Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health
Abstract
1. Introduction
2. Structure and Function of Anthocyanins
3. Anthocyanins in Whole Grain Cereals
3.1. Wheat
3.2. Barley
3.3. Maize
3.4. Rice
4. Processing of Coloured Cereals
5. Antioxidant Potential and Health Effects
5.1. Antioxidant Potential of Anthocyanins
5.2. Human and Animal Studies on the Health Effects of Cereal-Based Anthocyanins
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gani, A.; SM, W.; FA, M. Whole-Grain Cereal Bioactive Compounds and Their Health Benefits: A Review. J. Food Process. Technol. 2012, 03. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Dutta, H.; Saikia, D.; Mahanta, C.L. Quality characterisation and estimation of phytochemicals content and antioxidant capacity of aromatic pigmented and non-pigmented rice varieties. Food Res. Int. 2012, 46, 334–340. [Google Scholar] [CrossRef]
- Knievel, D.C.; Abdel-Aal, E.S.M.; Rabalski, I.; Nakamura, T.; Hucl, P. Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.). J. Cereal Sci. 2009, 50, 113–120. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M. Anthocyanin-Pigmented Grain Products. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; Awika, J.M., Piironen, V., Bean, S., Eds.; Oxford University Press, Inc.: Washington, DC, USA, 2011; pp. 76–109. ISBN 9780841226364. [Google Scholar]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2018, 1–6. [Google Scholar] [CrossRef]
- Damodoran, S.; Parkin, K.; Fennema, O.R. Fennema’s Food Chemistry; Srinivasan, D., Parkin, K.L., Eds.; Fourth.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 3-540-40818-5. [Google Scholar]
- Riaz, M.; Zia-ul-haq, M.; Saad, B. Biosynthesis and Stability of Anthocyanins. In Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Springer International: New York, NY, USA, 2016; pp. 71–86. ISBN 978-3-319-26454-7. [Google Scholar]
- Riaz, M.; Zia-ul-haq, M.; Saad, B. Occurence of Anthocyanins in Plants. In Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Springer International: New York, NY, USA, 2016; pp. 35–46. ISBN 978-3-319-26454-7. [Google Scholar]
- Shipp, J.; Abdel-Aal, E.-S.M. Food Applications and Physiological Effects of Anthocyanins as Functional Food Ingredients. Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-ul-haq, M.; Saad, B. Anthocyanins as Natural Colours. In Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Springer International: New York, NY, USA, 2016; pp. 47–55. ISBN 978-3-319-26454-7. [Google Scholar]
- Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanins in cereals. J. Chromatogr. A 2004, 1054, 129–141. [Google Scholar] [CrossRef]
- Stebbins, N.B. Characterization and Mechanisms of Anthocyanin Degradation and Stabilization. ProQuest Diss. Theses 2017, 12, 227. [Google Scholar]
- Zhao, C.L.; Yu, Y.Q.; Chen, Z.J.; Wen, G.S.; Wei, F.G.; Zheng, Q.; Wang, C.D.; Xiao, X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Ma, M.; Dolan, K.D. Effects of Spray Drying on Antioxidant Capacity and Anthocyanidin Content of Blueberry By-Products. J. Food Sci. 2011, 76. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, C.; Évora, A.; Faria, A.; Mateus, N.; Freitas, V. De Anthocyanins: Nutrition and Health; Springer: New York, NY, USA, 2018; ISBN 9783319545288. [Google Scholar]
- Charron, C.S.; Kurilich, A.C.; Clevidence, B.A.; Simon, P.W.; Harrison, D.J.; Britz, S.J.; Baer, D.J.; Novotny, J.A. Bioavailability of anthocyanins from purple carrot juice: Effects of acylation and plant matrix. J. Agric. Food Chem. 2009, 57, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.S.M.; Akhtar, H.; Rabalski, I.; Bryan, M. Accelerated, Microwave-Assisted, and Conventional Solvent Extraction Methods Affect Anthocyanin Composition from Colored Grains. J. Food Sci. 2014, 79, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Hucl, P. A Rapid Method for Quantifying Total Anthocyanins in Blue Aleurone and Purple Pericarp Wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Eisele, T.; Giusti, M.M.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; Martin, S.K.; Martinsen, B.K.; Miller, T.C.; Paquette, F.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of two anthocyanin quantification methods: HPLC and spectrophotometric methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of antioxidant activities of different colored wheat grains and analysis of phenolic compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Zhang, M.; Blanchard, C. Phenolics, flavonoids, proanthocyanidin and antioxidant activity of brown rice with different pericarp colors following storage. J. Stored Prod. Res. 2014, 59, 120–125. [Google Scholar] [CrossRef]
- Moore, J.; Hao, J. Antioxidant and Health Promoting Properties of Wheat (Triticum spp.). Cereals Pulses Nutraceutical Prop. Heal. Benefits 2012, 113–130. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. Cereal Grains for the Food and Beverage Industries, 1st ed.; Woodhead Publishing: Philidelphia, PA, USA, 2013; ISBN 9780857094131. [Google Scholar]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Choo, T.M.; Dhillon, S.; Rabalski, I. Free and bound phenolic acids and total phenolics in black, blue, and yellow barley and their contribution to free radical scavenging capacity. Cereal Chem. 2012, 89, 198–204. [Google Scholar] [CrossRef]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Varga, M.; Bánhidy, J.; Cseuz, L.; Matuz, J. The anthocyanin content of blue and purple coloured wheat cultivars and their hybrid generations. Cereal Res. Commun. 2013, 41, 284–292. [Google Scholar] [CrossRef]
- Delcour, J.A.; Hoseney, R.C. Principles of Cereal Science and Technology, 3rd ed.; Cereals and Grains Association: Saint Paul, MN, USA, 2009; ISBN 1891127632. [Google Scholar]
- Rao, S.; Schwarz, L.J.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Cereal Phenolic Contents as Affected by Variety and Environment. Cereal Chem. 2018, 95, 589–602. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, J.; Wang, C.; Qin, H.; Ding, H.; Xie, Y.; Guo, T. Accumulation of phenolic compounds and expression profiles of phenolic acid biosynthesis-related genes in developing grains of white, purple, and red wheat. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Clarke, M.W.; Singh, V.; Fang, Z. Growth temperature and genotype both play important roles in sorghum grain phenolic composition. Sci. Rep. 2016, 6, 21835. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.S.M.; Hucl, P. Composition and Stability of Anthocyanins in Blue-Grained Wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.S.M.; Abou-Arab, A.A.; Gamel, T.H.; Hucl, P.; Young, J.C.; Rabalski, I. Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties. J. Agric. Food Chem. 2008, 56, 11171–11177. [Google Scholar] [CrossRef]
- Siebenhandl, S.; Grausgruber, H.; Pellegrini, N.; Del Rio, D.; Fogliano, V.; Pernice, R.; Berghofer, E. Phytochemical profile of main antioxidants in different fractions of purple and blue wheat, and black barley. J. Agric. Food Chem. 2007, 55, 8541–8547. [Google Scholar] [CrossRef]
- Bellido, G.G.; Beta, T. Anthocyanin composition and oxygen radical scavenging capacity (ORAC) of milled and pearled purple, black, and common barley. J. Agric. Food Chem. 2009, 57, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Han, D.; Kim, B.; Baek, N.; Baik, B.K. Antioxidant and anti-hypertensive activity of anthocyanin-rich extracts from hulless pigmented barley cultivars. Int. J. Food Sci. Technol. 2013, 48, 984–991. [Google Scholar] [CrossRef]
- Diczházi, I.; Kursinszki, L. Anthocyanin content and composition in winter blue barley cultivars and lines. Cereal Chem. 2014, 91, 195–200. [Google Scholar] [CrossRef]
- Kim, M.J.Y.; Hyun, J.N.; Kim, J.G.J.A.; Park, J.C.; Kim, M.J.Y.; Kim, J.G.J.A.; Lee, S.J.; Chun, S.C.; Chung, I.M. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- Paulsmeyer, M.; Chatham, L.; Becker, T.; West, M.; West, L.; Juvik, J. Survey of Anthocyanin Composition and Concentration in Diverse Maize Germplasms. J. Agric. Food Chem. 2017, 65, 4341–4350. [Google Scholar] [CrossRef]
- Salinas Moreno, Y.; Sanchez, G.S.; Hernandez, D.R.; Lobato, N.R. Characterization of Anthocyanin Extracts from Maize Kernels. J. Chromatogr. Sci. 2005, 43, 483–487. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Foods 2014, 9, 109–118. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akillioǧlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Hu, Q.P.; Xu, J.G. Profiles of carotenoids, anthocyanins, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef]
- Chen, M.H.; McClung, A.M.; Bergman, C.J. Phenolic content, anthocyanins and antiradical capacity of diverse purple bran rice genotypes as compared to other bran colors. J. Cereal Sci. 2017, 77, 110–119. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Identification and quantification of phenolic acids and anthocyanins as antioxidants in bran, embryo and endosperm of white, red and black rice kernels (Oryza sativa L.). J. Cereal Sci. 2014, 59, 211–218. [Google Scholar] [CrossRef]
- Zhang, M.; Lei, N.; Zhu, T.; Zhang, Z. Thermal processing effects on the chemical constituent and antioxidant activity of s-alk(en)ylcysteine s-oxides (alliin) extract. LWT Food Sci. Technol. 2013, 51, 309–313. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Watanabe, S.; Crozier, A.; Fujimura, T.; Yokota, T.; Ashihara, H. Phytochemical profile of a Japanese black-purple rice. Food Chem. 2013, 141, 2821–2827. [Google Scholar] [CrossRef]
- Chen, X.Q.; Nagao, N.; Itani, T.; Irifune, K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012, 135, 2783–2788. [Google Scholar] [CrossRef]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Min, B.; Gu, L.; McClung, A.M.; Bergman, C.J.; Chen, M.H. Free and bound total phenolic concentrations, antioxidant capacities, and profiles of proanthocyanidins and anthocyanins in whole grain rice (Oryza sativa L.) of different bran colours. Food Chem. 2012, 133, 715–722. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; De Simone, V.; De Leonardis, A.M.; Giovanniello, V.; Del Nobile, M.A.; Padalino, L.; Lecce, L.; Borrelli, G.M.; De Vita, P. Use of purple durum wheat to produce naturally functional fresh and dry pasta. Food Chem. 2016, 205, 187–195. [Google Scholar] [CrossRef]
- Bartl, P.; Albreht, A.; Skrt, M.; Tremlova, B.; Ostadlova, M.; Smejkal, K.; Vovk, I.; Ulrih, N.P. Anthocyanins in purple and blue wheat grains and in resulting bread: Quantity, composition, and thermal stability. Int. J. Food Sci. Nutr. 2015, 66, 514–519. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. Bread fortified with anthocyanin-rich extract from black rice as nutraceutical sources: Its quality attributes and in vitro digestibility. Food Chem. 2016, 196, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cai, Y.Z.; Li, W.; Corke, H.; Kitts, D.D. Anthocyanin characterization and bioactivity assessment of a dark blue grained wheat (Triticum aestivum L. cv. Hedong Wumai) extract. Food Chem. 2007, 104, 955–961. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Food Chemistry Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Li, W.; Beta, T. Evaluation of antioxidant capacity and aroma quality of anthograin liqueur. Food Chem. 2011, 127, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pickard, M.D.; Beta, T. Evaluation of antioxidant activity and electronic taste and aroma properties of antho-beers from purple wheat grain. J. Agric. Food Chem. 2007, 55, 8958–8966. [Google Scholar] [CrossRef]
- Li, J.; Walker, C.E.; Faubion, J.M. Acidulant and oven type affect total anthocyanin content of blue corn cookies. J. Sci. Food Agric. 2011, 91, 38–43. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Ponce-García, N.; Ramírez-Wong, B.; Torres-Chávez, P.I.; Figueroa-Cárdenas, J.D.D.; Gutiérrez-Dorado, R. Specific Anthocyanin Contents of Whole Blue Maize Second-Generation Snacks: An Evaluation Using Response Surface Methodology and Lime Cooking Extrusion. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Tananuwong, K.; Tangsrianugul, N. Effects of storage conditions and cooking on colour and antioxidant activities of organic pigmented rice. Int. J. Food Sci. Technol. 2013, 48, 67–73. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y.; Chandrasekara, A. Antioxidants and Human Health. Cereals Pulses Nutraceutical Prop. Heal. Benefits 2012, 273–308. [Google Scholar] [CrossRef]
- Wallace, T.C.; Slavin, M.; Frankenfeld, C.L. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients 2016, 8, 32. [Google Scholar] [CrossRef]
- Blesso, C.N. Dietary Anthocyanins and Human Health. Nutrients 2019, 11, 2107. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U.; Mojzer, E.B.; Hrnc, M.K.; Škerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; De Simone, V.; Colecchia, S.A.; Pecorella, I.; Platani, C.; Nigro, F.; Finocchiaro, F.; Papa, R.; De Vita, P. Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (Triticum turgidum L. ssp. turgidum convar. durum) wheats. J. Agric. Food Chem. 2014, 62, 8686–8695. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Shibu, M.A.; Fan, M.J.; Chen, M.C.; Viswanadha, V.P.; Lin, Y.L.; Lai, C.H.; Lin, K.H.; Ho, T.J.; Kuo, W.W.; et al. Purple rice anthocyanin extract protects cardiac function in STZ-induced diabetes rat hearts by inhibiting cardiac hypertrophy and fibrosis. J. Nutr. Biochem. 2016, 31, 98–105. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Ji, Y.; Liu, Y.; Xu, L.; Guo, Y. Dietary Supplementation of Black Rice Anthocyanin Extract Regulates Cholesterol Metabolism and Improves Gut Microbiota Dysbiosis in C57BL/6J Mice Fed a High-Fat and Cholesterol Diet. Mol. Nutr. Food Res. 2020, 64, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M.; Feng, X.; Xia, X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Hum. Nutr. 2007, 62, 1–6. [Google Scholar] [CrossRef]
- Chen, W.; Müller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef]
- Sharma, S.; Khare, P.; Kumar, A.; Chunduri, V.; Kumar, A.; Kapoor, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin-Biofortified Colored Wheat Prevents High Fat Diet–Induced Alterations in Mice: Nutrigenomics Studies. Mol. Nutr. Food Res. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Xia, X.; Ling, W.; Ma, J.; Xia, M.; Hou, M.; Wang, Q.; Zhu, H.; Tang, Z. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. J. Nutr. 2006, 136, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhou, Y.; Wu, T.; Hao, L. Ameliorative effect of black rice anthocyanin on senescent mice induced by d-galactose. Food Funct. 2014, 5, 2892–2897. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.F.; Monteiro, V.V.S.; Souza Gomes, R.; Carmo, M.M.; Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.H.; Wang, L.L.; Ma, J. Supplementation of the black rice outer layer fraction to rabbits decreases atherosclerotic plaque formation and increases antioxidant status. J. Nutr. 2002, 132, 20–26. [Google Scholar] [CrossRef]
- Xia, M.; Ling, W.H.; Ma, J.; Kitts, D.D.; Zawistowski, J. Supplementation of diets with the black rice pigment fraction attenuates atherosclerotic plaque formation in apolipoprotein E deficient mice. J. Nutr. 2003, 133, 744–751. [Google Scholar] [CrossRef]
- Hu, C.; Zawistowski, J.; Ling, W.; Kitts, D.D. Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. J. Agric. Food Chem. 2003, 51, 5271–5277. [Google Scholar] [CrossRef]
- Fratantonio, D.; Cimino, F.; Molonia, M.S.; Ferrari, D.; Saija, A.; Virgili, F.; Speciale, A. Cyanidin-3-O-glucoside ameliorates palmitate-induced insulin resistance by modulating IRS-1 phosphorylation and release of endothelial derived vasoactive factors. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 351–357. [Google Scholar] [CrossRef]
- Fan, M.-J.; Yeh, P.-H.; Lin, J.-P.; Huang, A.-C.; Lien, J.-C.; Lin, H.-Y.; Chung, J.-G. Anthocyanins from black rice promote immune responses in leukemia through enhancing phagocytosis of macrophages in vivo. Exp. Ther. Med. 2017, 14, 59–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy Yongzhu. Food Chem. 2020, 339. [Google Scholar] [CrossRef]

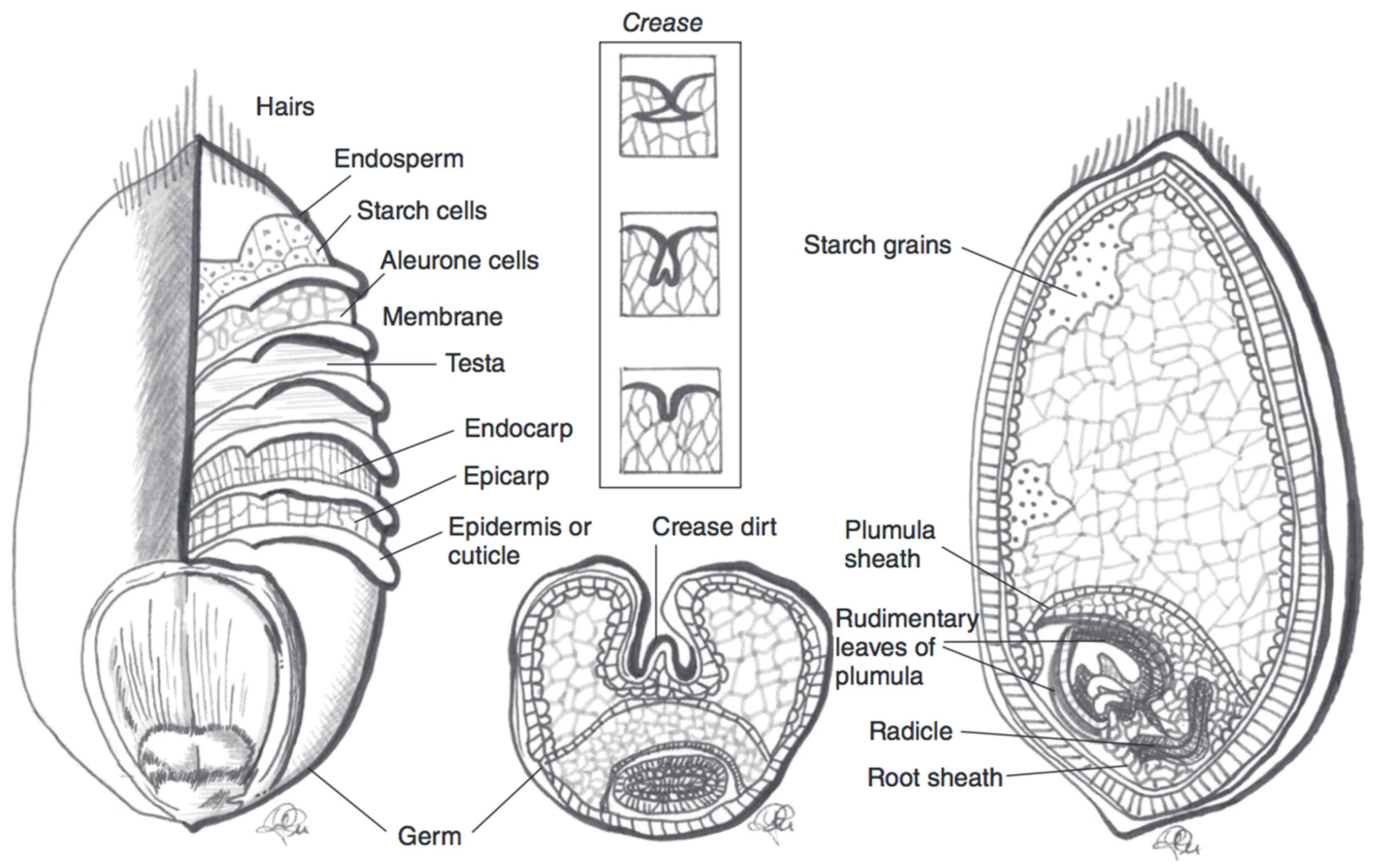
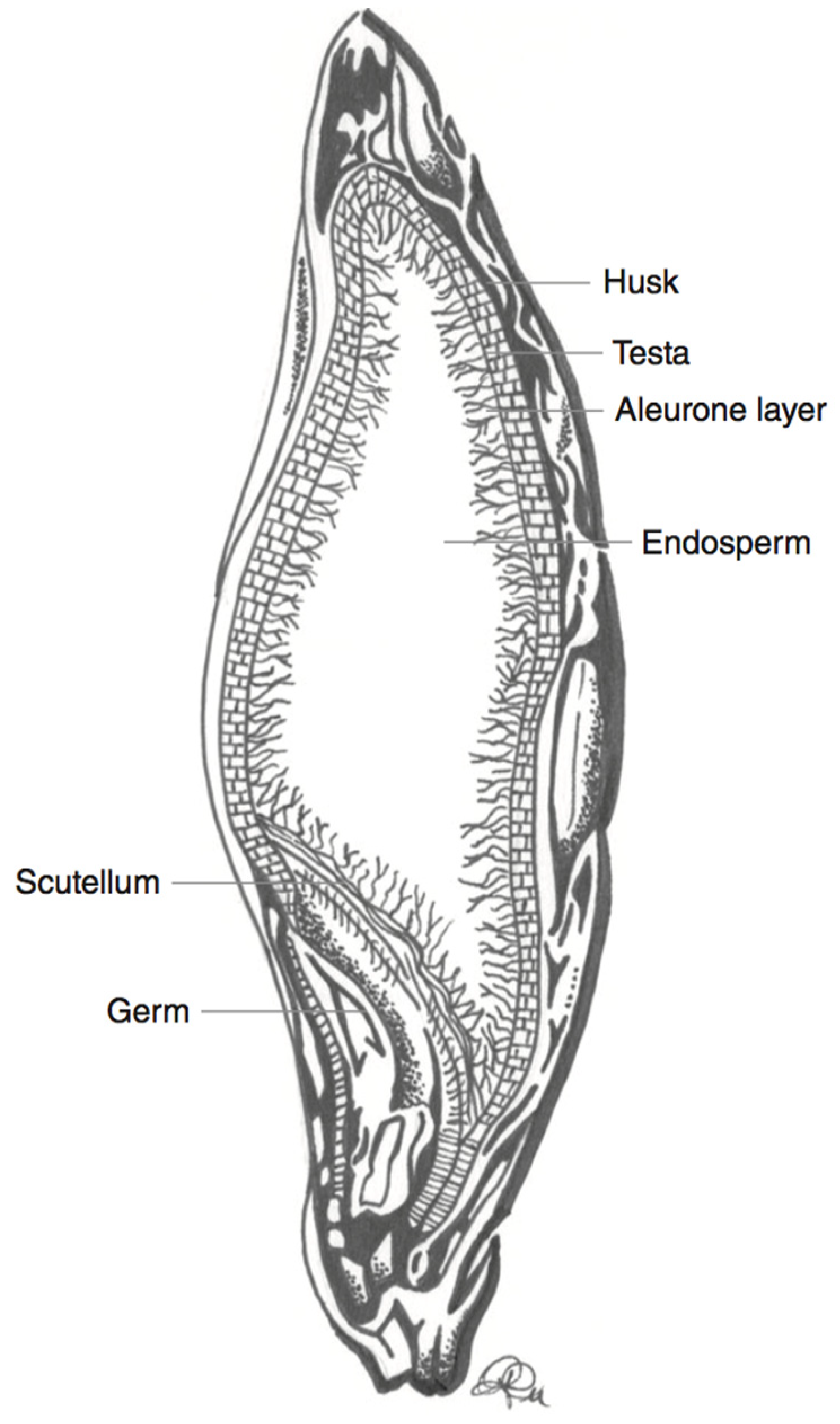
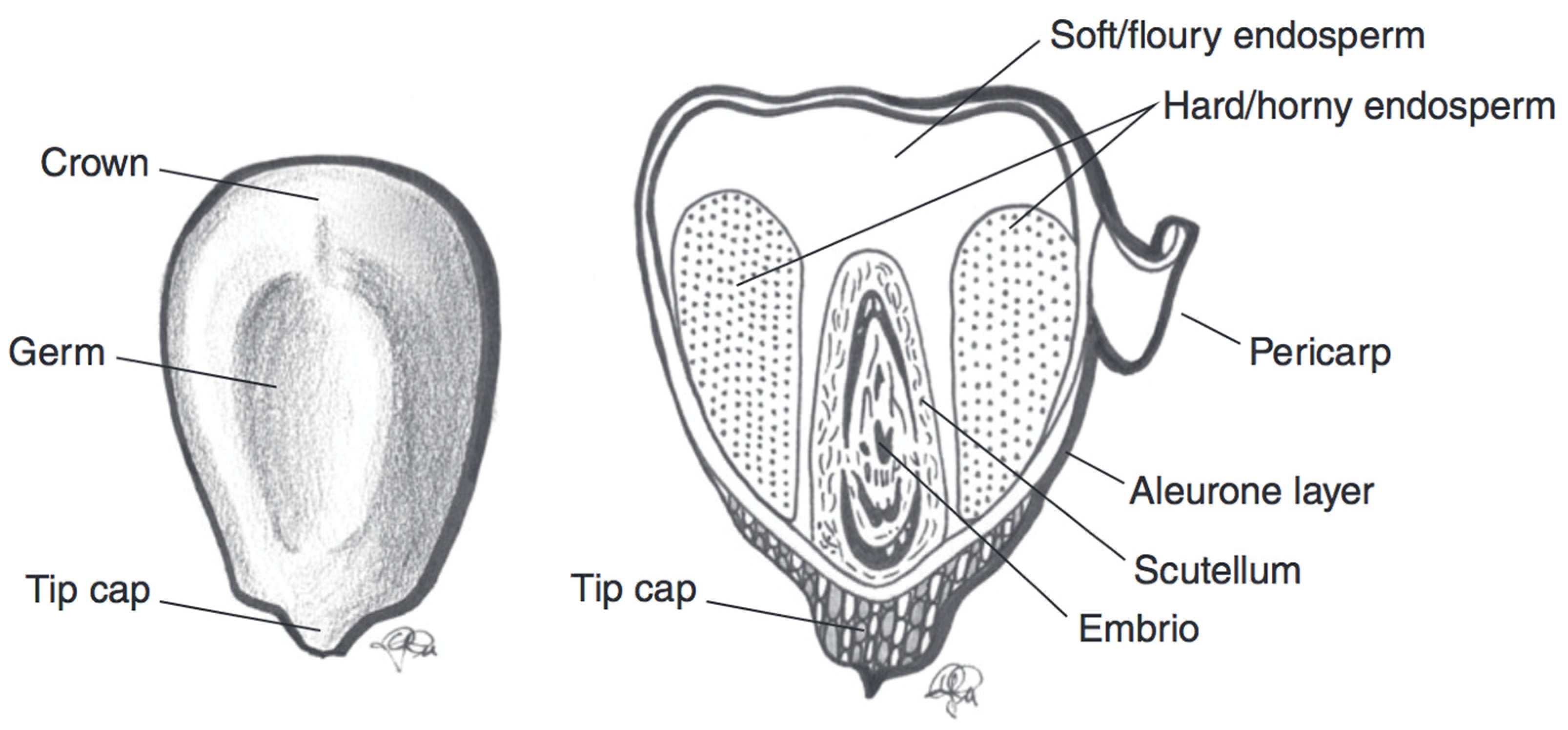
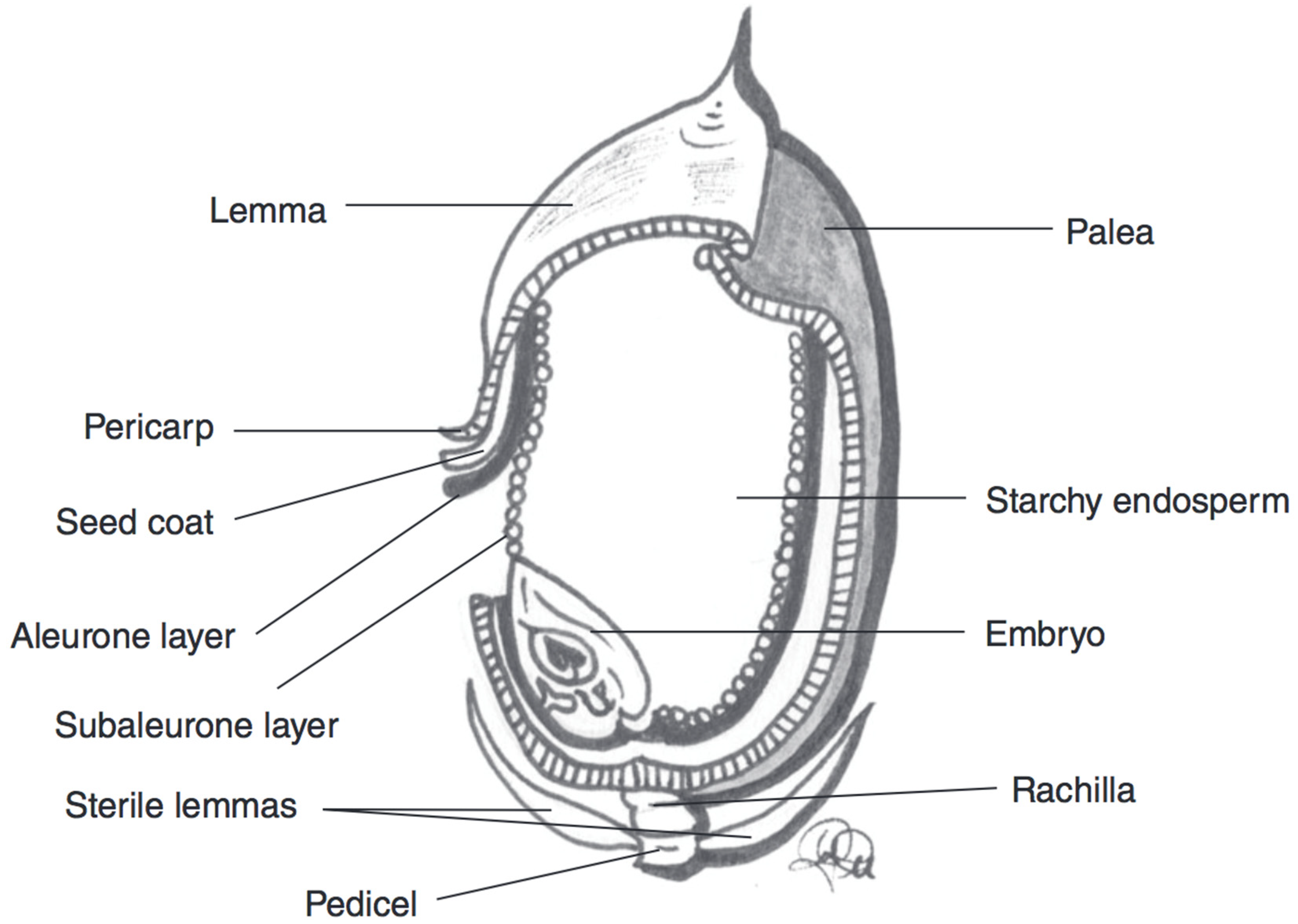
| Wheat Colour | Wheat Variety | TAC (mg/kg Kernel Weight) | TAC Measurement | Antioxidant Potential | Total Phenolic Content |
|---|---|---|---|---|---|
| Purple | Laval [28] | 96 ± 5 | Spectrophotometer | n.d. | n.d. |
| Purple | Laval 19 [31] | 10 | pH Differential | n.d. | n.d. |
| Purple | Charcoal [31] | 305 | pH Differential | n.d. | n.d. |
| Purple | Indigo [24] | 72 ± 1 | Spectrophotometer | 3959 µmol TE/100 g (ORAC) | 160 µmol FAE/100 g |
| Blue | Purendo [37] | 212 ± 3 | Spectrophotometer | n.d. | n.d. |
| Blue | Purendo [28] | 18 ± 4 | Spectrophotometer | n.d. | n.d |
| Blue | BVAL-214025 [38] | 74 ± 2 | Spectrophotometer | 5 mmol Fe2 + equiv/100 g (FRAP) | 163 mg FAE/100 g |
| Black | BW/2 PBW621-F5 [30] | 198 ± 9 | Spectrophotometer | n.d. | n.d. |
| Black | Black Wheat [30] | 186 ± 17 | Spectrophotometer | n.d. | n.d. |
| Black | UC 66049/Konini [31] | 56 | pH Differential | n.d. | n.d. |
| Barley Colour | Barley Variety | TAC (mg/kg Kernel Weight) | TAC Measurement | Antioxidant Potential | Total Phenolic Content |
|---|---|---|---|---|---|
| Purple | CI-1248 [39] | 573 ± 26 | HPLC | 3937 µmol TE/100 g (ORAC) | n.d. |
| Purple | Yu 5904-088 [40] | 679 ± 19 | Spectrophotometer | 78% (DPPH) | n.d. |
| Blue | Kompolti korai 1 [41] | 84 ± 3 | Spectrophotometer | n.d. | n.d. |
| Blue | Ubamer [40] | 77 ± 2 | Spectrophotometer | 35% (DPPH) | n.d. |
| Blue | Tankard [28] | 345 ± 1 | Spectrophotometer | n.d. | n.d. |
| Black | Peru-35 [39] | 0 | HPLC | 5430 µmol TE/100 g (ORAC) | n.d. |
| Black | Black [40] | 8 ± 1 | Spectrophotometer | 29% (DPPH) | n.d. |
| Maize Colour | Maize Variety | TAC (mg/kg Kernel Weight) | TAC Measurement | Antioxidant Potential | Total Phenolic Content |
|---|---|---|---|---|---|
| Purple | KKU-WX211003 [45] | 89 ± 1 | pH Differential | 14 μmol TE/g DW (DPPH) | 7 mg GAE/g DW |
| Purple | AREQ516540TL [46] | 850 ± 6 | Spectrophotometer | 92% (ABTS) | 3400 mg GAE/100g |
| Purple-black | KKU-WX111031 [45] | 1439 ± 8 | pH Differential | 22 μmol TE/g DW (DPPH) | 20 mg GAE/g DW |
| Purple-yellow | KKU-WX211004 [45] | 27 ± 1 | pH Differential | 12 μmol TE/g DW (DPPH) | 6 mg GAE/g DW |
| Red-yellow | ZPL-5 [47] | 3 ± 0 | HPLC | 23 mmol TE/kg (TEAC) | 6011 GAE/kg DM |
| Dark red | ZPP-1 selfed [47] | 696 ± 3 | HPLC | 27 mmol TE/kg (TEAC) | 6115 GAE/kg DM |
| Light blue | ZPP-2 selfed [47] | 379 ± 5 | HPLC | 36 mmol TE/kg (TEAC) | 10529 GAE/kg DM |
| Blue | Blue [46] | 100 ± 2 | Spectrophotometer | 63% (ABTS) | 343 mg GAE/100 g |
| Black | Black [46] | 77 ± 2 | Spectrophotometer | 60% (ABTS) | 457 mg GAE/100 g |
| Black | Negro normal OLI 04 PV [46] | 120 ± 2 | Spectrophotometer | 85% (ABTS) | 544 mg GAE/100 g |
| Rice Colour | Rice Variety | TAC (mg/kg Kernel Weight) | TAC Measurement | Antioxidant Potential | Total Phenolic Content |
|---|---|---|---|---|---|
| Purple | GPNO 20,175 [49] | 68 ± 29 | Spectrophotometer | 98 µmol TE/g bran (ORAC) | 6 mg GAE/g bran |
| Purple | Hung Hsien Ju PI 16,097 [49] | 151 ± 25 | Spectrophotometer | 174 µmol TE/g bran (ORAC) | 12 mg GAE/g bran |
| Purple | Hung Tsan [49] | 199 ± 36 | Spectrophotometer | 147 µmol TE/g bran (ORAC) | 11 mg GAE/g bran |
| Purple | IAC600 [51] | 4700 | Spectrophotometer | 101 µmol TE/g (ORAC) | 6 mg GAE/g |
| Red | SB7 [50] | 0 | pH Differential | 75 µmol TE/g (ORAC) | 1 mg GAE/g |
| Red | Red [28] | 94 ± 1 | Spectrophotometer | n.d. | n.d. |
| Black | Asamurasaki [52] | 1400 ± 41 | HPLC | n.d. | n.d. |
| Black | Asamurasaki [53] | 474 | HPLC | 65 mmol TE/g DW (ORAC) | n.d. |
| Black | Okunomurasaki [53] | 80 | HPLC | 55 mmol TE/g DW (ORAC) | n.d. |
| Black | Longjin 01 [54] | 5101 ± 79 | pH Differential | 1138 mmol TE/g DW (ORAC) | 4766 mg GAE/100 g DW |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francavilla, A.; Joye, I.J. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922. https://doi.org/10.3390/nu12102922
Francavilla A, Joye IJ. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients. 2020; 12(10):2922. https://doi.org/10.3390/nu12102922
Chicago/Turabian StyleFrancavilla, Alyssa, and Iris J. Joye. 2020. "Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health" Nutrients 12, no. 10: 2922. https://doi.org/10.3390/nu12102922
APA StyleFrancavilla, A., & Joye, I. J. (2020). Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients, 12(10), 2922. https://doi.org/10.3390/nu12102922




