oral, intake in morning) Intervention 2: 2.5 mg/week of folinic acid (oral, within 24 h of MTX intake) | Placebo | DAS28 ESR: I 1: 4.8 → −1.5 I 2: 4.6 → −1.4 C: 4.7 → −1.5 P1 = NS P2 = NS | Unadjusted? | B | Drop out unclear Unclear if analysis is adjusted |
| Gargari 2015 Iran [38] RCT DB | N randomized: 70N intervention: 35 N control: 35 Total drop out: 7% | 100% women 88% MTX use Disease activity: Remission | 8 weeks | Two capsules of 1200 mg alpha lipoic acid | Placebo (1200 mg maltodextrin) | DAS28: I: 2.11 → 1.86 C: 2.14 → 1.98 P = 0.442 | Adjusted for baseline value | A | Excluded those with poor compliance, but few No dropout analysis, but few dropouts Participants in remission at baseline Calculation of DAS28 unclear |
| Javadi 2016 Iran [39] RCT DB | N randomized: 50N intervention: 25 N control: 25 Total drop out: 20% | 100% women 92% MTX use Disease activity: Low–intermediate | 8 weeks | One capsule containing 500 mg quercetin | Placebo (lactose) | DAS28 ESR: I: 3.22 → 2.65 C: 3.13 → 3.11 P = 0.04 | Adjusted for baseline value | B | Conflict of interest statement missing No drop out analysis Excluded those with poor compliance |
| Nachvak 2019 Iran [40] RCT DB | N randomized: 54N intervention: 27 N control: 27 Total drop out: 17% | 89% women % MTX use not specified Disease activity: Intermediate | 2 months | 100 mg/day CoQ10 capsules | Placebo | DAS28 ESR: I: 5.01→2.34 C: 4.88→4.04 P < 0.001 | Adjusted for baseline, age, sex, disease duration, medications, and total energy intake | B | Compliance unclear No drop out analysis Placebo unclear |
| Khojah 2018 Egypt [41] RCT UB | N randomized: 100N intervention: 50 N control: 50 Total drop out: 0% | 68% women % MTX use not specified Disease activity: Intermediate | 3 months | One capsule containing 1 g resveratrol daily | Regular treatment | DAS28 ESR: I: 4.62 → 3.12 C: 4.91 → 4.78 P < 0.001 | Unadjusted | B | No placebo No relevant power analysis Compliance unclear |
| Author, Year, Country [Ref] | Participants | Population | Study Duration | Intervention | Control | DAS28 Main Results | Adjusted Analysis | Study Quality | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Alavi 2011 UK [42] RCT DB | N randomized: 78N intervention: 33 N control: 36 Total drop out: 22% | 81% women % MTX use not specified Disease activity: Intermediate | 6 months | Dietary fiber supplement, (Ambrotose complex: aloe vera gel extract, arabinogalactan, gum ghatti, gum tragacanth, glucosamine) – 1,3 g/day | Placebo (rice flour) | DAS28: I: ~4.1 → 4.0 C: ~4.3 → 3.8 P = 0.009 | Unadjusted | C | No drop out analysis, but ITT Baseline DAS28 and treatment unavailable Calculation of DAS28 unclear |
| Alipour 2014 Iran [43] RCT DB | N randomized: 60N intervention: 30 N control: 30 Total drop out: 23% | 100% women 76% MTX use Disease activity: Remisson | 8 weeks | One daily capsule of L. casei 01, min 108 colony forming units (plus maltrodextrin) | Placebo (maltrodextrin) | DAS28 CRP I: 2.56 → 2.07 C: 2.31 → 2.23 P = 0.039 | Adjusted (baseline, BMI change, anxiety and menopausal status) | B | Excluded those with poor compliance No drop out analysis Remission at baseline |
| Zamani 2016 Iran [44] RCT DB | N randomized: 60N intervention: 30 N control: 30 Total drop out: 0% | 85% women 97% MTX use Disease activity: Intermediate | 8 weeks | Probiotic capsules containing L. acidophilus (2 × 109 CFU), L. casei (2 × 109 CFU), B. bifidum (2 × 109 CFU) | Placebo (starch) | DAS28: I: 4.0 → 3.7 C: 4.1 → 4.0 P = 0.01 | Adjusted for baseline, age, BMI | A | Calculation of DAS28 unclear |
| De Los Angeles Pineda 2011 Canada [45] RCT DB | N randomized: 29N intervention: 15 N control: 14 Total drop out: 10% | 93% women 76% MTX use Disease activity: Intermediate | 3 months | Two daily capsules of L. rhamnosus GR-1 and L. reuteri RC-14 (each 2 billion CFU), plus dextrose, potato starch, microcrystalline cellulose and magnesium stearate | Placebo (dextrose, potato starch, microcrystalline cellulose and magnesium stearate) | DAS I: 4.18 → ∆ − 2.1 C: 4.83 → ∆ − 2.9 P = 0.77 | Unadjusted | B | No power calculationNo drop out analysis but few drop outs Compliance unclear |
| Zamani 2017 Iran [46] RCT DB | N randomized: 54N intervention: 27 N control: 27 Total drop out: 0% | 85% women 96% MTX Disease activity: Intermediate | 8 weeks | Synbiotic capsules containing L. acidophilus, L. casei, B. bifidum (each 2 × 109 CFU), and 800 mg inulin | Placebo (starch) | DAS28: I: 4.2 → 2.6 C: 3.5 → 3.2 P < 0.001 | Adjusted for baseline, BMI and age | A | Calculation of DAS28 unclear |
| Type of Intervention | Ref. | No Studies | Results for DAS28 | Quality of Evidence | Comments |
|---|---|---|---|---|---|
| Whole Diet Interventions | |||||
| 7Mediterranean style diet | [20,22] | 2 | Inconsistent, but suggestive | Moderate (+++) | Risk of bias: 0 Consistency: –1 Relevance: 0 Precision: 0 Publication bias: 0 |
| Raw food diet | [21] | 1 | Non-significant compared to control | Very low (+) | Risk of bias: –2 Consistency: 0 Relevance: -1 Precision: 0 Publication bias: 0 |
| Single food items | |||||
| Blue mussels | [24] | 1 | Improvement compared to control | Low (++) | Risk of bias: –1 Consistency: 0 Relevance: -1 Precision: 0 Publication bias: 0 |
| Cranberry juice | [23] | 1 | Non-significant compared to control | Moderate (+++) | Risk of bias: –1 Consistency: 0 Relevance: 0 Precision: 0 Publication bias: 0 |
| Ginger powder | [25] | 1 | Improvement compared to control | Moderate (+++) | Risk of bias: –1 Consistency: 0 Relevance: 0 Precision: 0 Publication bias: 0 |
| Cinnamon powder | [26] | 1 | Improvement compared to control | Moderate (+++) | Risk of bias: 0 Consistency: 0 Relevance: 0 Precision: –1 Publication bias: 0 |
| Saffron | [27] | 1 | Improvement compared to control | Moderate (+++) | Risk of bias: –1 Consistency: 0 Relevance: 0 Precision: 0 Publication bias: 0 |
| Nutrients | |||||
| n-3 supplementation | [28,29,30,31,32] | 5 | Inconsistent, most studies show no effect | Low (++) | Risk of bias: –1 Consistency: –1 Relevance: 0 Precision: 0 Publication bias: 0 |
| Vitamin D supplementation | [33,34] | 2 | Consistently no improvement compared to control | Low (++) | Risk of bias: –1 Consistency: 0 Relevance: 0 Precision: –1 Publication bias: 0 |
| Vitamin K supplementation | [36] | 1 | No improvement compared to control | Low (++) | Risk of bias: 0 Consistency: 0 Relevance: –1 Precision: –1 Publication bias: 0 |
| Folic acid supplementation | [37] | 1 | No improvement compared to control | Moderate (+++) | Risk of bias: –1 Consistency: 0 Relevance: 0 Precision: 0 Publication bias: 0 |
| Potassium supplementation | [35] | 1 | Significant improvement compared to control | Low (++) | Risk of bias: –1 Consistency: 0 Relevance: –1 Precision: 0 Publication bias: 0 |
| Single antioxidants | |||||
| Alpha lipoic acid | [38] | 1 | No improvement compared to control | Moderate (+++) | Risk of bias: 0 Consistency: 0 Relevance: –1 Precision: 0 Publication bias: 0 |
| Quercetin | [39] | 1 | Improvement compared to control | Moderate (+++) | Risk of bias: –1 Consistency: 0 Relevance: 0 Precision: 0 Publication bias: 0 |
| Resveratrol | [41] | 1 | Improvement compared to control | Low (++) | Risk of bias: –1 Consistency: 0 Relevance: 0 Precision: –1 Publication bias: 0 |
| Ubiquinone (Q10) | [40] | 1 | Improvement compared to control | Moderate (+++) | Risk of bias: –1 Consistency: 0 Relevance: 0 Precision: 0 Publication bias: 0 |
| Pro-, pre- and synbiotics | |||||
| Prebiotics | [42] | 1 | Significantly worse compared to control | Low (++) | Risk of bias: –2 Consistency: 0 Relevance: 0 Precision: 0 Publication bias: 0 |
| Probiotics and synbiotics, overall Probiotics and synbiotics containing L Casei | [43,44,45,46] [43,44,46] | 4 3 | Inconsistent overall Consistent improvement compared to control | Low (+++) Moderate (+++) | Risk of bias: 0 Consistency: –2 Relevance: 0 Precision: 0 Publication bias: 0 Risk of bias: 0 Consistency: 0 Relevance: 0 Precision: 0 Publication bias: –1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2991. https://doi.org/10.3390/nu12102991
Nelson J, Sjöblom H, Gjertsson I, Ulven SM, Lindqvist HM, Bärebring L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients. 2020; 12(10):2991. https://doi.org/10.3390/nu12102991
Chicago/Turabian StyleNelson, Josefine, Helen Sjöblom, Inger Gjertsson, Stine M. Ulven, Helen M. Lindqvist, and Linnea Bärebring. 2020. "Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials" Nutrients 12, no. 10: 2991. https://doi.org/10.3390/nu12102991
APA StyleNelson, J., Sjöblom, H., Gjertsson, I., Ulven, S. M., Lindqvist, H. M., & Bärebring, L. (2020). Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients, 12(10), 2991. https://doi.org/10.3390/nu12102991






