Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Literature Searches
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
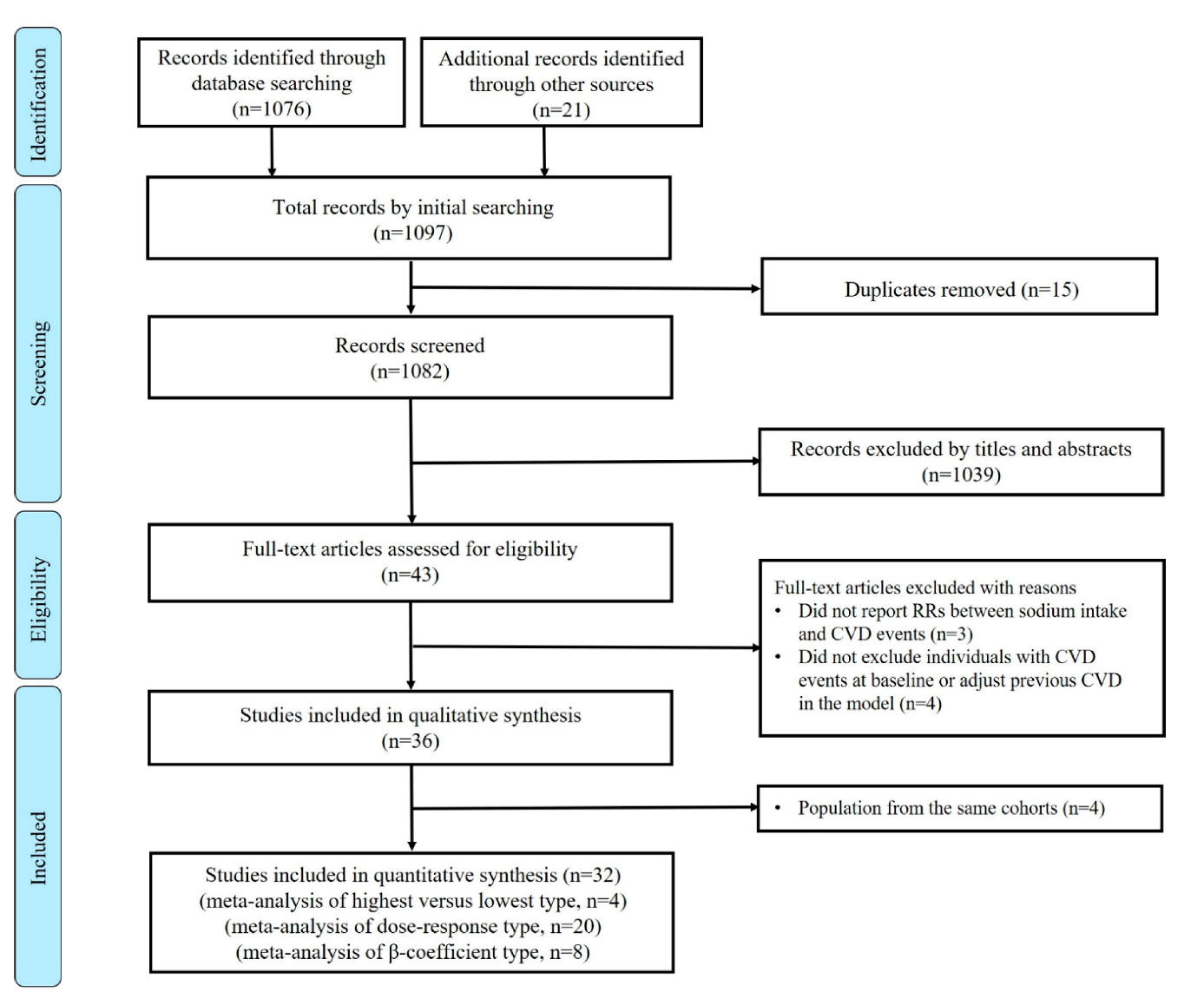
3.1. Study Characteristics and Quality Assessment
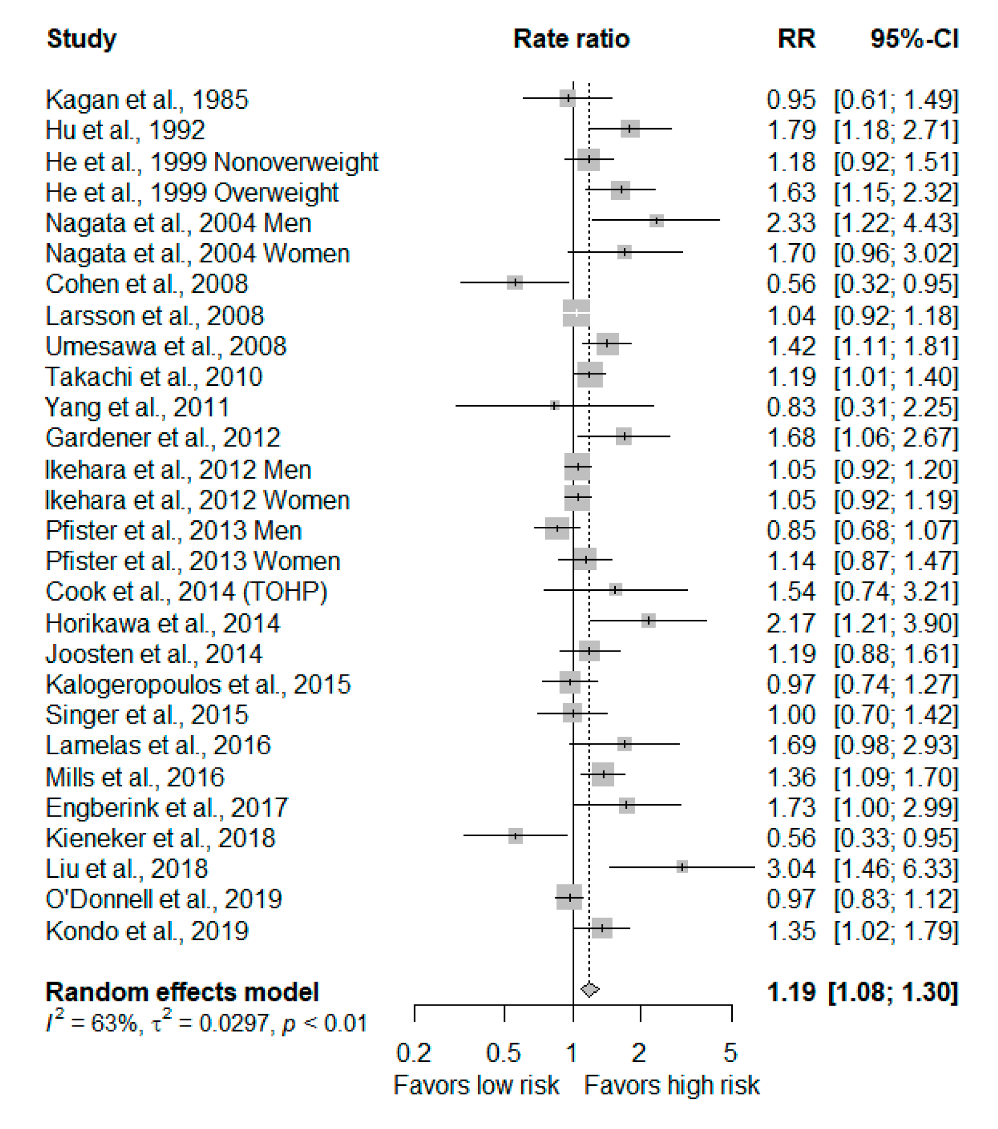
3.2. Highest Versus Lowest Meta-Analysis
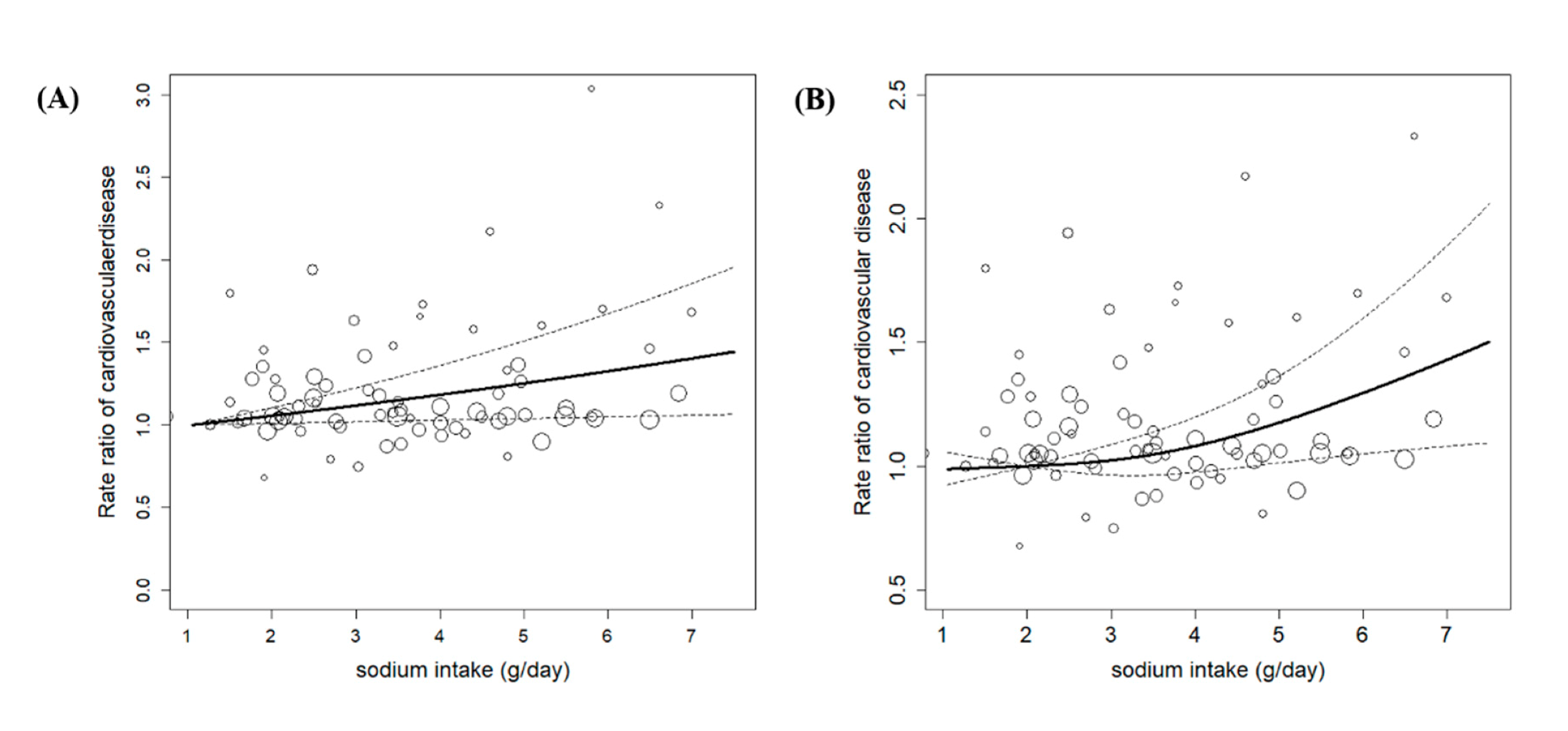
3.3. Dose-Response Meta-Analysis
3.4. Subgroup Analysis, Meta-Regression, and Sensitivity Analyses
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- World Health Organization. Atlas of African Health Statistics 2018: Universal Health Coverage and the Sustainable Development Goals in the WHO African Region; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J. The INTERSALT Study: Background, methods, findings, and implications. Am. J. Clin. Nutr. 1997, 65, 626s–642s. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Guideline: Potassium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary Guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Ayala, C.; Kuklina, E.; Peralez, J.; Keenan, N.L.; Labarthe, D.R. Application of lower sodium intake recommendations to adults—United States, 1999–2006. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 281–283. [Google Scholar]
- He, F.J.; Li, J.; MacGregor, G.A. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Graudal, N.; Hubeck-Graudal, T.; Jürgens, G.; Taylor, R.S. Dose-response relation between dietary sodium and blood pressure: A meta-regression analysis of 133 randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 1273–1278. [Google Scholar] [CrossRef]
- Huang, L.; Trieu, K.; Yoshimura, S.; Neal, B.; Woodward, M.; Campbell, N.R.C.; Li, Q.; Lackland, D.T.; Leung, A.A.; Anderson, C.A.M.; et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomised trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef]
- Elliott, P. Observational studies of salt and blood pressure. Hypertension 1991, 17, I3–I8. [Google Scholar] [CrossRef]
- Campbell, N.R.C.; He, F.J.; Tan, M.; Cappuccio, F.P.; Neal, B.; Woodward, M.; Cogswell, M.E.; McLean, R.; Arcand, J.; MacGregor, G.; et al. The international consortium for quality research on dietary sodium/salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 h) timed urine collections to assess dietary sodium intake. J. Clin. Hypertens. 2019, 21, 700–709. [Google Scholar] [CrossRef]
- Poggio, R.; Gutierrez, L.; Matta, M.G.; Elorriaga, N.; Irazola, V.; Rubinstein, A. Daily sodium consumption and CVD mortality in the general population: Systematic review and meta-analysis of prospective studies. Public Health Nutr. 2015, 18, 695–704. [Google Scholar] [CrossRef]
- Stolarz-Skrzypek, K.; Kuznetsova, T.; Thijs, L.; Tikhonoff, V.; Seidlerová, J.; Richart, T.; Jin, Y.; Olszanecka, A.; Malyutina, S.; Casiglia, E.; et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011, 305, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Ashton, K.E.; Moxham, T.; Hooper, L.; Ebrahim, S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ Br. Med J. 2013, 346, f1326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Li, Z.; Liu, Y.; Fan, X.; Zhang, Y.; Zhang, Y. Association of sodium intake and major cardiovascular outcomes: A dose-response meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2018, 18, 192. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Il’yasova, D.; Hertz-Picciotto, I.; Peters, U.; Berlin, J.A.; Poole, C. Choice of exposure scores for categorical regression in meta-analysis: A case study of a common problem. Cancer Causes Control 2005, 16, 383–388. [Google Scholar] [CrossRef]
- Orsini, N.; Li, R.; Wolk, A.; Khudyakov, P.; Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2011, 175, 66–73. [Google Scholar] [CrossRef]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E. Regression modeling strategies. BIOS 2017, 330, 2018. [Google Scholar]
- White, I.R. Multivariate random-effects meta-analysis. Stata J. 2009, 9, 40–56. [Google Scholar] [CrossRef]
- Orsini, N.; Bellocco, R.; Greenland, S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006, 6, 40–57. [Google Scholar] [CrossRef]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef]
- Hamling, J.; Lee, P.; Weitkunat, R.; Ambühl, M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat. Med. 2008, 27, 954–970. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629. [Google Scholar] [CrossRef]
- Kagan, A.; Popper, J.S.; Rhoads, G.G.; Yano, K. Dietary and other risk factors for stroke in Hawaiian Japanese men. Stroke 1985, 16, 390–396. [Google Scholar] [CrossRef]
- Hu, H.H.; Sheng, W.Y.; Chu, F.L.; Lan, C.F.; Chiang, B.N. Incidence of stroke in Taiwan. Stroke 1992, 23, 1237–1241. [Google Scholar] [CrossRef]
- Alderman, M.H.; Cohen, H.; Madhavan, S. Dietary sodium intake and mortality: The National Health and Nutrition Examination Survey (NHANES I). Lancet 1998, 351, 781–785. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Vupputuri, S.; Bazzano, L.A.; Loria, C.; Whelton, P.K. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. J. Am. Med Assoc. 1999, 282, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Jousilahti, P.; Rastenyte, D.; Moltchanov, V.; Tanskanen, A.; Pietinen, P.; Nissinen, A. Urinary sodium excretion and cardiovascular mortality in Finland: A prospective study. Lancet 2001, 357, 848–851. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch. Intern. Med. 2002, 162, 1619–1624. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Shimizu, N.; Shimizu, H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke 2004, 35, 1543–1547. [Google Scholar] [CrossRef]
- Cohen, H.W.; Hailpern, S.M.; Fang, J.; Alderman, M.H. Sodium intake and mortality in the NHANES II follow-up study. Am. J. Med. 2006, 119, 275.e7–275.e14. [Google Scholar] [CrossRef]
- Cook, N.R.; Cutler, J.A.; Obarzanek, E.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007, 334, 885–888. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Witteman, J.C.M.; Stijnen, T.; Kloos, M.W.; Hofman, A.; Grobbee, D.E. Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: The Rotterdam Study. Eur. J. Epidemiol. 2007, 22, 763–770. [Google Scholar] [CrossRef]
- Cohen, H.W.; Hailpern, S.M.; Alderman, M.H. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III). J. Gen. Intern. Med. 2008, 23, 1297–1302. [Google Scholar] [CrossRef]
- Larsson, S.C.; Virtanen, M.J.; Mars, M.; Männistö, S.; Pietinen, P.; Albanes, D.; Virtamo, J. Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch. Intern. Med. 2008, 168, 459–465. [Google Scholar] [CrossRef]
- Umesawa, M.; Iso, H.; Date, C.; Yamamoto, A.; Toyoshima, H.; Watanabe, Y.; Kikuchi, S.; Koizumi, A.; Kondo, T.; Inaba, Y.; et al. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: The Japan Collaborative Cohort study for evaluation of cancer risks. Am. J. Clin. Nutr. 2008, 88, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, Y.; Yang, Z.; Luo, J. Low dietary sodium intake increases the death risk in peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 2010, 5, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Takachi, R.; Inoue, M.; Shimazu, T.; Sasazuki, S.; Ishihara, J.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Iso, H.; Tsubono, Y.; et al. Consumption of sodium and salted foods in relation to cancer and cardiovascular disease: The Japan public health center-based prospective study. Am. J. Clin. Nutr. 2010, 91, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, T.; Kuklina, E.V.; Flanders, W.D.; Hong, Y.; Gillespie, C.; Chang, M.H.; Gwinn, M.; Dowling, N.; Khoury, M.J.; et al. Sodium and potassium intake and mortality among US adults: Prospective data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2011, 171, 1183–1191. [Google Scholar] [CrossRef]
- Gardener, H.; Rundek, T.; Wright, C.B.; Elkind, M.S.V.; Sacco, R.L. Dietary sodium and risk of stroke in the Northern Manhattan Study. Stroke 2012, 43, 1200–1205. [Google Scholar] [CrossRef]
- Ikehara, S.; Iso, H.; Date, C.; Kikuchi, S.; Watanabe, Y.; Inaba, Y.; Tamakoshi, A. Salt preference and mortality from stroke and coronary heart disease for Japanese men and women: The JACC study. Prev. Med. 2012, 54, 32–37. [Google Scholar] [CrossRef]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014, 129, 981–989. [Google Scholar] [CrossRef]
- Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; Ohashi, Y.; et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: Analysis of the Japan diabetes complications study (JDCS). J. Clin. Endocrinol. Metab. 2014, 99, 3635–3643. [Google Scholar] [CrossRef]
- Joosten, M.M.; Gansevoort, R.T.; Mukamal, K.J.; Heerspink, H.J.L.; Geleijnse, J.M.; Feskens, E.J.M.; Navis, G.; Bakker, S.J.L. Sodium excretion and risk of developing coronary heart disease. Circulation 2014, 129, 1121–1128. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; Wang, X.; Liu, L.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. Med. 2014, 371, 612–623. [Google Scholar] [CrossRef]
- Pfister, R.; Michels, G.; Sharp, S.J.; Luben, R.; Wareham, N.J.; Khaw, K.T. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur. J. Heart Fail. 2014, 16, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Murphy, R.A.; Newman, A.B.; Bauer, D.C.; Harris, T.B.; Yang, Z.; Applegate, W.B.; Kritchevsky, S.B. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: The health, aging, and body composition (Health ABC) study. JAMA Intern. Med. 2015, 175, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Cohen, H.; Alderman, M. Assessing the associations of sodium intake with long-term all-cause and cardiovascular mortality in a hypertensive cohort. Am. J. Hypertens. 2015, 28, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lamelas, P.M.; Mente, A.; Diaz, R.; Orlandini, A.; Avezum, A.; Oliveira, G.; Lanas, F.; Seron, P.; Lopez-Jaramillo, P.; Camacho-Lopez, P.; et al. Association of urinary sodium excretion with blood pressure and cardiovascular clinical events in 17,033 Latin Americans. Am. J. Hypertens. 2016, 29, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Chen, J.; Yang, W.; Appel, L.J.; Kusek, J.W.; Alper, A.; Delafontaine, P.; Keane, M.G.; Mohler, E.; Ojo, A.; et al. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA J. Am. Med Assoc. 2016, 315, 2200–2210. [Google Scholar] [CrossRef]
- Polonia, J.; Monteiro, J.; Almeida, J.; Silva, J.A.; Bertoquini, S. High salt intake is associated with a higher risk of cardiovascular events: A 7.2-year evaluation of a cohort of hypertensive patients. Blood Press. Monit. 2016, 21, 301–306. [Google Scholar] [CrossRef]
- Olde Engberink, R.H.G.; Van Den Hoek, T.C.; Van Noordenne, N.D.; Van Den Born, B.J.H.; Peters-Sengers, H.; Vogt, L. Use of a single baseline versus multiyear 24-hour urine collection for estimation of long-term sodium intake and associated cardiovascular and renal risk. Circulation 2017, 136, 917–926. [Google Scholar] [CrossRef]
- Prentice, R.L.; Huang, Y.; Neuhouser, M.L.; Manson, J.E.; Mossavar-Rahmani, Y.; Thomas, F.; Tinker, L.F.; Allison, M.; Johnson, K.C.; Wassertheil-Smoller, S.; et al. Associations of Biomarker-Calibrated Sodium and Potassium Intakes with Cardiovascular Disease Risk among Postmenopausal Women. Am. J. Epidemiol. 2017, 186, 1035–1043. [Google Scholar] [CrossRef]
- Kieneker, L.M.; Eisenga, M.F.; Gansevoort, R.T.; de Boer, R.A.; Navis, G.; Dullaart, R.P.F.; Joosten, M.M.; Bakker, S.J.L. Association of Low Urinary Sodium Excretion with Increased Risk of Stroke. Mayo Clin. Proc. 2018, 93, 1803–1809. [Google Scholar] [CrossRef]
- Lelli, D.; Antonelli-Incalzi, R.; Bandinelli, S.; Ferrucci, L.; Pedone, C. Association between sodium excretion and cardiovascular disease and mortality in the elderly: A cohort study. J. Am. Med Dir. Assoc. 2018, 19, 229–234. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Zhou, L.; Wu, Y.; Li, Y.; Mai, J.; Nie, Z.; Wu, Y.; Liu, X.; Zhao, L. Urinary sodium excretion and risk of cardiovascular disease in the Chinese population: A prospective study. Hypertens. Res. 2018, 41, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Ah, S.T.L.; Wei, L.; Diaz, R.; et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: A community-level prospective epidemiological cohort study. Lancet 2018, 392, 496–506. [Google Scholar] [CrossRef]
- Kondo, K.; Miura, K.; Tanaka-Mizuno, S.; Kadota, A.; Arima, H.; Okuda, N.; Fujiyoshi, A.; Miyagawa, N.; Yoshita, K.; Okamura, T.; et al. Cardiovascular risk assessment chart by dietary factors in Japan—NIPPON DATA80. Circ. J. 2019, 83, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; O’Leary, N.; Yin, L.; Liu, X.; Swaminathan, S.; Khatib, R.; Rosengren, A.; et al. Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: Prospective cohort study. BMJ 2019, 364, l772. [Google Scholar] [CrossRef]
- Milajerdi, A.; Djafarian, K.; Shab-Bidar, S. Dose–response association of dietary sodium intake with all-cause and cardiovascular mortality: A systematic review and meta-analysis of prospective studies. Public Health Nutr. 2019, 22, 295–306. [Google Scholar] [CrossRef]
- Graudal, N.; Jürgens, G.; Baslund, B.; Alderman, M.H. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: A meta-analysis. Am. J. Hypertens. 2014, 27, 1129–1137. [Google Scholar] [CrossRef]
- Graudal, N. The data show a u-shaped association of sodium intake with cardiovascular disease and mortality. Am. J. Hypertens. 2014, 28, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Moran, J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.; Ahola, A.; Wadén, J.; Tolonen, N.; Saraheimo, M.; Gordin, D.; et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011, 34, 861–866. [Google Scholar] [CrossRef]
- Cobb, L.K.; Anderson, C.A.; Elliott, P.; Hu, F.B.; Liu, K.; Neaton, J.D.; Whelton, P.K.; Woodward, M.; Appel, L.J. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: A science advisory from the American Heart Association. Circulation 2014, 129, 1173–1186. [Google Scholar] [CrossRef]
- Jayedi, A.; Ghomashi, F.; Zargar, M.S.; Shab-Bidar, S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: A systematic review and nonlinear dose-response meta-analysis. Clin. Nutr. 2019, 38, 1092–1100. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Peach, M.J. Renin-angiotensin system: Biochemistry and mechanisms of action. Physiol. Rev. 1977, 57, 313–370. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.G.; Whelton, P.K.; Saito, H.; Russell, R.P.; Hermann, J. Relation between blood pressure and renin, renin substrate, angiotensin II, aldosterone and urinary sodium and potassium in 574 ambulatory subjects. Hypertension 1979, 1, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Haddy, F.J. Mechanism, prevention and therapy of sodium-dependent hypertension. Am. J. Med. 1980, 69, 746–758. [Google Scholar] [CrossRef]
- Weinberger, M.H. Salt sensitivity of blood pressure in humans. Hypertension 1996, 27, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.; Tannen, R. Low Renin Essential Hypertension. Hypertension; McGraw-Hill: New York, NY, USA, 1977; p. 349. [Google Scholar]
- Chen, J.; Gu, D.; Huang, J.; Rao, D.C.; Jaquish, C.E.; Hixson, J.E.; Chen, C.-S.; Chen, J.; Lu, F.; Hu, D. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: A dietary intervention study. Lancet 2009, 373, 829–835. [Google Scholar] [CrossRef]
- Koomans, H.; Roos, J.; Boer, P.; Geyskes, G.; Mees, E. Salt sensitivity of blood pressure in chronic renal failure. Evidence for renal control of body fluid distribution in man. Hypertension 1982, 4, 190–197. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Miller, J.Z.; Luft, F.C.; Grim, C.E.; Fineberg, N.S. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 1986, 8, II127. [Google Scholar] [CrossRef]
- Rodriguez, B.L.; Labarthe, D.R.; Huang, B.; Lopez-Gomez, J. Rise of blood pressure with age. New evidence of population differences. Hypertension 1994, 24, 779–785. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Miller, J.Z.; Fineberg, N.S.; Luft, F.C.; Grim, C.E.; Christian, J.C. Association of haptoglobin with sodium sensitivity and resistance of blood pressure. Hypertension 1987, 10, 443–446. [Google Scholar] [CrossRef]
- Land, M.-A.; Webster, J.; Christoforou, A.; Johnson, C.; Trevena, H.; Hodgins, F.; Chalmers, J.; Woodward, M.; Barzi, F.; Smith, W.; et al. The association of knowledge, attitudes and behaviours related to salt with 24-hour urinary sodium excretion. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Spiegelman, D.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Barnett, J.B.; Chavarro, J.E.; Subar, A.F.; Sampson, L.K.; Willett, W.C. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am. J. Epidemiol. 2017, 185, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Micheli, E.T.; Rosa, A.A. Estimation of sodium intake by urinary excretion and dietary records in children and adolescents from Porto Alegre, Brazil: A comparision of two methods. Nutr. Res. 2003, 23, 1477–1487. [Google Scholar] [CrossRef]
- McLean, R.; Cameron, C.; Butcher, E.; Cook, N.R.; Woodward, M.; Campbell, N.R.C. Comparison of 24-hour urine and 24-hour diet recall for estimating dietary sodium intake in populations: A systematic review and meta-analysis. J. Clin. Hypertens. 2019, 21, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
| Subgroup Analysis | Number of Estimates | Summary RR (95% CI) | I2 (%) * | p Value * |
|---|---|---|---|---|
| Overall | 33 | 1.04 (1.01, 1.07) | 77 | 0.01 * |
| Subregion | 0.06 | |||
| Asia | 9 | 1.15 (1.01, 1.29) | 72 | |
| Non-Asia | 23 | 1.03 (0.99, 1.07) | 75 | |
| Multi-nation | 1 | 1.00 (0.97, 1.02) | - | |
| Follow-up years | 0.88 | |||
| Above ten years | 20 | 1.05 (1.00, 1.10) | 67 | |
| Less than 10 years | 13 | 1.01 (1.01, 1.02) | 85 | |
| Sodium assessment | 0.82 | |||
| From diet | 19 | 1.05 (0.99, 1.11) | 76 | |
| From Urine | 14 | 1.04 (1.00, 1.08) | 63 | |
| Outcomes | 0.91 | |||
| Total CVD | 14 | 1.05 (1.01, 1.09) | 81 | |
| CVD mortality | 11 | 1.06 (0.94, 1.20) | 81 | |
| Stroke | 5 | 1.04 (0.91, 1.19) | 73 | |
| Coronary heart disease | 1 | 1.05 (0.97, 1.15) | - | |
| Heart failure | 2 | 0.99 (0.89, 1.10) | 62 |
| Meta Regression | Number of Estimates | Point Estimates | I2 (%) * | p Value * |
|---|---|---|---|---|
| Mean age (year) | 32 | 1.00 (0.99, 1.01) | 90 | 0.92 |
| Percentage of women (%) | 33 | 1.00 (1.00, 1.00) | 90 | 0.89 |
| Follow-up years (year) | 33 | 1.00 (0.99, 1.01) | 90 | 0.89 |
| Study quality score | 33 | 1.01 (0.96, 1.06) | 86 | 0.60 |
| Body mass index (kg/m2) | 22 | 0.99 (0.97, 1.02) | 91 | 0.62 |
| Percentage of white participants (%) | 10 | 1.00 (1.00, 1.01) | 83 | 0.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-J.; Yeh, T.-L.; Shih, M.-C.; Tu, Y.-K.; Chien, K.-L. Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2020, 12, 2934. https://doi.org/10.3390/nu12102934
Wang Y-J, Yeh T-L, Shih M-C, Tu Y-K, Chien K-L. Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients. 2020; 12(10):2934. https://doi.org/10.3390/nu12102934
Chicago/Turabian StyleWang, Yi-Jie, Tzu-Lin Yeh, Ming-Chieh Shih, Yu-Kang Tu, and Kuo-Liong Chien. 2020. "Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis" Nutrients 12, no. 10: 2934. https://doi.org/10.3390/nu12102934
APA StyleWang, Y.-J., Yeh, T.-L., Shih, M.-C., Tu, Y.-K., & Chien, K.-L. (2020). Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients, 12(10), 2934. https://doi.org/10.3390/nu12102934







