Gastrointestinal Tolerance of Low, Medium and High Dose Acute Oral l-Glutamine Supplementation in Healthy Adults: A Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Participants and Ethical Approval
2.2. Experimental Overview
2.3. Dietary and Lifestyle Controls
2.4. Anthropometric Measurements
2.5. l-Glutamine Supplementation
2.6. Gastrointestinal Tolerance Questionnaire
2.7. Characterisation of GLN Beverages
3. Statistics
Power Analysis
4. Results
4.1. GLN Supplementation
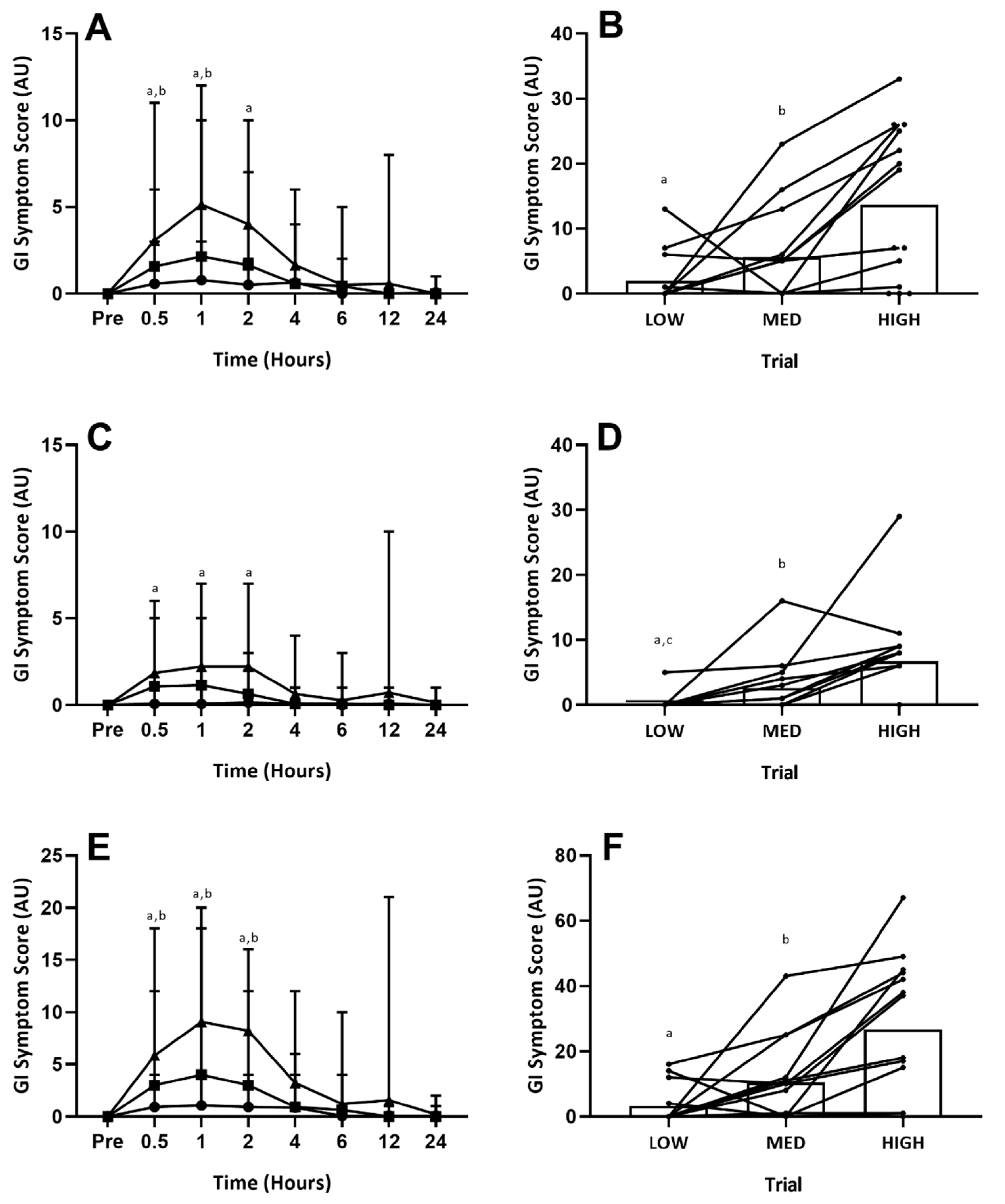
4.2. Accumulated Gastrointestinal Symptoms (24-h Total)
4.3. Characterisation of GLN Beverages
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BI | Blinding Index |
| CV | Co-efficient of variation |
| ESPEN | European Society for Parenteral and Enteral Nutrition |
| GI | Gastrointestinal |
| GLN | l-Glutamine |
| HIGH | High Glutamine Trial |
| LOW | Low Glutamine Trial |
| MED | Medium Glutamine Trial |
| mVAS | Modified Visual Analogue Scale |
References
- Smith, R.J.; Wilmore, D.W. Glutamine nutrition and requirements. J. Parenter. Enter. Nutr. 1990, 14, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.M.; Wilmore, D.W. Is glutamine a conditionally essential amino acid? Nutr. Rev. 1990, 48, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E. Glutamine: Role in gut protection in critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Rodas, P.C.; Rooyackers, O.; Hebert, C.; Norberg, Å.; Wernerman, J. Glutamine and glutathione at ICU admission in relation to outcome. Clin. Sci. 2012, 122, 591–597. [Google Scholar] [CrossRef]
- McRae, M.P. Therapeutic benefits of glutamine: An umbrella review of meta-analyses. Biomed. Rep. 2017, 6, 576–584. [Google Scholar] [CrossRef]
- Wischmeyer, P.E. The glutamine debate in surgery and critical care. Curr. Opin. Crit. Care 2019, 25, 322–328. [Google Scholar] [CrossRef]
- Holecek, M. Side effects of long-term glutamine supplementation. J. Parenter. Enter. Nutr. 2013, 37, 607–616. [Google Scholar] [CrossRef]
- Gleeson, M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J. Nutr. 2008, 138, 2045–2049. [Google Scholar] [CrossRef]
- Gillis, C.; Wischmeyer, P.E. Pre-operative nutrition and the elective surgical patient: Why, how and what? Anaesthesia 2019, 74, 27–35. [Google Scholar] [CrossRef]
- Zuhl, M.; Dokladny, K.; Mermier, C.; Schneider, S.; Salgado, R.; Moseley, P. The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell Stress Chaperones 2015, 20, 85–93. [Google Scholar] [CrossRef]
- Pugh, J.N.; Sage, S.; Hutson, M.; Doran, D.A.; Fleming, S.C.; Highton, J.; Morton, J.P.; Close, G.L. Glutamine supplementation reduces markers of intestinal permeability during running in the heat in a dose-dependent manner. Eur. J. Appl. Physiol. 2017, 117, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.O.; Stewart, I.B.; Beagley, K.W.; Borg, D.N.; Minett, G.M. Acute glutamine supplementation does not improve 20-km self-paced cycling performance in the heat. Eur. J. Appl. Physiol. 2019, 119, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L.; Bosco-Garate, I.; Souza-Gallardo, L.M.; Méndez, J.D.; Juárez-Oropeza, M.A.; Román-Ramos, R.; Ferat-Osorio, E. Effect of Preoperative Administration of Oral Arginine and Glutamine in Patients with Enterocutaneous Fistula Submitted to Definitive Surgery: A Prospective Randomized Trial. J. Gastrointest. Surg. 2019, 26, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Hathcock, J.N. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul. Toxicol. Pharmacol. 2008, 50, 376–399. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.R.; Benfell, K.; Smith, R.J.; Young, L.S.; Brown, E.; Ferrari-Baliviera, E.; Lowe, D.K.; Wilmore, D.W. Safety and metabolic effects of L-glutamine administration in humans. J. Parenter. Enter. Nutr. 1990, 14, 137–146. [Google Scholar] [CrossRef]
- Ward, E.; Picton, S.; Reid, U.; Thomas, D.; Gardener, C.; Smith, M.; Henderson, M.; Holden, V.; Kinsey, S.; Lewis, I.; et al. Oral glutamine in paediatric oncology patients: A dose finding study. Eur. J. Clin. Nutr. 2003, 57, 31–36. [Google Scholar] [CrossRef]
- Galera, S.C.; Fechine, F.; Teixeira, M.J.; Coelho, Z.C.B.; de Vasconcelos, R.C.; de Vasconcelos, P.R.L. The safety of oral use of L-glutamine in middle-aged and elderly individuals. Nutrition 2010, 26, 375–381. [Google Scholar] [CrossRef]
- Costa, R.J.; Gaskell, S.K.; McCubbin, A.J.; Snipe, R.M. Exertional-heat stress-associated gastrointestinal perturbations during Olympic sports: Management strategies for athletes preparing and competing in the 2020 Tokyo Olympic Games. Temperature 2020, 7, 58–88. [Google Scholar] [CrossRef]
- Marfell-Jones, M.; Olds, T.; Stewart, A.; Carter, L. ISAK Manua, International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Potchefstroom, South Africa, 2006. [Google Scholar]
- Durnin, J.V.; Womersley, J.V. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef]
- Bang, H.; Flaherty, S.P.; Kolahi, J.; Park, J. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin. Res. Regul. Aff. 2010, 27, 42–51. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Snipe, R.M.; Costa, R.J. Test–Retest Reliability of a Modified Visual Analog Scale Assessment Tool for Determining Incidence and Severity of Gastrointestinal Symptoms in Response to Exercise Stress. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Ohlsson, B. The brief Visual Analogue Scale for Irritable Bowel Syndrome questionnaire can be used to evaluate psychological well-being in patients with irritable bowel syndrome. Eur. J. Intern. Med. 2013, 24, 82–83. [Google Scholar]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar]
- Déchelotte, P.; Darmaun, D.; Rongier, M.; Hecketsweiler, B.; Rigal, O.; Desjeux, J.F. Absorption and metabolic effects of enterally administered glutamine in humans. Am. J. Physiol. 1991, 260, G677–G682. [Google Scholar] [CrossRef]
- Valencia, E.; Marin, A.; Hardy, G. Impact of oral L-glutamine on glutathione, glutamine, and glutamate blood levels in volunteers. Nutrition 2002, 18, 367–370. [Google Scholar]
- Harris, R.C.; Hoffman, J.R.; Allsopp, A.; Routledge, N.B. L-glutamine absorption is enhanced after ingestion of L-alanyl-glutamine compared with the free amino acid or wheat protein. Nutr. Res. 2012, 32, 272–277. [Google Scholar]
- Irimia, R.; Stanciu, C.; Cojocariu, C.; Sfarti, C.; Trifan, A. Oral glutamine challenge improves the performance of psychometric tests for the diagnosis of minimal hepatic encephalopathy in patients with liver cirrhosis. J. Gastrointest. Liver Dis. 2013, 22, 277–281. [Google Scholar]
- Savy, G.K. Enteral glutamine supplementation: Clinical review and practical guidelines. Nutr. Clin. Pract. 1997, 12, 259–262. [Google Scholar]
- Tjäder, I.E.; Essén, P.; Hultman, E.; Forsberg, A.; Wernerman, J. Glutamine supplementation to ICU patients affects lactate metabolism in skeletal muscle. Clin. Nutr. 2000, 19, 46. [Google Scholar]
- Darmaun, D.; Hayes, V.; Schaeffer, D.; Welch, S.; Mauras, N. Effects of glutamine and recombinant human growth hormone on protein metabolism in prepubertal children with cystic fibrosis. J. Clin. Endocrinol. Metab. 2004, 89, 1146–1152. [Google Scholar] [CrossRef][Green Version]
- Nava, R.C.; Zuhl, M.N.; Moriarty, T.A.; Amorim, F.T.; Bourbeau, K.C.; Welch, A.M.; McCormick, J.J.; King, K.E.; Mermier, C.M. The effect of acute glutamine supplementation on markers of inflammation and fatigue during consecutive days of simulated wildland firefighting. J. Occup. Environ. Med. 2019, 61, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zuhl, M.N.; Lanphere, K.R.; Kravitz, L.; Mermier, C.M.; Schneider, S.; Dokladny, K.; Moseley, P.L. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J. Appl. Physiol. 2014, 116, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Fürst, P.; Pogan, K.; Stehle, P. Glutamine dipeptides in clinical nutrition. Nutrition 1997, 13, 731–737. [Google Scholar] [CrossRef]
- Ward, E.; Smith, M.; Henderson, M.; Reid, U.; Lewis, I.; Kinsey, S.; Allgar, V.; Bowers, D.; Picton, S.V. The effect of high-dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. Eur. J. Clin. Nutr. 2009, 63, 134–140. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Ahmetovic, Z. Gastrointestinal distress after creatine supplementation in athletes: Are side effects dose dependent? Res. Sports Med. 2008, 16, 15–22. [Google Scholar] [CrossRef]
- Evans, R.W.; Fernstrom, J.D.; Thompson, J.; Morris, S.M., Jr.; Kuller, L.H. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J. Nutr. Biochem. 2004, 15, 534–539. [Google Scholar] [CrossRef]
- Grimble, G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007, 137, 1693–1701. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.J.C.; Senden, J.; Saris, W.H.M.; Wagenmakers, A.J.M. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Ogden, H.B.; Child, R.B.; Fallowfield, J.L.; Delves, S.K.; Westwood, C.S.; Layden, J.D. The gastrointestinal exertional heat stroke paradigm: Pathophysiology, assessment, severity, aetiology and nutritional countermeasures. Nutrients 2020, 12, 537. [Google Scholar] [CrossRef]
- Burke, L.M.; Jeukendrup, A.E.; Jones, A.M.; Mooses, M. Contemporary nutrition strategies to optimize performance in distance runners and race walkers. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 117–129. [Google Scholar] [CrossRef]

| Measure | Mean ± SD |
|---|---|
| Age (years) | 25 ± 5 |
| Height (m) | 1.79 ± 0.07 |
| Body Mass (kg) | 77.7 ± 9.8 |
| Body Fat (%) | 14.8 ± 4.6 |
| Fat Free Mass (kg) | 65.8 ± 5.8 |
| Assignment | Response | ||||
|---|---|---|---|---|---|
| LOW | MED | HIGH | DK | BI (95% CI) | |
| LOW | 3 | 2 | 0 | 9 | 0.07 (−0.27, 0.34) |
| MED | 1 | 2 | 2 | 9 | −0.07 (−0.49, 0.35) |
| HIGH | 0 | 2 | 4 | 8 | 0.14 (−0.18, 0.32) |
| Pre | 0.5 h | 1 h | 2 h | 4 h | 6 h | 12 h | 24 h | Incidence (%) | Total Trial | |
| Gut Discomfort | ||||||||||
| LOW | 0 (0–0) | 1 (1–2) | 2 (1–2) a | 1 (1–2) a | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 28 | 4 (1–6) a |
| MED | 0 (0–0) | 2 (1–2) | 1 (1–3) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 64 | 3 (1–8) b |
| HIGH | 0 (0–0) | 2 (1–3) | 2 (1–4) | 3 (1–5) | 2 (1–4) | 1 (1–2) | 2 (1–3) | 1 (1–1) | 79 | 8 (1–12) |
| Belching | ||||||||||
| LOW | 0 (0–0) | 1 (1–2) | 2 (1–2) a | 1 (1–2) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 21 | 7 (4–12) a |
| MED | 0 (0–0) | 2 (1–2) | 1 (1–3) b | 1 (1–2) | 3 (2–4) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 50 | 4 (1–8) b |
| HIGH | 0 (0–0) | 2 (1–5) | 3 (1–5) | 2 (1–3) | 3 (1–5) | 2 (1–4) | 1 (1–2) | 3 (3–3) | 64 | 8 (1–14) |
| Bloating (Upper) | ||||||||||
| LOW | 0 (0–0) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 1 (1–1) | 43 | 2 (1–2) a,c |
| MED | 0 (0–0) | 2 (1–2) | 1 (1–3) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 71 | 3 (1–7) b |
| HIGH | 0 (0–0) | 2 (1–5) | 3 (1–4) | 2 (1–4) | 2 (1–4) | 2 (2–2) | 4 (4–4) | 0 (0–0) | 64 | 8 (3–13) |
| Pain (Upper) | ||||||||||
| LOW | 0 (0–0) | 1 (1–1) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 14 | 4 (1–6) a |
| MED | 0 (0–0) | 1 (1–1) | 1 (1–2) | 2 (1–2) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 50 | 2 (1–5) |
| HIGH | 0 (0–0) | 1 (1–2) | 2 (1–3) | 3 (1–4) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 50 | 4 (1–8) |
| Urge to Regurgitate | ||||||||||
| LOW | 0 (0–0) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 7 | 1 (1–1) a |
| MED | 0 (0–0) | 1 (1–2) | 3 (1–5) | 2 (1–3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 28 | 2 (2–3) b |
| HIGH | 0 (0–0) | 2 (1–4) | 3 (1–5) | 2 (1–4) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 64 | 5 (1–13) |
| Flatulence | ||||||||||
| LOW | 0 (0–0) | 0 (0–0) | 0 (0–0) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 1 (1–1) | 0 (0–0) | 14 | 1 (1–1) |
| MED | 0 (0–0) | 0 (0–0) | 1 (1–1) | 1 (1–2) | 0 (0–0) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 28 | 2 (2–2) |
| HIGH | 0 (0–0) | 2 (1–2) | 2 (1–2) | 2 (1–3) | 2 (1–2) | 1 (1–1) | 4 (4–4) | 1 (1–1) | 28 | 6 (1–11) |
| Bloating (Lower) | ||||||||||
| LOW | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 | 0 (0–0) |
| MED | 0 (0–0) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 21 | 2 (2–3) |
| HIGH | 0 (0–0) | 2 (1–3) | 2 (1–2) | 2 (2–2) | 2 (1–2) | 3 (3–3) | 1 (1–1) | 0 (0–0) | 21 | 7 (4–11) |
| Pain (Lower) | ||||||||||
| LOW | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 | 0 (0–0) |
| MED | 0 (0–0) | 3 (3–3) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 7 | 4 (4–4) |
| HIGH | 0 (0–0) | 0 (0–0) | 0 (0–0) | 2 (2–2) | 0 (0–0) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 7 | 3 (3–3) |
| Nausea | ||||||||||
| LOW | 0 (0–0) | 1 (1–1) a | 1 (1–1) a | 1 (1–1) a | 1 (1–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 7 | 4 (4–4) a,c |
| MED | 0 (0–0) | 2 (1–3) | 2 (1–3) | 1 (1–2) | 1 (1–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 57 | 3 (1–8) b |
| HIGH | 0 (0–0) | 2 (1–4) | 2 (1–4) | 2 (1–5) | 1 (1–1) | 0 (0–0) | 2 (2–2) | 0 (0–0) | 71 | 6 (4–8) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogden, H.B.; Child, R.B.; Fallowfield, J.L.; Delves, S.K.; Westwood, C.S.; Millyard, A.; Layden, J.D. Gastrointestinal Tolerance of Low, Medium and High Dose Acute Oral l-Glutamine Supplementation in Healthy Adults: A Pilot Study. Nutrients 2020, 12, 2953. https://doi.org/10.3390/nu12102953
Ogden HB, Child RB, Fallowfield JL, Delves SK, Westwood CS, Millyard A, Layden JD. Gastrointestinal Tolerance of Low, Medium and High Dose Acute Oral l-Glutamine Supplementation in Healthy Adults: A Pilot Study. Nutrients. 2020; 12(10):2953. https://doi.org/10.3390/nu12102953
Chicago/Turabian StyleOgden, Henry B., Robert B. Child, Joanne L. Fallowfield, Simon K. Delves, Caroline S. Westwood, Alison Millyard, and Joseph D. Layden. 2020. "Gastrointestinal Tolerance of Low, Medium and High Dose Acute Oral l-Glutamine Supplementation in Healthy Adults: A Pilot Study" Nutrients 12, no. 10: 2953. https://doi.org/10.3390/nu12102953
APA StyleOgden, H. B., Child, R. B., Fallowfield, J. L., Delves, S. K., Westwood, C. S., Millyard, A., & Layden, J. D. (2020). Gastrointestinal Tolerance of Low, Medium and High Dose Acute Oral l-Glutamine Supplementation in Healthy Adults: A Pilot Study. Nutrients, 12(10), 2953. https://doi.org/10.3390/nu12102953




