Nutritional Strategies in Prediabetes: A Scoping Review of Recent Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
3. Results
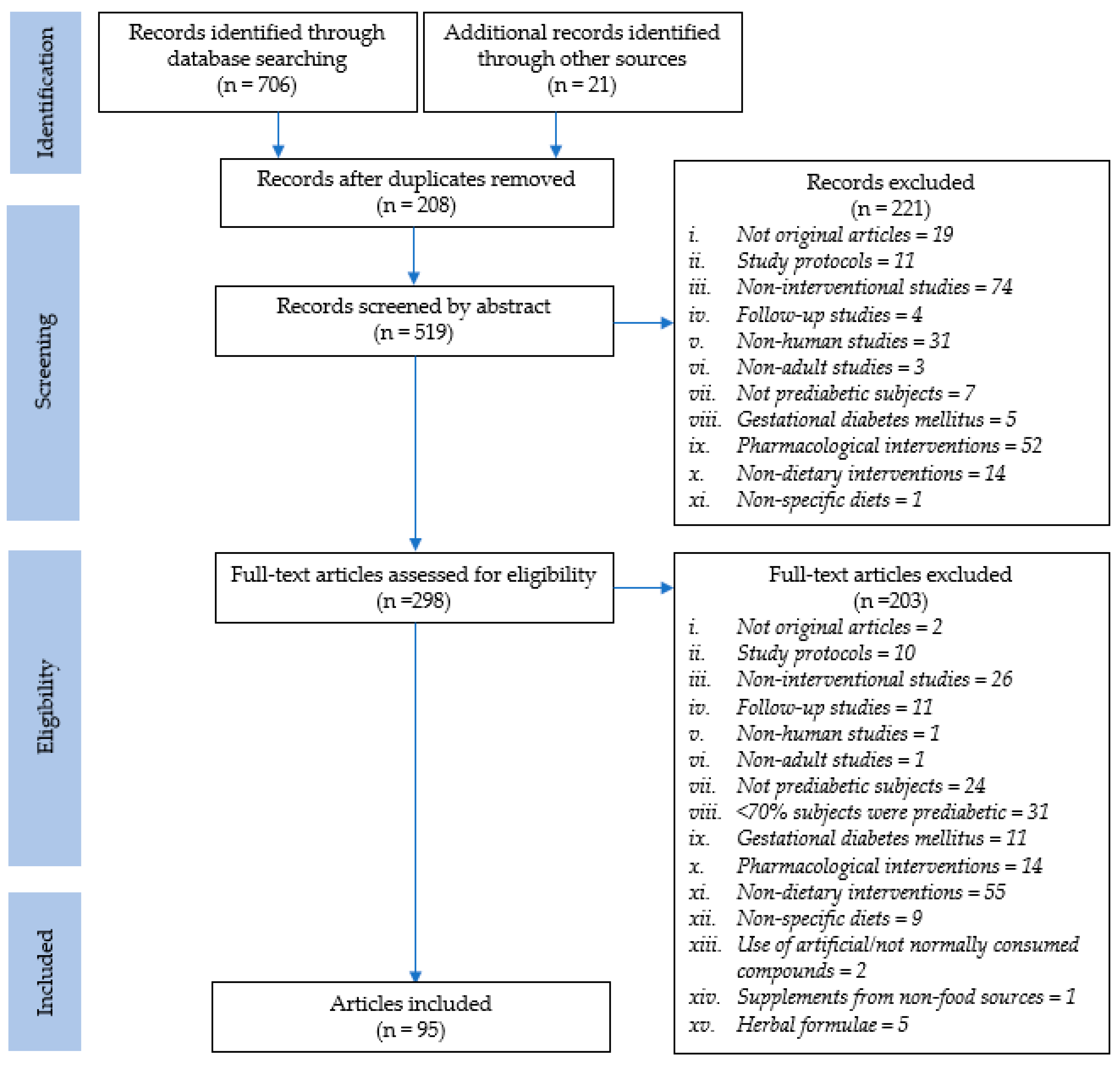
3.1. Study Selection and Characteristics
3.2. Intervention Characteristics
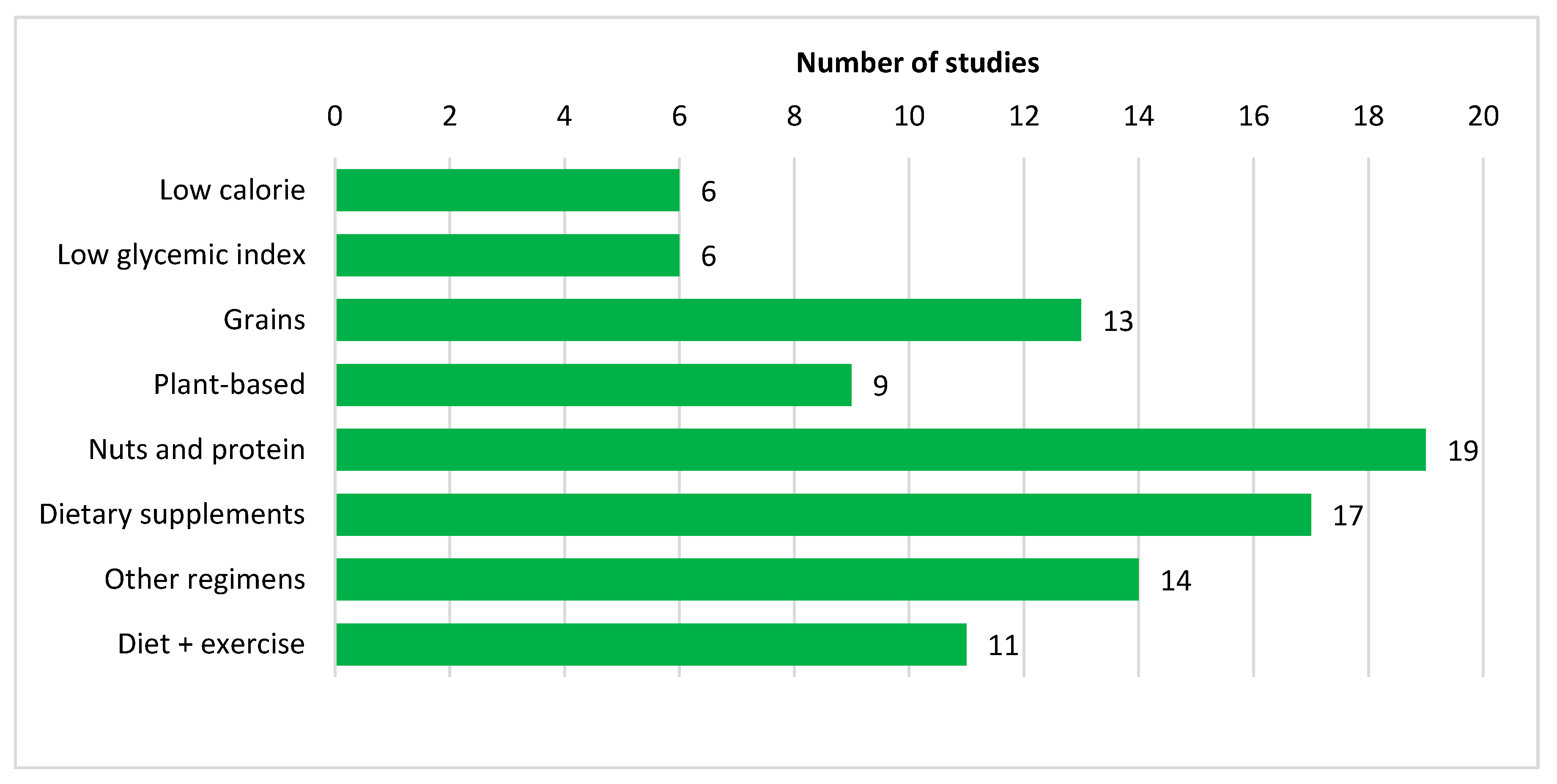
3.3. Nutritional Strategies
3.3.1. Low Calorie Diet
3.3.2. Low Glycemic Index Diet
3.3.3. Other Dietary Regimens
3.3.4. Diet and Physical Activity
3.3.5. Specific Food/Food Groups
Grains
Plant-Based Foods
Nuts and Protein-Based Foods
Dietary Supplements
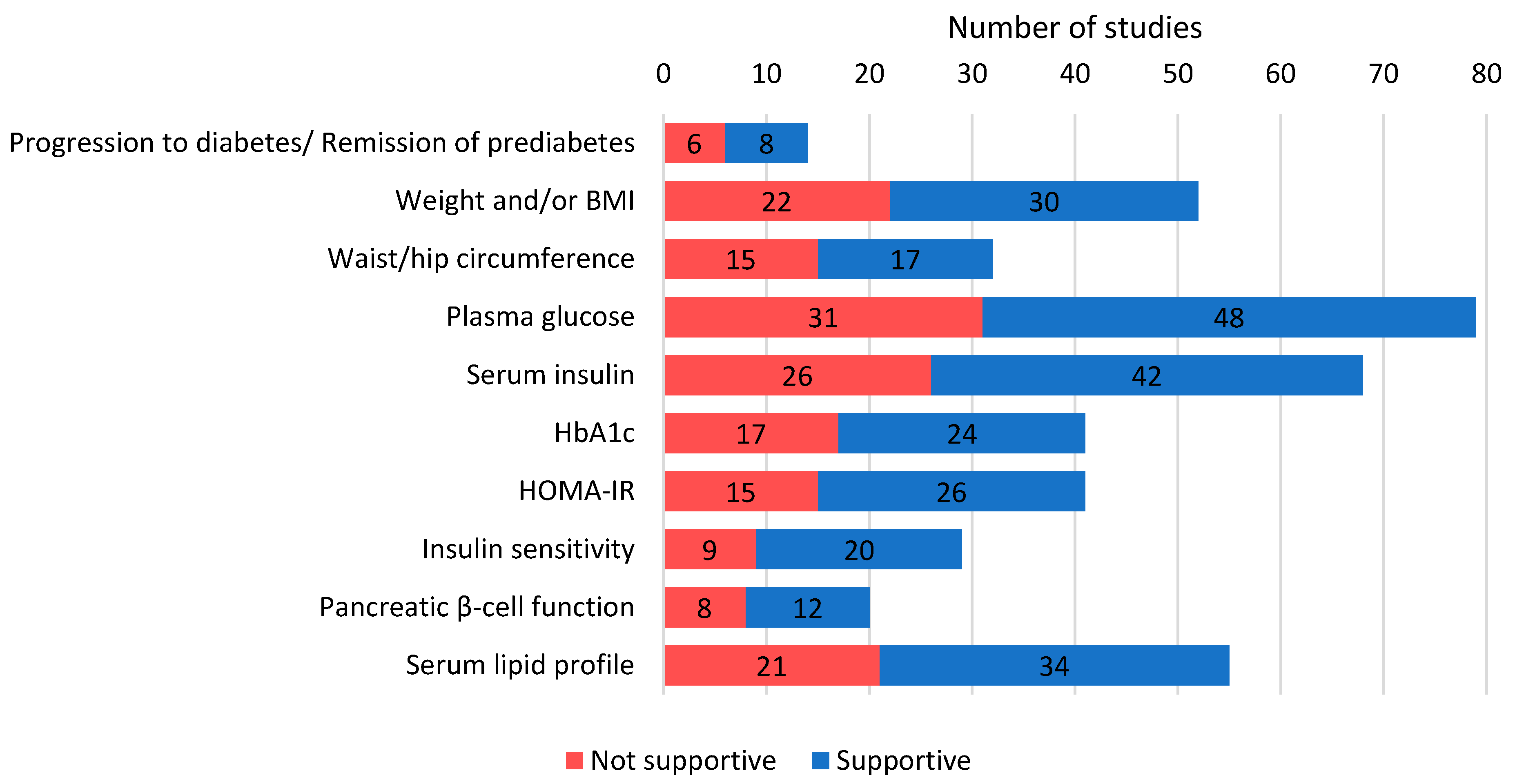
3.4. Outcomes
3.4.1. General Outcomes
3.4.2. Anthropometry
3.4.3. Glucose- and Insulin-Related Parameters
3.4.4. Additional Blood Parameters
3.4.5. Other Parameters
3.4.6. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Definition. Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus. Available online: https://apps.who.int/iris/handle/10665/66040 (accessed on 25 April 2020).
- American Diabetes Association. Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers. Clin. Diabetes 2019, 37, 11. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Lee, C.M.Y.; Colagiuri, S.; Woodward, M.; Gregg, E.W.; Adams, R.; Azizi, F.; Gabriel, R.; Gill, T.K.; Gonzalez, C.; Hodge, A.; et al. Comparing different definitions of prediabetes with subsequent risk of diabetes: An individual participant data meta-analysis involving 76 513 individuals and 8208 cases of incident diabetes. BMJ Open Diabetes Res. Care 2019, 7, e000794. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. Available online: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed on 25 April 2020).
- Hsueh, W.A.; Orloski, L.; Wyne, K. Prediabetes: The importance of early identification and intervention. Postgrad. Med. 2010, 122, 129–143. [Google Scholar] [CrossRef]
- Yudkin, J.S. “Prediabetes”: Are there problems with this label? Yes, the label creates further problems! Diabetes Care 2016, 39, 1468. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Guess, N.D. Dietary interventions for the prevention of type 2 diabetes in high-risk groups: Current state of evidence and future research needs. Nutrients 2018, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software. Melbourne, Australia. Available online: www.covidence.org (accessed on 27 April 2020).
- An, S.Y.; Lee, M.S.; Jeon, J.Y.; Ha, E.S.; Kim, T.H.; Yoon, J.Y.; Ok, C.O.; Lee, H.K.; Hwang, W.S.; Choe, S.J.; et al. Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Ann. Nutr. Metab. 2013, 63, 111–119. [Google Scholar] [CrossRef]
- Choi, M.S.; Ryu, R.; Seo, Y.R.; Jeong, T.S.; Shin, D.H.; Park, Y.B.; Kim, S.R.; Jung, U.J. The beneficial effect of soybean (Glycine max (L.) Merr.) leaf extracts in adults with prediabetes: A randomized placebo-controlled trial. Food Funct. 2014, 5, 1621–1630. [Google Scholar] [CrossRef]
- Kim, M.; Jeung, S.R.; Jeong, T.S.; Lee, S.H.; Lee, J.H. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J. Lipid Res. 2014, 55, 1762–1771. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, J.H.; Ahn, C.W.; Park, S.H.; Shim, S.T.; Song, Y.D.; Han, E.N.; Lee, K.H.; Chae, J.S. Black soy peptide supplementation improves glucose control in subjects with prediabetes and newly diagnosed type 2 diabetes mellitus. J. Med. Food 2010, 13, 1307–1312. [Google Scholar] [CrossRef]
- Kwak, J.H.; Paik, J.K.; Kim, H.I.; Kim, O.Y.; Shin, D.Y.; Kim, H.J.; Lee, J.H.; Lee, J.H. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis 2012, 224, 457–464. [Google Scholar] [CrossRef]
- Nah, E.H.; Chu, J.; Kim, S.; Cho, S.; Kwon, E. Efficacy of lifestyle interventions in the reversion to normoglycemia in Korean prediabetics: One-year results from a randomised controlled trial. Prim. Care Diabetes 2019, 13, 212–220. [Google Scholar] [CrossRef]
- Minjoo, K.; Gayoung, S.; Miso, K.; Hye Jin, Y.; Tae-Sook, J.; Sang-Hyun, L.; Jong Ho, L. Replacing carbohydrate with protein and fat in prediabetes or type-2 diabetes: Greater effect on metabolites in PBMC than plasma. Nutr. Metab. 2016, 13, 3. [Google Scholar] [CrossRef]
- Kobayakawa, A.; Suzuki, T.; Ikami, T.; Saito, M.; Yabe, D.; Seino, Y. Improvement of fasting plasma glucose level after ingesting moderate amount of dietary fiber in Japanese men with mild hyperglycemia and visceral fat obesity. J. Diet. Suppl. 2013, 10, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi-Fukatsu, A.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and viscous vegetables in a Japanese-style breakfast improved insulin sensitivity, lipid metabolism and oxidative stress in overweight subjects with impaired glucose tolerance. Br. J. Nutr. 2012, 107, 1184–1191. [Google Scholar] [CrossRef]
- Saito, T.; Watanabe, M.; Nishida, J.; Izumi, T.; Omura, M.; Takagi, T.; Fukunaga, R.; Bandai, Y.; Tajima, N.; Nakamura, Y.; et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: A randomized controlled trial. Arch. Int. Med. 2011, 171, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Sakane, N.; Sato, J.; Tsushita, K.; Tsujii, S.; Kotani, K.; Tsuzaki, K.; Tominaga, M.; Kawazu, S.; Sato, Y.; Usui, T.; et al. Prevention of type 2 diabetes in a primary healthcare setting: Three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health 2011, 11, 40. [Google Scholar] [CrossRef]
- Bi, M.; Niu, Y.; Li, X.; Li, Y.; Sun, C. Effects of barley flake on metabolism of glucose and lipids in the patients with impaired fasting glucose. J. Hyg. Res. 2013, 42, 719–723. [Google Scholar]
- Cheng, S.; Ge, J.; Zhao, C.; Le, S.; Yang, Y.; Ke, D.; Wu, N.; Tan, X.; Zhang, X.; Du, X.; et al. Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease: A randomized controlled trial. Sci. Rep. 2017, 7, 15952. [Google Scholar] [CrossRef]
- Gong, Q.; Gregg, E.W.; Wang, J.; An, Y.; Zhang, P.; Yang, W.; Li, H.; Li, H.; Jiang, Y.; Shuai, Y.; et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: The China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011, 54, 300–307. [Google Scholar] [CrossRef]
- Ren, X.; Yin, R.; Hou, D.; Xue, Y.; Zhang, M.; Diao, X.; Zhang, Y.; Wu, J.; Hu, J.; Hu, X.; et al. The glucose-lowering effect of foxtail millet in subjects with impaired glucose tolerance: A self-controlled clinical trial. Nutrients 2018, 10, 15. [Google Scholar] [CrossRef]
- Bui, T.N.; Le, T.H.; do Nguyen, H.; Tran, Q.B.; Nguyen, T.L.; Le, D.T.; do Nguyen, V.A.; Vu, A.L.; Aoto, H.; Okuhara, Y.; et al. Pre-germinated brown rice reduced both blood glucose concentration and body weight in Vietnamese women with impaired glucose tolerance. J. Nutr. Sci. Vitaminol. 2014, 60, 183–187. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Ford, C.N.; Weber, M.B.; Staimez, L.R.; Anjana, R.M.; Lakshmi, K.; Mohan, V.; Narayan, K.M.V.; Harish, R. Dietary changes in a diabetes prevention intervention among people with prediabetes: The Diabetes Community Lifestyle Improvement Program trial. Acta Diabetol. 2019, 56, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Chen, Y.M.; Ho, S.C.; Ho, Y.P.; Woo, J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: A 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am. J. Clin. Nutr. 2010, 91, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Niroomand, M.; Fotouhi, A.; Irannejad, N.; Hosseinpanah, F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes Res. Clin. Pract. 2019, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.A.; Aminorroaya, A.; Iraj, B.; Amini, M. The effects of oral vitamin D on insulin resistance in pre-diabetic patients. J. Res. Med. Sci. 2013, 18, 47–51. [Google Scholar]
- Alfawaz, H.A.; Wani, K.; Alnaami, A.M.; Al-Saleh, Y.; Aljohani, N.J.; Al-Attas, O.S.; Alokail, M.S.; Kumar, S.; Al-Daghri, N.M. Effects of different dietary and lifestyle modification therapies on metabolic syndrome in prediabetic arab patients: A 12-month longitudinal study. Nutrients 2018, 10, 20. [Google Scholar] [CrossRef]
- Canfora, E.E.; van der Beek, C.M.; Hermes, G.D.A.; Goossens, G.H.; Jocken, J.W.E.; Holst, J.J.; van Eijk, H.M.; Venema, K.; Smidt, H.; Zoetendal, E.G.; et al. Supplementation of diet with galacto-oligosaccharides increases bifidobacteria, but not insulin sensitivity, in obese prediabetic individuals. Gastroenterology 2017, 153, 87–97. [Google Scholar] [CrossRef]
- Den Boer, A.T.; Herraets, I.J.; Stegen, J.; Roumen, C.; Corpeleijn, E.; Schaper, N.C.; Feskens, E.; Blaak, E.E. Prevention of the metabolic syndrome in IGT subjects in a lifestyle intervention: Results from the SLIM study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1147–1153. [Google Scholar] [CrossRef]
- Van Can, J.G.; van Loon, L.J.; Brouns, F.; Blaak, E.E. Reduced glycaemic and insulinaemic responses following trehalose and isomaltulose ingestion: Implications for postprandial substrate use in impaired glucose-tolerant subjects. Br. J. Nutr. 2012, 108, 1210–1217. [Google Scholar] [CrossRef]
- Oosterwerff, M.M.; Eekhoff, E.M.; Van Schoor, N.M.; Boeke, A.J.; Nanayakkara, P.; Meijnen, R.; Knol, D.L.; Kramer, M.H.; Lips, P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 152–160. [Google Scholar] [CrossRef]
- Abellan Ruiz, M.S.; Barnuevo Espinosa, M.D.; Garcia Santamaria, C.; Contreras Fernandez, C.J.; Aldeguer Garcia, M.; Soto Mendez, F.; Guillen Guillen, I.; Luque Rubia, A.J.; Quinde Razuri, F.J.; Martinez Garrido, A.; et al. Effect of quinua (Chenopodium quinoa)consumption as a coadjuvant in nutritional intervention in prediabetic subjects. Nutr. Hosp. 2017, 34, 1163–1169. [Google Scholar] [CrossRef]
- Canudas, S.; Hernandez-Alonso, P.; Galie, S.; Muralidharan, J.; Morell-Azanza, L.; Zalba, G.; Garcia-Gavilan, J.; Marti, A.; Salas-Salvado, J.; Bullo, M. Pistachio consumption modulates DNA oxidation and genes related to telomere maintenance: A crossover randomized clinical trial. Am. J. Clin. Nutr. 2019, 109, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, P.; Salas-Salvado, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bullo, M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: A randomized clinical trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef]
- Hernandez-Alonso, P.; Salas-Salvado, J.; Baldrich-Mora, M.; Mallol, R.; Correig, X.; Bullo, M. Effect of pistachio consumption on plasma lipoprotein subclasses in pre-diabetic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, P.; Canueto, D.; Giardina, S.; Salas-Salvado, J.; Canellas, N.; Correig, X.; Bullo, M. Effect of pistachio consumption on the modulation of urinary gut microbiota-related metabolites in prediabetic subjects. J. Nutr. Biochem. 2017, 45, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, P.; Giardina, S.; Salas-Salvado, J.; Arcelin, P.; Bullo, M. Chronic pistachio intake modulates circulating microRNAs related to glucose metabolism and insulin resistance in prediabetic subjects. Eur. J. Nutr. 2017, 56, 2181–2191. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Alcala-Diaz, J.F.; Rangel-Zuniga, O.A.; Gomez-Delgado, F.; Jimenez-Lucena, R.; Garcia-Rios, A.; Vals-Delgado, C.; Romero-Baldonado, C.; Luque, R.M.; Ordovas, J.M.; et al. Prediabetes diagnosis criteria, type 2 diabetes risk and dietary modulation: The CORDIOPREV study. Clin. Nutr. 2020, 39, 492–500. [Google Scholar] [CrossRef]
- Carnevale, R.; Loffredo, L.; Del Ben, M.; Angelico, F.; Nocella, C.; Petruccioli, A.; Bartimoccia, S.; Monticolo, R.; Cava, E.; Violi, F. Extra virgin olive oil improves post-prandial glycemic and lipid profile in patients with impaired fasting glucose. Clin. Nutr. 2017, 36, 782–787. [Google Scholar] [CrossRef]
- Di Pino, A.; Currenti, W.; Urbano, F.; Mantegna, C.; Purrazzo, G.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Low advanced glycation end product diet improves the lipid and inflammatory profiles of prediabetic subjects. J. Clin. Lipidol. 2016, 10, 1098–1108. [Google Scholar] [CrossRef]
- Monti, L.D.; Casiraghi, M.C.; Setola, E.; Galluccio, E.; Pagani, M.A.; Quaglia, L.; Bosi, E.; Piatti, P. L-arginine enriched biscuits improve endothelial function and glucose metabolism: A pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism 2013, 62, 255–264. [Google Scholar] [CrossRef]
- Monti, L.D.; Galluccio, E.; Villa, V.; Fontana, B.; Spadoni, S.; Piatti, P.M. Decreased diabetes risk over 9 year after 18-month oral L-arginine treatment in middle-aged subjects with impaired glucose tolerance and metabolic syndrome (extension evaluation of L-arginine study). Eur. J. Nutr. 2018, 57, 2805–2817. [Google Scholar] [CrossRef]
- Erkkila, A.T.; Lee, J.C.; Lankinen, M.; Manninen, S.; Leung, H.H.; Oger, C.; de Mello, V.D.; Schwab, U.S. Camelina sativa oil, fatty fish, and lean fish do not markedly affect urinary prostanoids in subjects with impaired glucose metabolism. Lipids 2019, 54, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.S.; Lankinen, M.A.; de Mello, V.D.; Manninen, S.M.; Kurl, S.; Pulkki, K.J.; Laaksonen, D.E.; Erkkilä, A.T. Camelina sativa oil, but not fatty fish or lean fish, improves serum lipid profile in subjects with impaired glucose metabolism-a randomized controlled trial. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- De Mello, V.D.; Schwab, U.; Kolehmainen, M.; Koenig, W.; Siloaho, M.; Poutanen, K.; Mykkänen, H.; Uusitupa, M. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: The Sysdimet study. Diabetologia 2011, 54, 2755–2767. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Schwab, U.; Kolehmainen, M.; Paananen, J.; Poutanen, K.; Mykkänen, H.; Seppänen-Laakso, T.; Gylling, H.; Uusitupa, M.; Orešič, M. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: The Sysdimet study. PLoS ONE 2011, 6, e22646. [Google Scholar] [CrossRef] [PubMed]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef]
- Konig, D.; Kookhan, S.; Schaffner, D.; Deibert, P.; Berg, A. A meal replacement regimen improves blood glucose levels in prediabetic healthy individuals with impaired fasting glucose. Nutrition 2014, 30, 1306–1309. [Google Scholar] [CrossRef]
- Sartorius, T.; Weidner, A.; Dharsono, T.; Boulier, A.; Wilhelm, M.; Schon, C. Postprandial effects of a proprietary milk protein hydrolysate containing bioactive peptides in prediabetic subjects. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef]
- Dodevska, M.S.; Sobajic, S.S.; Djordjevic, P.B.; Dimitrijevic-Sreckovic, V.S.; Spasojevic-Kalimanovska, V.V.; Djordjevic, B.I. Effects of total fibre or resistant starch-rich diets within lifestyle intervention in obese prediabetic adults. Eur. J. Nutr. 2016, 55, 127–137. [Google Scholar] [CrossRef]
- Polovina, S.; Micić, D. The influence of diet with reduction in calorie intake on metabolic syndrome parameters in obese subjects with impaired glucose tolerance. Med. Pregl. 2010, 63, 465–469. [Google Scholar] [CrossRef][Green Version]
- Mitrou, P.; Petsiou, E.; Papakonstantinou, E.; Maratou, E.; Lambadiari, V.; Dimitriadis, P.; Spanoudi, F.; Raptis, S.A.; Dimitriadis, G. The role of acetic acid on glucose uptake and blood flow rates in the skeletal muscle in humans with impaired glucose tolerance. Eur. J. Clin. Nutr. 2015, 69, 734–739. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Kontogianni, M.D.; Mitrou, P.; Magriplis, E.; Vassiliadi, D.; Nomikos, T.; Lambadiari, V.; Georgousopoulou, E.; Dimitriadis, G. Effects of 6 vs 3 eucaloric meal patterns on glycaemic control and satiety in people with impaired glucose tolerance or overt type 2 diabetes: A randomized trial. Diabetes Metab. 2018, 44, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, M.D.; Liatis, S.; Grammatikou, S.; Perrea, D.; Katsilambros, N.; Makrilakis, K. Changes in dietary habits and their association with metabolic markers after a non-intensive, community-based lifestyle intervention to prevent type 2 diabetes, in Greece. The DEPLAN study. Diabetes Res. Clin. Pract. 2012, 95, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Pietraszek, A.; Gregersen, S.; Hermansen, K. Acute effects of dietary fat on inflammatory markers and gene expression in first-degree relatives of type 2 diabetes patients. Rev. Diabet. Stud. 2011, 8, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Bliddal, H.; Riecke, B.F.; Leeds, A.R.; Astrup, A.; Christensen, R. Comparison of a low-energy diet and a very low-energy diet in sedentary obese individuals: A pragmatic randomized controlled trial. Clin. Obes. 2011, 1, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sollid, S.T.; Hutchinson, M.Y.S.; Fuskevag, O.M.; Figenschau, Y.; Joakimsen, R.M.; Schirmer, H.; Njølstad, I.; Svartberg, J.; Kamycheva, E.; Jorde, R.; et al. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care 2014, 37, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Alvarsson, M.; Mannheimer, B.; Degerblad, M.; Ostenson, C.G. No effect of high-dose vitamin d treatment on beta-cell function, insulin sensitivity, or glucose homeostasis in subjects with abnormal glucose tolerance: A randomized clinical trial. Diabetes Care 2016, 39, 345–352. [Google Scholar] [CrossRef]
- Baugh, M.E.; Steele, C.N.; Angiletta, C.J.; Mitchell, C.M.; Neilson, A.P.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Inulin supplementation does not reduce plasma trimethylamine n-oxide concentrations in individuals at risk for type 2 diabetes. Nutrients 2018, 10, 793. [Google Scholar] [CrossRef]
- Dube, J.J.; Amati, F.; Toledo, F.G.; Stefanovic-Racic, M.; Rossi, A.; Coen, P.; Goodpaster, B.H. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 2011, 54, 1147–1156. [Google Scholar] [CrossRef]
- Errazuriz, I.; Dube, S.; Slama, M.; Visentin, R.; Nayar, S.; O’Connor, H.; Cobelli, C.; Das, S.K.; Basu, A.; Kremers, W.K.; et al. Randomized controlled trial of a MUFA or fiber-rich diet on hepatic fat in prediabetes. J. Clin. Endocrinol. Metab. 2017, 102, 1765–1774. [Google Scholar] [CrossRef]
- Grizales, A.M.; Patti, M.E.; Lin, A.P.; Beckman, J.A.; Sahni, V.A.; Cloutier, E.; Fowler, K.M.; Dreyfuss, J.M.; Pan, H.; Kozuka, C.; et al. Metabolic effects of betaine: A randomized clinical trial of betaine supplementation in prediabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3038–3049. [Google Scholar] [CrossRef]
- Hari, A.; Fealy, C.; Solomon, T.P.J.; Haus, J.M.; Kelly, K.R.; Barkoukis, H.; Kirwan, J.P. Exercise-induced improvements in glucose effectiveness are blunted by a high glycemic diet in adults with prediabetes. Acta Diabetol. 2019, 56, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Haus, J.M.; Solomon, T.P.; Marchetti, C.M.; Edmison, J.M.; Gonzalez, F.; Kirwan, J.P. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J. Clin. Endocrinol. Metab. 2010, 95, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; Brown, B.D.; Cunnane, S.C.; Domitrovich, S.G.; Adams, E.R.; Bobowiec, C.E. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: A randomized study. Nutr. Res. 2013, 33, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Kullman, E.L.; Scelsi, A.R.; Godin, J.P.; Ross, A.B.; Kirwan, J.P. A whole-grain diet increases glucose-stimulated insulin secretion independent of gut hormones in adults at risk for type 2 diabetes. Mol. Nutr. Food Res. 2019, 63, e1800967. [Google Scholar] [CrossRef]
- McDonald, J.D.; Chitchumroonchokchai, C.; Li, J.; Mah, E.; Labyk, A.N.; Reverri, E.J.; Ballard, K.D.; Volek, J.S.; Bruno, R.S. Replacing carbohydrate during a glucose challenge with the egg white portion or whole eggs protects against postprandial impairments in vascular endothelial function in prediabetic men by limiting increases in glycaemia and lipid peroxidation. Br. J. Nutr. 2018, 119, 259–270. [Google Scholar] [CrossRef]
- McDonald, J.D.; Mah, E.; Chitchumroonchokchai, C.; Dey, P.; Labyk, A.N.; Villamena, F.A.; Volek, J.S.; Bruno, R.S. Dairy milk proteins attenuate hyperglycemia-induced impairments in vascular endothelial function in adults with prediabetes by limiting increases in glycemia and oxidative stress that reduce nitric oxide bioavailability. J. Nutr. Biochem. 2019, 63, 165–176. [Google Scholar] [CrossRef]
- McDonald, J.D.; Mah, E.; Chitchumroonchokchai, C.; Reverri, E.J.; Li, J.; Volek, J.S.; Villamena, F.A.; Bruno, R.S. Co-ingestion of whole eggs or egg whites with glucose protects against postprandial hyperglycaemia-induced oxidative stress and dysregulated arginine metabolism in association with improved vascular endothelial function in prediabetic men. Br. J. Nutr. 2018, 120, 901–913. [Google Scholar] [CrossRef]
- McDonald, J.D.; Mah, E.; Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Villamena, F.A.; Bruno, R.S. Dairy milk, regardless of fat content, protects against postprandial hyperglycemia-mediated impairments in vascular endothelial function in adults with prediabetes by limiting oxidative stress responses that reduce nitric oxide bioavailability. J. Nutr. Biochem. 2019, 63, 129–139. [Google Scholar] [CrossRef]
- Mehrotra, A.; Calvo, M.S.; Beelman, R.B.; Levy, E.; Siuty, J.; Kalaras, M.D.; Uribarri, J. Bioavailability of vitamin D2 from enriched mushrooms in prediabetic adults: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 1154–1160. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Pittas, A.G.; Pratley, R.E.; Seyhan, A.A. Circulating levels of miR-7, miR-152 and miR-192 respond to vitamin D supplementation in adults with prediabetes and correlate with improvements in glycemic control. J. Nutr. Biochem. 2017, 49, 117–122. [Google Scholar] [CrossRef]
- Parker, A.R.; Byham-Gray, L.; Denmark, R.; Winkle, P.J. The effect of medical nutrition therapy by a registered dietitian nutritionist in patients with prediabetes participating in a randomized controlled clinical research trial. J. Acad. Nutr. Diet. 2014, 114, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Brunt, A. Flaxseed supplementation improved insulin resistance in obese glucose intolerant people: A randomized crossover design. Nutr. J. 2011, 10, 44. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef]
- Wang, B.; Medapalli, R.; Xu, J.; Cai, W.; Chen, X.; He, J.C.; Uribarri, J. Effects of a whole rice diet on metabolic parameters and inflammatory markers in prediabetes. e-SPEN J. 2013, 8, e15–e20. [Google Scholar] [CrossRef]
- Weinhold, K.R.; Miller, C.K.; Marrero, D.G.; Nagaraja, H.N.; Focht, B.C.; Gascon, G.M. A randomized controlled trial translating the diabetes prevention program to a university worksite, Ohio, 2012–2014. Prev. Chronic Dis. 2015, 12, E210. [Google Scholar] [CrossRef]
- Wien, M.; Bleich, D.; Raghuwanshi, M.; Gould-Forgerite, S.; Gomes, J.; Monahan-Couch, L.; Oda, K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J. Am. Coll. Nutr. 2010, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Tippens, K.M.; Erlandsen, A.; Hanes, D.A.; Graybill, R.; Jackson, C.; Briley, J.; Zwickey, H. Impact of a short-term naturopathic whole-foods-based nutrition education intervention on dietary behavior and diabetes risk markers: A pilot study. J. Altern. Complement. Med. 2019, 25, 234–240. [Google Scholar] [CrossRef]
- Davidson, M.B.; Duran, P.; Lee, M.L.; Friedman, T.C. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 2013, 36, 260–266. [Google Scholar] [CrossRef]
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 486–494. [Google Scholar] [CrossRef]
- Stentz, F.B.; Brewer, A.; Wan, J.; Garber, C.; Daniels, B.; Sands, C.; Kitabchi, A.E. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: Randomized control trial. BMJ Open Diabetes Res. Care 2016, 4, e000258. [Google Scholar] [CrossRef]
- Harris, S.S.; Pittas, A.G.; Palermo, N.J. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes. Metab. 2012, 14, 789–794. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ma, Y.; Reynolds, J.; Wise, J.P., Sr.; Inzucchi, S.E.; Katz, D.L. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocr. Pract. 2011, 17, 16–25. [Google Scholar] [CrossRef]
- Solomon, T.P.; Haus, J.M.; Kelly, K.R.; Cook, M.D.; Filion, J.; Rocco, M.; Kashyap, S.R.; Watanabe, R.M.; Barkoukis, H.; Kirwan, J.P. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am. J. Clin. Nutr. 2010, 92, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Noll, C.; Kunach, M.; Frisch, F.; Bouffard, L.; Dubreuil, S.; Jean-Denis, F.; Phoenix, S.; Cunnane, S.C.; Guerin, B.; Turcotte, E.E.; et al. Seven-day caloric and saturated fat restriction increases myocardial dietary fatty acid partitioning in impaired glucose-tolerant subjects. Diabetes 2015, 64, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Medina Larque, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonne, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, T.H.M.; Da Silva, F.V.P.; Reis, C.E.G.; Casulari, L.A. Improved metabolic response after 16 weeks of calorie-restricted low-glycaemic index diet and metformin in impaired glucose tolerance subjects. Nutr. Hosp. 2014, 29, 1081–1087. [Google Scholar] [CrossRef]
- Velázquez-López, L.; González-Figueroa, E.; Medina-Bravo, P.; Pineda-Del Aguila, I.; Ávila-Jiménez, L.; Ramos-Hernández, R.; Klunder-Klunder, M.; Escobedo-De La Peña, J. Low calorie and carbohydrate diet: To improve the cardiovascular risk indicators in overweight or obese adults with prediabetes. Endocrine 2013, 43, 593–602. [Google Scholar] [CrossRef]
- Alaei Shahmiri, F.; Soares, M.J.; Zhao, Y.; Sherriff, J. High-dose thiamine supplementation improves glucose tolerance in hyperglycemic individuals: A randomized, double-blind cross-over trial. Eur. J. Nutr. 2013, 52, 1821–1824. [Google Scholar] [CrossRef]
- Alaei-Shahmiri, F.; Soares, M.J.; Zhao, Y.; Sherriff, J. The impact of thiamine supplementation on blood pressure, serum lipids and C-reactive protein in individuals with hyperglycemia: A randomised, double-blind cross-over trial. Diabetes Metab. Syndr. 2015, 9, 213–217. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Differential effects of red meat/refined grain diet and dairy/chicken/nuts/whole grain diet on glucose, insulin and triglyceride in a randomized crossover study. Nutrients 2016, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Orazio, L.K.; Isbel, N.M.; Armstrong, K.A.; Tarnarskyj, J.; Johnson, D.W.; Hale, R.E.; Kaisar, M.; Banks, M.D.; Hickman, I.J. Evaluation of dietetic advice for modification of cardiovascular disease risk factors in renal transplant recipients. J. Ren. Nutr. 2011, 21, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Pinto, S.K.; Dunstan, D.W.; Hawley, J.A. Impact of first meal size during prolonged sitting on postprandial glycaemia in individuals with prediabetes: A randomised, crossover study. Nutrients 2018, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Willis, J.; Gearry, R.B.; Hughes, A.; Lawley, B.; Skidmore, P.; Frampton, C.; Fleming, E.; Anderson, A.; Jones, L.; et al. SunGold kiwifruit supplementation of individuals with prediabetes alters gut microbiota and improves vitamin c status, anthropometric and clinical markers. Nutrients 2018, 10, 895. [Google Scholar] [CrossRef]
- Christensen, P.; Meinert Larsen, T.; Westerterp-Plantenga, M.; Macdonald, I.; Martinez, J.A.; Handjiev, S.; Poppitt, S.; Hansen, S.; Ritz, C.; Astrup, A.; et al. Men and women respond differently to rapid weight loss: Metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW). Diabetes Obes. Metab. 2018, 20, 2840–2851. [Google Scholar] [CrossRef]
- Drummen, M.; Dorenbos, E.; Vreugdenhil, A.C.E.; Stratton, G.; Raben, A.; Westerterp-Plantenga, M.S.; Adam, T.C. Associations of brain reactivity to food cues with weight loss, protein intake and dietary restraint during the PREVIEW Intervention. Nutrients 2018, 10, 1771. [Google Scholar] [CrossRef]
- Moller, G.; Rikardt Andersen, J.; Ritz, C.; Silvestre, P.M.; Navas-Carretero, S.; Jalo, E.; Christensen, P.; Simpson, E.; Taylor, M.; Martinez, J.A.; et al. Higher protein intake is not associated with decreased kidney function in pre-diabetic older adults following a one-year intervention-a preview sub-study. Nutrients 2018, 10, 54. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef]
- Kaiser, A.B.; Zhang, N.; Der Pluijm, W.V. Global prevalence of type 2 diabetes over the next ten years (2018–2028). Diabetes 2018, 67 (Suppl. 1), 202-LB. [Google Scholar] [CrossRef]
- Hostalek, U. Global epidemiology of prediabetes—Present and future perspectives. Clin. Diabetes Endocrinol. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.P.; Pathak, S. Applied research in low-income countries: Why and how? Front. Res. Metr. Anal. 2019, 4, 3. [Google Scholar] [CrossRef]
- Sun, Y.; You, W.; Almeida, F.; Estabrooks, P.; Davy, B. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: A systematic review and meta-analysis. J. Acad. Nutr. Diet. 2017, 117, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Karakelides, H.; Irving, B.A.; Short, K.R.; O’Brien, P.; Nair, K.S. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 2010, 59, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, D.H.; Lee, E.K.; Kim, N.D.; Im, D.S.; Lee, J.; Yu, B.P.; Chung, H.Y. Age-related inflammation and insulin resistance: A review of their intricate interdependency. Arch. Pharm. Res. 2014, 37, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef]
- Hu, F.B.; Meigs, J.B.; Li, T.Y.; Rifai, N.; Manson, J.E. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004, 53, 693. [Google Scholar] [CrossRef]
- Wang, X.; Bao, W.; Liu, J.; OuYang, Y.-Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.-L.; Zhang, Y.; Yao, P.; et al. Inflammatory markers and risk of type 2 diabetes. Diabetes Care 2013, 36, 166. [Google Scholar] [CrossRef]
- Healey, G.R.; Murphy, R.; Brough, L.; Butts, C.A.; Coad, J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 2017, 75, 1059–1080. [Google Scholar] [CrossRef]
- Milenkovic, D.; Morand, C.; Cassidy, A.; Konic-Ristic, A.; Tomás-Barberán, F.; Ordovas, J.M.; Kroon, P.; De Caterina, R.; Rodriguez-Mateos, A. Interindividual variability in biomarkers of cardiometabolic health after consumption of major plant-food bioactive compounds and the determinants involved. Adv. Nutr. 2017, 8, 558–570. [Google Scholar] [CrossRef]
- Walther, B.; Lett, A.M.; Bordoni, A.; Tomás-Cobos, L.; Nieto, J.A.; Dupont, D.; Danesi, F.; Shahar, D.R.; Echaniz, A.; Re, R.; et al. GutSelf: Interindividual variability in the processing of dietary compounds by the human gastrointestinal tract. Mol. Nutr. Food Res. 2019, 63, 1900677. [Google Scholar] [CrossRef] [PubMed]
- Pem, D.; Jeewon, R. Fruit and vegetable intake: Benefits and progress of nutrition education interventions- narrative review article. Iran. J. Public Health 2015, 44, 1309–1321. [Google Scholar] [PubMed]
- Henríquez, S.; Jara, N.; Bunout, D.; Hirsch, S.; de la Maza, M.P.; Leiva, L.; Barrera, G. Variability of formulas to assess insulin sensitivity and their association with the Matsuda index. Nutr. Hosp. 2013, 28, 1594–1598. [Google Scholar] [CrossRef]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Fowler, S.E.; Hamman, R.F.; Christophi, C.A.; Hoffman, H.J.; Brenneman, A.T.; Brown-Friday, J.O.; Goldberg, R.; Venditti, E.; Nathan, D.M. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q.; Li, H.; Li, H.; Jiang, Y.; An, Y.; et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Kosaka, K.; Noda, M.; Kuzuya, T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005, 67, 152–162. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [PubMed]

| Country | Studies | Participants(N) | RCTs | Randomized, Nonplacebo-Controlled Trials | Non-RCTs | Pre–Post Trials | References |
|---|---|---|---|---|---|---|---|
| Asia | 22 | 4373 | 19 | 1 | 1 | 1 | [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] |
| Korea | 7 | 1171 | 6 | 1 | [17,18,19,20,21,22,23] | ||
| Japan | 4 | 986 | 4 | [24,25,26,27] | |||
| China | 4 | 827 | 3 | 1 | [28,29,30,31] | ||
| Vietnam | 1 | 60 | 1 | [32] | |||
| Thailand | 1 | 240 | 1 | [33] | |||
| India | 1 | 485 | 1 | [34] | |||
| Hong Kong | 1 | 180 | 1 | [35] | |||
| Iran | 2 | 207 | 2 | [36,37] | |||
| Saudi Arabia | 1 | 217 | 1 | [38] | |||
| Europe | 31 | 2951 | 25 | 5 | 1 | [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] | |
| The Netherlands | 4 | 330 | 3 | 1 | [39,40,41,42] | ||
| Spain | 7 | 731 | 6 | 1 | [43,44,45,46,47,48,49] | ||
| Italy | 4 | 258 | 4 | [50,51,52,53] | |||
| Finland | 4 | 388 | 4 | [54,55,56,57] | |||
| Germany | 3 | 180 | 3 | [58,59,60] | |||
| Serbia | 2 | 102 | 2 | 1 | [61,62] | ||
| Greece | 3 | 181 | 2 | 1 | 1 | [63,64,65] | |
| Denmark | 2 | 226 | 2 | 1 | [66,67] | ||
| Norway | 1 | 511 | 1 | [68] | |||
| Sweden | 1 | 44 | 1 | [69] | |||
| Americas (North and South) | 33 | 1413 | 25 | 5 | 3 | [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102] | |
| United States | 29 | 1255 | 23 | 5 | 1 | [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] | |
| Canada | 2 | 56 | 2 | [99,100] | |||
| Brazil | 1 | 16 | 1 | [101] | |||
| Mexico | 1 | 86 | 1 | [102] | |||
| Australasia | 7 | 216 | 4 | 2 | 1 | [103,104,105,106,107,108] | |
| Australia | 5 | 190 | 3 | 2 | [103,104,105,106,107] | ||
| New Zealand | 1 | 26 | 1 | 1 | [108] | ||
| Multinational | 3 | 2560 | 3 | [109,110,111] |
| Characteristics | Number of Studies | Percentage of Included Studies (%) |
|---|---|---|
| Intervention providers | ||
| Physician | 11 | 11.6 |
| Nurse | 9 | 9.5 |
| Dietitian/nutritionist | 30 | 31.6 |
| Pharmacist | 5 | 5.3 |
| Unknown | 49 | 51.6 |
| Study recruitment location | ||
| Primary care clinic | 15 | 15.8 |
| District/tertiary hospital | 14 | 14.7 |
| Community | 47 | 49.5 |
| Unknown | 24 | 25.3 |
| Prediabetes diagnosis criteria | ||
| IFG + IGT + HbA1c | 10 | 10.5 |
| IFG + IGT | 30 | 31.6 |
| IFG + HbA1c | 8 | 8.4 |
| IFG – ADA (FPG 5.6–6.9mmol/L) | 26 | 27.4 |
| IFG – WHO (FPG ≥ 6.1 but <7.0 mmol/L) | 1 | 1.1 |
| IGT (2hPG ≥ 7.8 but <11.1 mmol/L) | 15 | 15.8 |
| HbA1c (5.7–6.4%) | 2 | 2.1 |
| Others (not defined, or did not follow ADA nor WHO definitions) | 3 | 3.2 |
| Study/Year | Intervention | Main Findings |
|---|---|---|
| The Finnish Diabetes Prevention Study (FDPS) [130] 1993–2002 | Aim: 5% weight loss
| Body weight and diabetes risk were significantly reduced by lifestyle changes in overweight participants with IGT. |
| U.S. Diabetes Prevention Program (DPP) [9] 1996–1999 | Aim: 7% weight loss
| Lifestyle intervention and metformin significantly decreased the incidence of T2DM in prediabetic participants, with more notable reductions in the former. |
| The Da-Qing Impaired Glucose Tolerance (IGT) and Diabetes Study [131,132] 1986–1992 | Aim: achieve BMI of 23 kg/m2 (if ≥25 kg/m2)
| Diet and/or exercise interventions reduced the incidence of T2DM in Chinese participants with IGT. |
| Japanese Diabetes Prevention Trial [133] 1993–1996 | Aim: maintain BMI < 22.0 kg/m2
| Lifestyle intervention successfully reduced body weight and the 4-year cumulative incidence of T2DM in Japanese males with IGT. |
| Indian Diabetes Prevention Study [134] 2002–2005 |
| Incidence of T2DM in Asian Indians was significantly reduced in the lifestyle modification and metformin groups, with no additional benefits in the combined group. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yau, J.W.; Thor, S.M.; Ramadas, A. Nutritional Strategies in Prediabetes: A Scoping Review of Recent Evidence. Nutrients 2020, 12, 2990. https://doi.org/10.3390/nu12102990
Yau JW, Thor SM, Ramadas A. Nutritional Strategies in Prediabetes: A Scoping Review of Recent Evidence. Nutrients. 2020; 12(10):2990. https://doi.org/10.3390/nu12102990
Chicago/Turabian StyleYau, Jun Wern, Sze Mun Thor, and Amutha Ramadas. 2020. "Nutritional Strategies in Prediabetes: A Scoping Review of Recent Evidence" Nutrients 12, no. 10: 2990. https://doi.org/10.3390/nu12102990
APA StyleYau, J. W., Thor, S. M., & Ramadas, A. (2020). Nutritional Strategies in Prediabetes: A Scoping Review of Recent Evidence. Nutrients, 12(10), 2990. https://doi.org/10.3390/nu12102990







