Next Generation Health Claims Based on Resilience: The Example of Whole-Grain Wheat
Abstract
1. Introduction
2. Whole-Grain Wheat and Its Health Effects
3. Chronic Low-Grade Inflammation as a Targetable Example
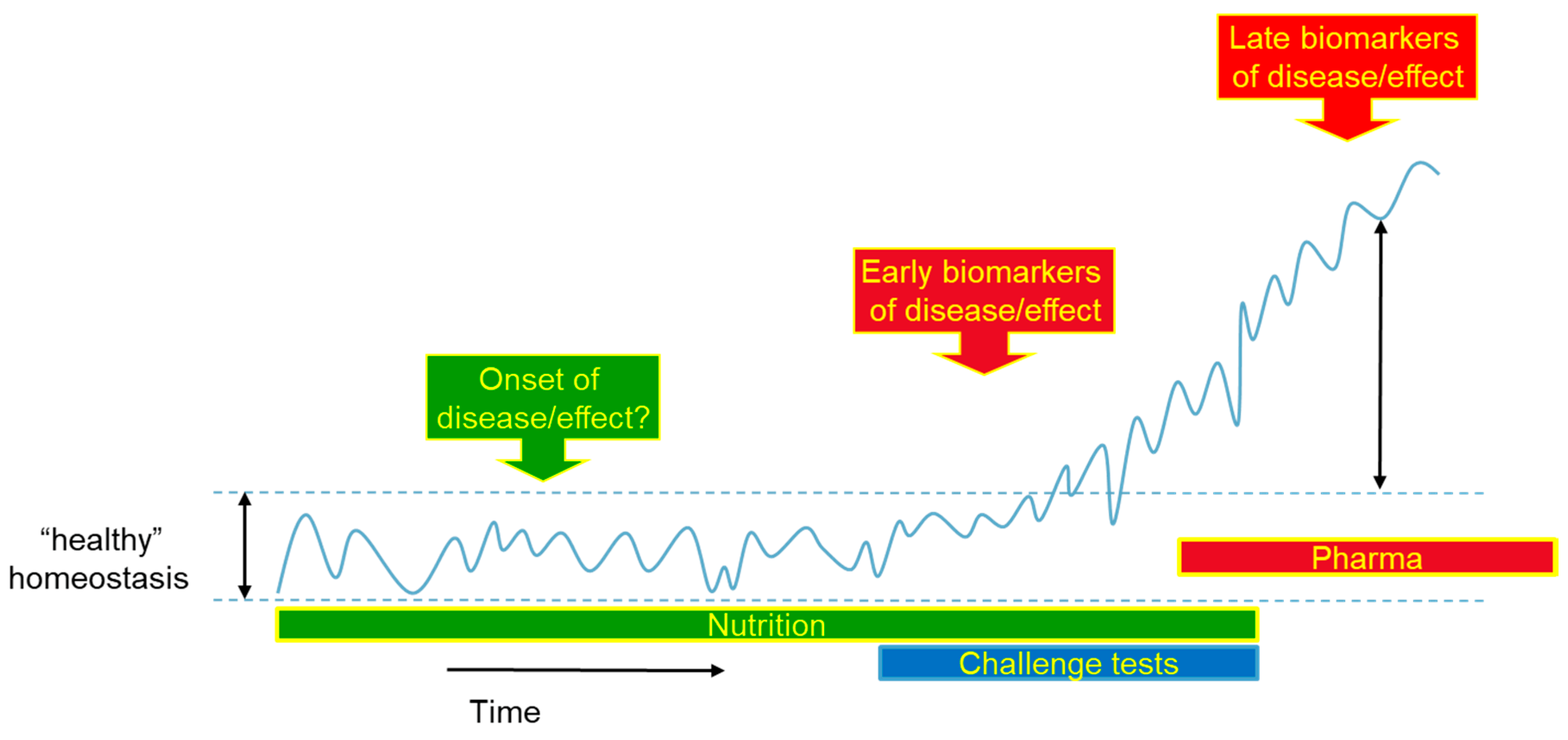
4. Alternative Method for Measurement of Health Effects
5. Health Claims on Whole-Grain Wheat: Status, Issues and Perspectives
Characterization of Whole Grains, Whole Wheat and Whole Wheat Products
6. What are the Issues in Substantiation of Health Benefits for Health Claims with Whole-Grain Wheat?
6.1. EFSA Requirements for Sufficient Evidence
6.2. Substantiation of Scientific Evidence for Whole-Grain Wheat
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to whole grain (ID 831, 832, 833, 1126, 1268, 1269, 1270, 1271, 1431) pursuant to Article 13(1) of Regulation (EC) No 1924/2. EFSA J. 2010, 8, 1766. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific and technical guidance for the preparation and presentation of a health claim application (Revision 2). EFSA J. 2017, 15, e04680. [Google Scholar] [CrossRef]
- Parma, U.; Martini, D.; Del Rio, D.; Bedogni, G.; Pruneti, C.; Ventura, M.; Passeri, G.; Vitale, M.; Dei Cas, A.; Zavaroni, I.; et al. GP/EFSA/NUTRI/2014/01 Scientific substantiation of health claims made on food: Collection, collation and critical analysis of information in relation to claimed effects, outcome variables and methods of measurement. EFSA Support. Publ. 2018, 15, 1272E. [Google Scholar] [CrossRef]
- Committee, E.S.; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA J. 2017, 15, e04970. [Google Scholar] [CrossRef]
- Huber, M.; Knottnerus, J.A.; Green, L.; van der Horst, H.; Jadad, A.R.; Kromhout, D.; Leonard, B.; Lorig, K.; Loureiro, M.I.; van der Meer, J.W.M.; et al. How should we define health? BMJ 2011, 343, d4163. [Google Scholar] [CrossRef]
- The Lancet. What Is Health? The Ability to Adapt. Lancet 2009, 781. [Google Scholar] [CrossRef]
- Stroeve, J.H.M.; van Wietmarschen, H.; Kremer, B.H.A.; van Ommen, B.; Wopereis, S. Phenotypic flexibility as a measure of health: The optimal nutritional stress response test. Genes Nutr. 2015, 10. [Google Scholar] [CrossRef]
- Van Ommen, B.; Wopereis, S. Next-Generation Biomarkers of Health. Nestlé Nutr. Inst. Work. Ser. 2016, 84, 25–33. [Google Scholar] [CrossRef]
- Van Ommen, B.; Keijer, J.; Heil, S.G.; Kaput, J. Challenging homeostasis to define biomarkers for nutrition related health. Mol. Nutr. Food Res. 2009, 53, 795–804. [Google Scholar] [CrossRef]
- Wang, L.; Gaziano, J.M.; Liu, S.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Whole- and refined-grain intakes and the risk of hypertension in women. Am. J. Clin. Nutr. 2007, 86, 472–479. [Google Scholar] [CrossRef]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.J.; Hu, F.B.; Glynn, R.J.; Jensen, M.K.; Franz, M.; Sampson, L.; Rimm, E.B. Whole grains and incident hypertension in men. Am. J. Clin. Nutr. 2009, 90, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Qi, L.; Fahey, G.C.; Klurfeld, D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef]
- Seal, C.J.; Brownlee, I.A. Whole-grain foods and chronic disease: Evidence from epidemiological and intervention studies. Proc. Nutr. Soc. 2015, 74, 313–319. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353353, i2716. [Google Scholar] [CrossRef]
- Zong, G.; Gao, A.; Hu, F.B.; Sun, Q.; Slavin, J.; Fardet, A.; Ferruzzi, M.; Jonnalagadda, S.; Liu, S.; Marquart, L.; et al. Whole Grain Intake and Mortality From All Causes, Cardiovascular Disease, and Cancer: A Meta-Analysis of Prospective Cohort Studies. Circulation 2016, 133, 2370–2380. [Google Scholar] [CrossRef]
- Chen, G.-C.; Tong, X.; Xu, J.-Y.; Han, S.-F.; Wan, Z.-X.; Qin, J.-B.; Qin, L.-Q. Whole-grain intake and total, cardiovascular, and cancer mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2016, 104, 164–172. [Google Scholar] [CrossRef]
- Åberg, S.; Mann, J.; Neumann, S.; Ross, A.B.; Reynolds, A.N. Whole-Grain Processing and Glycemic Control in Type 2 Diabetes: A Randomized Crossover Trial. Diabetes Care 2020, dc200263. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of whole grain foods and risk of type 2 diabetes: Results from three prospective cohort studies. BMJ 2020, 370. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Livingston, K.A.; Jacques, P.F.; Koecher, K.; McKeown, N.M. Evidence Mapping of Whole Grain Intervention Studies, Health Outcomes, and Reporting Practices. FASEB J. 2017, 31, 446–452. [Google Scholar]
- Yamini, S.; Trumbo, P.R. Qualified health claim for whole-grain intake and risk of type 2 diabetes: An evidence-based review by the US Food and Drug Administration. Nutr. Rev. 2016, 74, 601–611. [Google Scholar] [CrossRef] [PubMed]
- De Moura, F.F.; Lewis, K.D.; Falk, M.C. Applying the FDA Definition of Whole Grains to the Evidence for Cardiovascular Disease Health Claims. J. Nutr. 2009, 139, 2220S–2226S. [Google Scholar] [CrossRef]
- Sinclair, S.E.; Mansfield, E.D.; Wells, G.A. Evidence for a Whole Grains and Coronary Heart Disease Health Claim. Int. Food Risk Anal. 2013, 3. [Google Scholar] [CrossRef]
- Musa-Veloso, K.; Poon, T.; Harkness, L.S.; O’Shea, M.; Chu, Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Hui, S.; Liu, K.; Lang, H.; Liu, Y.; Wang, X.; Zhu, X.; Doucette, S.; Yi, L.; Mi, M. Comparative effects of different whole grains and brans on blood lipid: A network meta-analysis. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Sadeghi, O.; Sadeghian, M.; Rahmani, S.; Maleki, V.; Larijani, B.; Esmaillzadeh, A. Whole-Grain Consumption Does Not Affect Obesity Measures: An Updated Systematic Review and Meta-analysis of Randomized Clinical Trials. Adv. Nutr. 2019. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Hernández Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3. [Google Scholar]
- Van den Brink, W.; van Bilsen, J.; Salic, K.; Hoevenaars, F.P.M.; Verschuren, L.; Kleemann, R.; Bouwman, J.; Ronnett, G.V.; van Ommen, B.; Wopereis, S. Current and Future Nutritional Strategies to Modulate Inflammatory Dynamics in Metabolic Disorders. Front. Nutr. 2019, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, P.; Azadbakht, L.; Hashemipor, M.; Kelishadi, R.; Esmaillzadeh, A. Whole-grain intake favorably affects markers of systemic inflammation in obese children: A randomized controlled crossover clinical trial. Mol. Nutr. Food Res. 2014, 58, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Han, S.I.; Song, B.C.; Yeum, K.J. Bioactives in Commonly Consumed Cereal Grains: Implications for Oxidative Stress and Inflammation. J. Med. Food 2015, 18, 1179–1186. [Google Scholar] [CrossRef]
- Hoevenaars, F.P.M.; Esser, D.; Schutte, S.; Priebe, M.G.; Vonk, R.J.; van den Brink, W.J.; van der Kamp, J.-W.; Stroeve, J.H.M.; Afman, L.A.; Wopereis, S. Whole Grain Wheat Consumption Affects Postprandial Inflammatory Response in a Randomized Controlled Trial in Overweight and Obese Adults with Mild Hypercholesterolemia in the Graandioos Study. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome–Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef]
- Benetti, E.; Chiazza, F.; Patel, N.S.A.; Collino, M. The NLRP3 inflammasome as a novel player of the intercellular crosstalk in metabolic disorders. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Schutte, S.; Esser, D.; Hoevenaars, F.P.M.; Hooiveld, G.J.E.J.; Priebe, M.G.; Vonk, R.J.; Wopereis, S.; Afman, L.A. A 12 week whole grain wheat intervention protects against hepatic fat; the Graandioos study, a randomized trial in overweight subjects. Am. J. Clin. Nutr. 2018, 108, 1264–1274. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef]
- Cowan, S.F.; Leeming, E.R.; Sinclair, A.; Dordevic, A.L.; Truby, H.; Gibson, S.J. Effect of whole foods and dietary patterns on markers of subclinical inflammation in weight-stable overweight and obese adults: A systematic review. Nutr. Rev. 2019, 1–20. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, Q.; Feng, J.; Du, L.; Li, K.; Zhou, Y. Whole grain diet reduces systemic inflammation: A meta-analysis of 9 randomized trials. Medicine 2018, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Jonnalagadda, S. Effect of whole grains on markers of subclinical inflammation. Nutr. Rev. 2012, 70, 387–396. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Outcome of a public consultation on the discussion paper for the revision of the guidance on the scientific requirements for health claims related to gut and immune function. EFSA Support. Publ. 2015, 12. [Google Scholar] [CrossRef]
- Seal, C.J.; Nugent, A.P.; Tee, E.-S.; Thielecke, F. Whole-grain dietary recommendations: The need for a unified global approach. Br. J. Nutr. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.J.J.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Pellis, L.; van Erk, M.J.; van Ommen, B.; Bakker, G.C.M.; Hendriks, H.F.J.; Cnubben, N.H.P.; Kleemann, R.; van Someren, E.P.; Bobeldijk, I.; Rubingh, C.M.; et al. Plasma metabolomics and proteomics profiling after a postprandial challenge reveal subtle diet effects on human metabolic status. Metabolomics 2012, 8, 347–359. [Google Scholar] [CrossRef]
- Cruz-Teno, C.; Pérez-Martínez, P.; Delgado-Lista, J.; Yubero-Serrano, E.M.; García-Ríos, A.; Marín, C.; Gómez, P.; Jiménez-Gómez, Y.; Camargo, A.; Rodríguez-Cantalejo, F.; et al. Dietary fat modifies the postprandial inflammatory state in subjects with metabolic syndrome: The LIPGENE study. Mol. Nutr. Food Res. 2012, 56, 854–865. [Google Scholar] [CrossRef]
- Esser, D.; Mars, M.; Oosterink, E.; Stalmach, A.; Müller, M.; Afman, L.A. Dark chocolate consumption improves leukocyte adhesion factors and vascular function in overweight men. FASEB J. 2014, 28, 1464–1473. [Google Scholar] [CrossRef]
- Kardinaal, A.F.M.; Van Erk, M.J.; Dutman, A.E.; Stroeve, J.H.M.; Van De Steeg, E.; Bijlsma, S.; Kooistra, T.; Van Ommen, B.; Wopereis, S. Quantifying phenotypic flexibility as the response to a high-fat challenge test in different states of metabolic health. FASEB J. 2015, 29, 4600–4613. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809. [Google Scholar] [CrossRef]
- Bouwman, J.; Vogels, J.T.W.E.; Wopereis, S.; Rubingh, C.M.; Bijlsma, S.; van Ommen, B. Visualization and identification of health space, based on personalized molecular phenotype and treatment response to relevant underlying biological processes. BMC Med. Genom. 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Esser, D.; van Dijk, S.J.; Oosterink, E.; Müller, M.; Afman, L.A. A High-Fat SFA, MUFA, or n3 PUFA Challenge Affects the Vascular Response and Initiates an Activated State of Cellular Adherence in Lean and Obese Middle-Aged Men. J. Nutr. 2013, 143, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Esser, D.; van Dijk, S.J.; Oosterink, E.; Lopez, S.; Müller, M.; Afman, L.A. High fat challenges with different fatty acids affect distinct atherogenic gene expression pathways in immune cells from lean and obese subjects. Mol. Nutr. Food Res. 2015, 59, 1563–1572. [Google Scholar] [CrossRef]
- Spruit, M.A.; Franssen, F.M.E.; Rutten, E.P.A.; Wopereis, S.; Wouters, E.F.M.; Vanfleteren, L.E.G.W. A new perspective on COPD exacerbations: Monitoring impact by measuring physical, psychological and social resilience. Eur. Respir. J. 2016, 47, 1024–1027. [Google Scholar] [CrossRef]
- Van den Broek, T.J.; Bakker, G.C.M.; Rubingh, C.M.; Bijlsma, S.; Stroeve, J.H.M.; van Ommen, B.; van Erk, M.J.; Wopereis, S. Ranges of phenotypic flexibility in healthy subjects. Genes Nutr. 2017, 12, 32. [Google Scholar] [CrossRef]
- Wopereis, S.; Stroeve, J.H.M.; Stafleu, A.; Bakker, G.C.M.; Burggraaf, J.; van Erk, M.J.; Pellis, L.; Boessen, R.; Kardinaal, A.A.F.; van Ommen, B. Multi-parameter comparison of a standardized mixed meal tolerance test in healthy and type 2 diabetic subjects: The PhenFlex challenge. Genes Nutr. 2017, 12. [Google Scholar] [CrossRef]
- Van der Greef, J.; Davidov, E.; Verheij, E.; Vogels, J.; van der Heijden, R.; Adourian, A.S.; Oresic, M.; Marple, E.W.; Naylor, S. The Role of Metabolomics in Systems Biology. In Metabolic Profiling: Its Role in Biomarker Discovery and Gene Function Analysis; Harrigan, G.G., Goodacre, R., Eds.; Springer US: Boston, MA, USA, 2003; pp. 171–198. ISBN 978-1-4615-0333-0. [Google Scholar] [CrossRef]
- Van Der Kamp, J.W.; Poutanen, K.; Seal, C.J.; Richardson, D.P. The HEALTHGRAIN definition of “whole grain”. Food Nutr. Res. 2014, 58. [Google Scholar] [CrossRef]
- Van Der Kamp, J.W.; Lupton, J. Definitions, regulations, and health claims associated with dietary fibre and wholegrain foods. In Fibre-Rich and Wholegrain Foods: Improving Quality; Woodhead: Cambridge, UK, 2013; pp. 3–24. ISBN 9780857090386. [Google Scholar]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to dietary fibre and maintenance of normal blood cholesterol concentrations (ID 747, 750, 811) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1255. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to fruits and/or vegetables (ID 1212, 1213, 1214, 1217, 1218, 1219, 1301, 1425, 1426, 1427, 1428, 1429, 1430) and to the “Mediterranean diet” (ID 1423) pursuant to Article 13(1) of Regulati. EFSA J. 2011, 9, 2245. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to nuts and essential fatty acids (omega-3/omega-6) in nut oil (ID 741, 1129, 1130, 1305, 1407) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2032. [Google Scholar] [CrossRef][Green Version]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to meat or fish and the improvement of non haem iron absorption (ID 1223) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2040. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to walnuts and maintenance of normal blood LDL-cholesterol concentrations (ID 1156, 1158) and improvement of endothelium-dependent vasodilation (ID 1155, 1157) pursuant to Article 13(1) of. EFSA J. 2011, 9, 2074. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to dietary fibre (ID 744, 745, 746, 748, 749, 753, 803, 810, 855, 1415, 1416, 4308, 4330) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1735. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to wheat bran fibre and increase in faecal bulk (ID 3066), reduction in intestinal transit time (ID 828, 839, 3067, 4699) and contribution to the maintenance or achievement of a normal body. EFSA J. 2010, 8, 1817. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to rye fibre and changes in bowel function (ID 825), reduction of post prandial glycaemic responses (ID 826) and maintenance of normal blood LDL-cholesterol concentrations (ID 827) pursuant. EFSA J. 2011, 9, 2258. [Google Scholar] [CrossRef][Green Version]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to oat and barley grain fibre and increase in faecal bulk (ID 819, 822) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2249. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852. EFSA J. 2011, 9, 2207. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta glucans and maintenance of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant. EFSA J. 2009, 7, 1254. [Google Scholar] [CrossRef]
- EUR-Lex. Consolidated Text: Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; EUR-Lex: Brussels, Belgium, 2014. [Google Scholar]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to appetite ratings, weight management, and blood glucose concentrations. EFSA J. 2012, 10, 2604. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health. EFSA J. 2018, 16, e05136. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to bone, joints, skin, and oral health. EFSA J. 2012, 10, 2702. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to functions of the nervous system, including psychological functions. EFSA J. 2012, 10, 2816. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to physical performance. EFSA J. 2012, 10, 2817. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms. EFSA J. 2016, 14, 4369. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Outcome of a public consultation on the draft guidance on the assessment of the biological relevance of data in scientific assessments. EFSA Support. Publ. 2017, 14, 1296E. [Google Scholar] [CrossRef]
- Gezondheidsraad. Granen en Graanproducten—Achtergronddocument Bij Richtlijnen Goede Voeding; Gezondheidsraad: Den Haag, The Netherlands, 2015; A15/11. [Google Scholar]
| Health Benefit | Biomarker |
|---|---|
| Nervous system, including psychological functions [76] | Standard psychometric tests, established test batteries or valid and reliable tests for the specific domain. Standard tests of visual acuity and contrast sensitivity or valid clinical diagnostic tools. |
| Physical performance [77] | Characteristics of the exercise or physical activity in combination with the target population should be specified. Exercise time to fatigue. Outcome measures of muscle function which may be appropriate for the assessment (i.e., change in muscle structure) of the claimed effect in humans in the context of a particular type of exercise or physical activity should be indicated. |
| Bone, joints, skin and oral health [75] | Measurements of bone mass or bone mineral density using appropriate measures. Maintenance (i.e., reduced loss) of joint function could be assessed via validated protocols and questionnaires. Saliva flow or measurement of self-perceived oral dryness by validated questionnaires. Measurement of trans epidermal water loss using validated methods. |
| Appetite ratings, weight management, and blood glucose concentrations [73] | Behavioral assessment using methods with appropriate validity and precision. Biochemical markers in support (i.e., cholecystokinin). Body fat (primary; dual energy Xray absorptiometry (DEXA), magnetic resonance imaging MRI, computed tomography (CT), secondary; bodyweight, skinfold thickness, bioelectrical impedance analysis (BIA), air displacement plethysmography (ADP)). Bodyweight regain (prolonged time period, 6 months). Body fat (MRI, CT), waist circumference, sustained effect (12 weeks). Lean mass (DEXA, MRI, CT), specified conditions (physical activity etc.). Blood glucose changes (during time, oral glucose tolerance test (OGTT)). Glycosylated hemoglobin (HbA1c). |
| Immune system, gastro intestinal, and defense against pathogens [78] | Breath hydrogen levels, gas volume assessed by imaging (i.e., MRI). Transit time, frequency of bowel movements, stool bulk. Validated subjective global symptom severity questionnaires. Composition of the gut microbiota including pathogenic and toxicogenic microorganisms. Changes in immune markers, e.g., numbers of various lymphoid subpopulations in the circulation, changes in markers of inflammation, changes in short chain fatty acid production in the gut, changes in structure of intestinal epithelium, changes in microbiota composition of the gut accompanied by evidence of a beneficial physiological effect or clinical outcome. |
| Antioxidants, oxidative damage and cardiovascular health [74] | Low Density Lipoprotein-cholesterol Ratio total cholesterol/High Density Lipoprotein HDL-cholesterol Oxidized-LDL Triglycerides Systolic blood pressure Diastolic blood pressure Flow mediated dilatation Decreased platelet aggregation Homocysteine cis-Monounsaturated Fatty Acids |
| WGW | RW | |
|---|---|---|
| Glucose metabolism | ~ | ~ |
| Lipid metabolism | ~ | ~ |
| Liver health | ↓ | ↑↑ |
| Cardiovascular disease markers | O | ↑ |
| Inflammatory resilience (based on Interleukin-10, Interleukin-6, Interleukin-8, Tumor necrosis factor-α) | ↓↓ | ↑ |
| Metabolic Resilience (based on glucose, insulin, triglycerides, Low density lipoprotein-cholesterol, High density lipoprotein-cholesterol, total cholesterol) | ~ | ~ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoevenaars, F.; van der Kamp, J.-W.; van den Brink, W.; Wopereis, S. Next Generation Health Claims Based on Resilience: The Example of Whole-Grain Wheat. Nutrients 2020, 12, 2945. https://doi.org/10.3390/nu12102945
Hoevenaars F, van der Kamp J-W, van den Brink W, Wopereis S. Next Generation Health Claims Based on Resilience: The Example of Whole-Grain Wheat. Nutrients. 2020; 12(10):2945. https://doi.org/10.3390/nu12102945
Chicago/Turabian StyleHoevenaars, Femke, Jan-Willem van der Kamp, Willem van den Brink, and Suzan Wopereis. 2020. "Next Generation Health Claims Based on Resilience: The Example of Whole-Grain Wheat" Nutrients 12, no. 10: 2945. https://doi.org/10.3390/nu12102945
APA StyleHoevenaars, F., van der Kamp, J.-W., van den Brink, W., & Wopereis, S. (2020). Next Generation Health Claims Based on Resilience: The Example of Whole-Grain Wheat. Nutrients, 12(10), 2945. https://doi.org/10.3390/nu12102945






