Effect of a Supplementation with Two Quelites on Urinary Excretion of Arsenic in Adolescents Exposed to Water Contaminated with the Metalloid in a Community in the State of Guanajuato, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipments
2.2. Intervention Design, Study Groups, and Acquisition of Data
2.2.1. Eligibility Criteria
2.2.2. Pre-Selection Phase and Ethical Statement
2.2.3. Selection Phase and Clinical Intervention
2.3. Supplement of Quelites
2.4. Dietary Supplementation
2.5. Urine Sample
2.6. Anthropometric Assessment
2.7. Dietary Assessment
2.8. Hemoglobin Concentration
2.9. Urine Malondialdehyde Concentration
2.10. Concentrations of Arsenic in Drinking Water and Urine
2.10.1. Water
2.10.2. Urine
2.11. Statistical Analysis
3. Results
3.1. Study Population
3.2. Diet
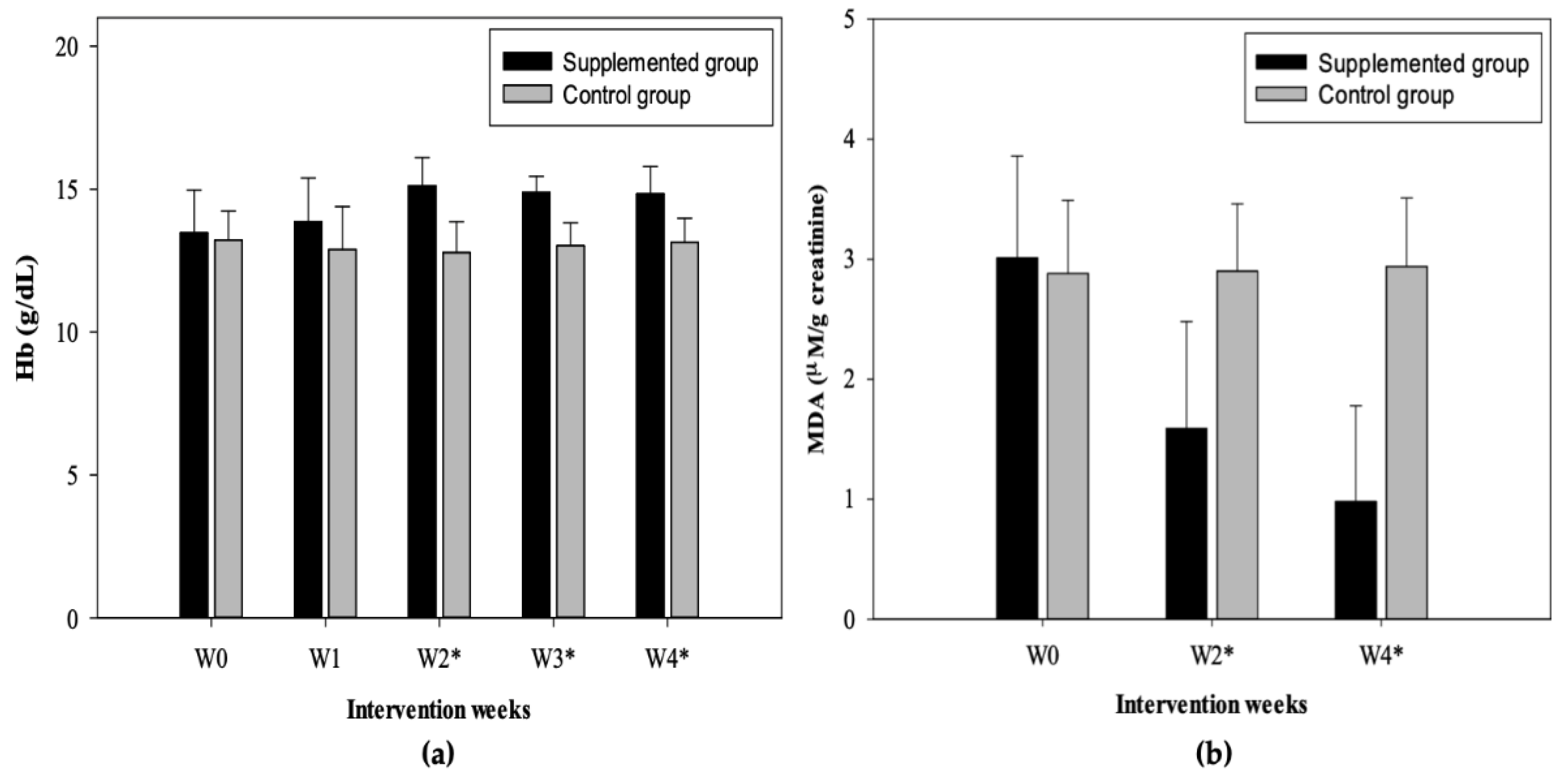
3.3. Hb Concentration
3.4. MDA Concentration
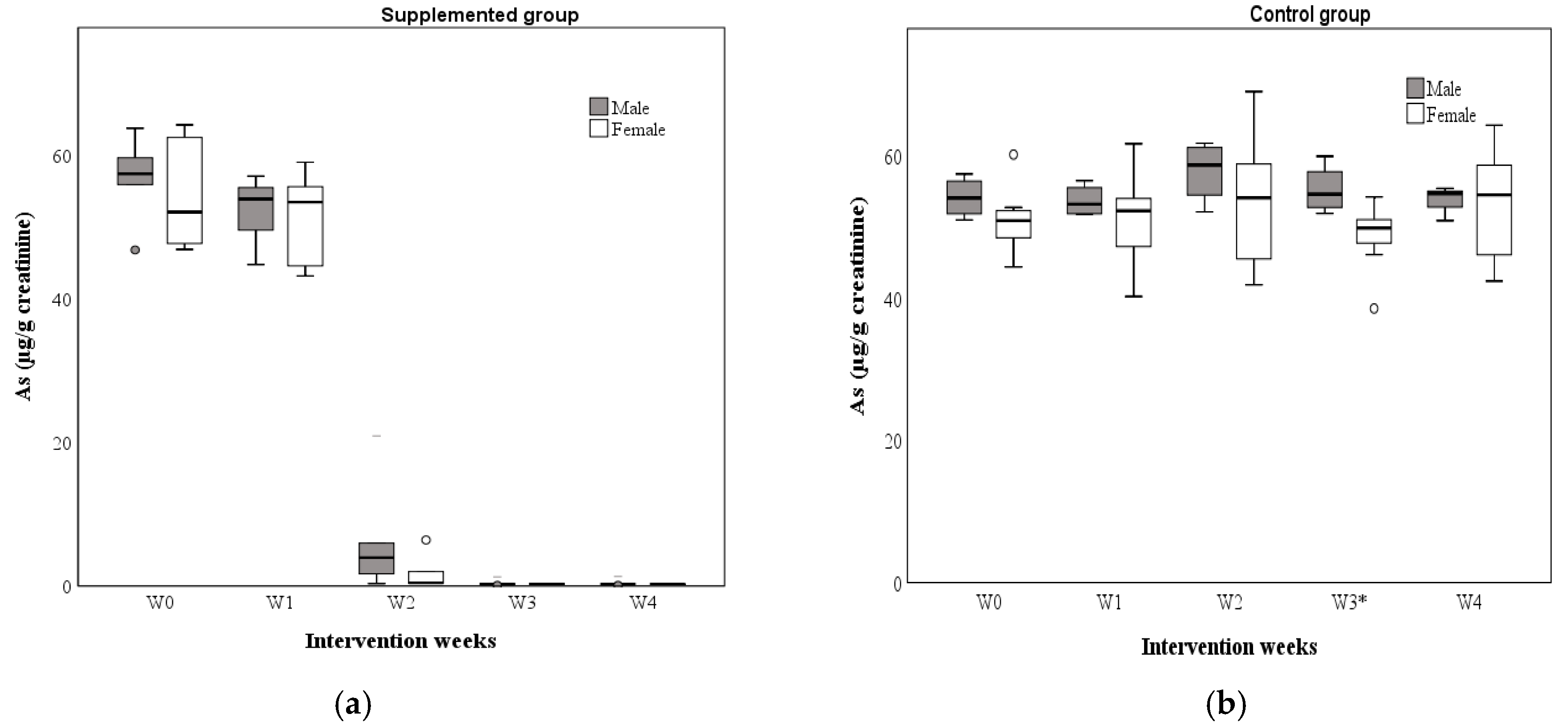
3.5. As Concentrations in Water and Urine
3.6. NNT
4. Discussion
4.1. As in Drinking Water
4.2. Hb, UMDA, and UAs
4.2.1. Hb Concentration
4.2.2. MDA Concentration
4.2.3. UAs Concentration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orteaga-Guerrero, M.A. Presencia, distribución, hidrogeoquímica y origen de arsénico, fluoruro y otros elementos traza disueltos en agua subterránea, a escala de cuenca hidrológica tributaria de Lerma-Chapala, México. Rev. Mex. Cienc. Geol. 2009, 26, 143–161. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Arsenic. ATSDR: U.S. Department of Health and Human Services Public. Health Service. 2007. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3 (accessed on 15 September 2019).
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Van Halem, D.; Bakker, S.A.; Amy, G.L.; Van Dijk, J.C. Arsenic in drinking water: Not just a problem for Bangladesh. Drink. Water Eng. Sci. Discuss. 2009, 2, 51–64. [Google Scholar] [CrossRef]
- Sepúlveda-Saa, R. El arsénico en la contaminación de aguas subterráneas. Ciencia UANL 2009, 12, 239–244. [Google Scholar]
- Méndez, M.; Armienta, M.A. Arsenic phase distribution in Zimapán mine tailings, Mexico. Geofísica Int. 2003, 42, 131–140. [Google Scholar]
- Arreguín-Cortés, F.I.; Chávez-Guillén, R.; Soto-Navarro, P.R. Una Revisión de la Presencia de Arsénico en el Agua Subterránea en México; Comisión Nacional del Agua (CONAGUA), Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT): Ciudad de México, México, 2013. [Google Scholar]
- Monroy-Torres, R.; Macías, A.E.; Gallaga-Solorzano, J.C.; Santiago-García, E.J.; Hernández, I. Arsenic in mexican children exposed to contaminated well water. Ecol. Food Nutr. 2009, 48, 59–75. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Arsenic. WHO, 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 15 September 2019).
- Chávez-Capilla, T.; Beshai, M.; Maher, W.; Kelly, T.; Foster, S. Bioaccessibility and degradation of naturally occurring arsenic species from food in the human gastrointestinal tract. Food Chem. 2016, 212, 189–197. [Google Scholar] [CrossRef]
- Cubadda, F.; Jackson, B.; Cottingham, K.L.; Ornelas-Van Horne, Y.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci. Total Environ. 2016, 579, 1228–1239. [Google Scholar] [CrossRef]
- Denny, A.; Buttriss, J. Plant Foods and Health: Focus on Plant Bioactives; European Food Information Resource (EuroFIR): Norwich, UK, 2007. [Google Scholar]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In Vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Nandi, D.; Patra, R.C.; Swarup, D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology 2005, 211, 26–35. [Google Scholar] [CrossRef]
- Nandi, D.; Patra, R.C.; Swarup, D. Oxidative stress indices and plasma biochemical parameters during oral exposure to arsenic in rats. Food Chem. Toxicol. 2006, 44, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Fakoya, A. Free radical scavenging and antigenotoxic activities of natural phenolic compounds in dried flowers of Hibiscus saidariffa L. Mol. Nutr. Food Res. 2005, 49, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Sahu, R.; Dua, T.K.; Bag, S.; Gangopadhyay, M.; Sinha, M.K.; Dewanjee, S. Arsenic-induced myocardial injury: Protective role of Corchorus olitorius leaves. Food Chem. Toxicol. 2010, 48, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Alamolhodaei, N.S.; Shirani, K.; Karimi, G. Arsenic cardiotoxicity: An overview. Environ. Toxicol. Pharmacol. 2015, 40, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Spratlen, M.J.; Gamble, M.V.; Grau-Perez, M.; Kuo, C.C.; Best, L.G.; Yracheta, J.; Francesconi, K.; Goessler, W.; Mossavar-Rahmani, Y.; Hall, M.; et al. Arsenic metabolism and one-carbon metabolism at low-moderate arsenic exposure: Evidence from the strong heart study. Food Chem. Toxicol. 2017, 105, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Linares, M.E.; Bye, R. The under-utilized species of the milpa. Rev. Digit. Univ. 2015, 16, 22. [Google Scholar]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; Alatorre-Cruz, J.M.; Monroy-Torres, R. Nutritional importance and biological activity of bioactive compounds from quelites consumed in Mexico. Rev. Chil. Nutr. 2019, 46, 593–605. [Google Scholar] [CrossRef]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; Monroy-Torres, R.; Cariño-Cortés, R.; Jiménez-Alvarado, R. Physicochemical, nutritional and antioxidant characterization of three vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as potential sources of phytochemicals and bioactive compounds. J. Food Meas. Charact. 2018, 12, 2855–2864. [Google Scholar] [CrossRef]
- Kokanova-Nedialkova, Z.; Nedialkov, P.; Kondeva-Burdina, M.; Simeonova, R.; Tzankova, V.; Aluani, D. Chenopodium bonus-henricus L.—A source of hepatoprotective flavonoids. Fitoterapia 2017, 118, 13–20. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Swieca, M.; Sułkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts—In Vitro study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar] [CrossRef]
- Wanyin, W.; Liwei, D.; Lin, J.; Hailiang, X.; Changquan, L.; Min, L. Ethanol extract of Portulaca oleracea L. protects against hypoxia-induced neurodamage through modulating endogenous erythropoietin expression. J. Nutr. Biochem. 2012, 23, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zhou, Q.; Gong, W.; Wang, Y.; Nie, Z.; He, H.; Li, J.; Wu, J.; Wu, C.; Zhang, J. Studies on the antioxidant and hepatoprotective activities of polysaccharides from Talinum triangulare. J. Ethnopharmacol. 2011, 136, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel-Moneim, A.E. Protective effects of Portulaca oleracea against rotenone mediated depletion of glutathione in the striatum of rats as an animal model of Parkinson’s disease. Pest. Biochem. Physiol. 2012, 103, 108–114. [Google Scholar] [CrossRef]
- Monroy-Torres, R.; Espinoza-Pérez, J.A.; Ramírez-Gómez, X.; Carrizalez-Yañez, L.; Linares-Segovia, B.; Mejía-Saavedra, J.J. Efecto de una suplementación multivitamínica de cuatro semanas sobre el estado nutricio y excreción urinaria de arsénico en adolescentes. Nutr. Hosp. 2018, 35, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana (NOM-127-SSA1-1994). Salud ambiental. Agua para uso y consumo humano. Límites permisibles de calidad y tratamientos a que debe someterse el agua para su potabilización. Norma Oficial Mexicana NOM 1994; SSA, 2000. Available online: http://www.salud.gob.mx/unidades/cdi/nom/127ssa14.html (accessed on 17 September 2019).
- Santiago-Saenz, Y.O.; López-Palestina, C.U.; Gutiérrez-Tlahque, J.; Monroy-Torres, R.; Pinedo-Espinoza, J.M.; Hernández-Fuentes, A.D. Nutritional and functional evaluation of three powder mixtures based on Mexican quelites: Alternative ingredients to formulate food supplements. Food Sci. Technol. (Campinas) 2020, in press. [Google Scholar]
- Grossman, C.M. The effect of amino acids on serum and urine creatine. J. Clin. Investig. 1945, 24, 380–383. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Organización Mundial de la Salud (OMS). Capacitación Sobre la Evaluación del Crecimiento del Niño. OMS, 2008. Available online: http://www.who.int/childgrowth/training/c_interpretando.pdf (accessed on 15 September 2019).
- Shamah-Levy, T.; Villalpando-Hernández, S.; Rivera-Dommarco, J. Manual de Procedimientos Para Proyectos de Nutrición; Instituto Nacional de Salud Pública: Cuernavaca, México, 2006. [Google Scholar]
- Cox, D.H. Arsine evolution-electrothermal atomic absorption method for the determination of nanogram levels of total arsenic in urine and water. J. Anal. Toxicol. 1980, 4, 207–211. [Google Scholar] [CrossRef]
- National Academies (NAP). Dietary Reference Intakes Tables and Application. Dietary Reference Intakes: Macronutrients, Vitamins and Elements. NAP, 2019. Available online: http://nationalacademies.org/hmd/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx (accessed on 15 September 2019).
- Meza-Lozano, B.; Ortíz-Pérez, M.D.; Ponce-Palomares, M.; Castillo-Gutiérrez, S.G.; Flores-Ramírez, R.; Cubillas-Tejeda, A.C. Implementación y evaluación de un programa de comunicación de riesgos por exposición a flúor en la comunidad de el fuerte, Santa María del Río, San Luís Potosí, México. Rev. Int. Contam. Ambie. 2016, 32, 87–100. [Google Scholar]
- Organización Mundial de la Salud (OMS). Concentraciones de Hemoglobina Para Diagnosticar la Anemia y Evaluar su Gravedad. OMS, 2011. Available online: https://www.who.int/vmnis/indicators/haemoglobin_es.pdf (accessed on 15 September 2019).
- Egbi, G.; Gbogbo, S.; Mensah, G.; Glover-Amengor, M.; Steiner-Asiedu, M. Effect of green leafy vegetables powder on anaemia and vitamin-A status of Ghanaian school children. BMC Nutr. 2018, 4, 27. [Google Scholar] [CrossRef]
- You, C.S.; Parker, R.S.; Swanson, J.E. Bioavailability and vitamin A value of carotenes from red palm oil assessed by an intrinsic isotope reference method. Asia Pac. J. Clin. Nutr. 2002, 11, S438–S442. [Google Scholar] [CrossRef]
- Wang, J.P.; Maddalena, R.; Zheng, B.; Zai, C.; Liu, F.; Ng, J.C. Arsenicosis status and urinary malondialdehyde (MDA) in people exposed to arsenic contaminated-coal in China. Environ. Int. 2009, 35, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Bhadauria, S.; Kannan, G.M.; Singh, N. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: A review. J. Environ. Biol. 2007, 28, 333–347. [Google Scholar] [PubMed]
- Khuda-Bukhsh, A.R.; Pathak, S.; Guha, B.; Karmakar, S.R.; Das, J.K.; Banerjee, P.; Biswas, S.J.; Mukherjee, P.; Bhattacharjee, N.; Choudhury, S.C.; et al. Can homeopathic arsenic remedy combat arsenic poisoning in humans exposed to groundwater arsenic contamination? A preliminary report on first human trial. eCAM 2005, 2, 537–548. [Google Scholar] [CrossRef]
- Pace, C.; Dagda, R.; Angermann, J. Antioxidants protect against arsenic induced mitochondrial cardio toxicity. Toxics 2017, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Emergency Preparedness and Response: Arsenic. CDC, 2018. Available online: https://emergency.cdc.gov/agent/arsenic/casedef.asp (accessed on 15 September 2019).
- Calderon, R.L.; Hudgens, E.; Le, X.C.; Schreinemachers, D.; Thomas, D.J. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ. Health Perspect. 1999, 107, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Skröder, H.L.; Kippler, M.; Lu, Y.; Ahmed, S.; Kuehnelt, D.; Raqib, R.; Vahter, M. Arsenic metabolism in children differs from that in adults. Toxicol. Sci. 2016, 152, 29–39. [Google Scholar] [CrossRef]
- Vahter, M.E. Interactions between arsenic-induced toxicity and nutrition in early life. J. Nutr. 2007, 137, 2798–2804. [Google Scholar] [CrossRef]
- Queen-Samour, P.; King-Helm, K. Handbook of Pediatric Nutrition; Jones & Bartlett: Sudbury, MA, USA, 2005. [Google Scholar]
- Kumar, U.C.; Mahmudur, M.R.; Kumar, M.S.; Lodh, D.; Ranjan, C.C.; Roy, S.; Quamruzzaman, Q.; Tokunaga, H.; Ando, M.; Chakraborti1, D. Pattern of excretion of arsenic compounds [Arsenite, Arsenate, MMA (V), DMA (V)] in urine of children compared to adults from an arsenic exposed area in Bangladesh. J. Environ. Sci. Health Part A 2003, 38, 87–113. [Google Scholar] [CrossRef]
- Kile, M.; Hoffman, E.; Hsueh, Y.M.; Afroz, S.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Ryan, L.; Christiani, D. Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environ. Health Perspect. 2009, 117, 455–460. [Google Scholar] [CrossRef]
- Kurzius-Spencer, M.; da Silva, V.; Thomson, C.A.; Hartz, V.; Hsu, C.H.; Burgess, J.L.; O’Rourke, M.K.; Harris, R.B. Nutrients in one-carbon metabolism and urinary arsenic methylation in the National Health and Nutrition Examination Survey (NHANES) 2003–2004. Sci Total Environ. 2017, 607, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Torres, R.; Espinoza-Pérez, J.A.; Pérez-González, R.M. Evaluación de las prácticas de alimentación y nutrición en una población expuesta a arsénico: Una propuesta para integrar indicadores de exposición nutricional. Nutrición Clínica y Dietética Hospitalaria 2016, 36, 140–149. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; García, O.P.; Rosado, J.L.; Goñi, I. The contribution of fruits and vegetables to dietary intake of polyphenols and antioxidant capacity in a Mexican rural diet: Importance of fruit and vegetable variety. Food Res. Int. 2011, 44, 1182–1189. [Google Scholar] [CrossRef]
- López-Carrillo, L.; Gamboa-Loira, B.; Becerra, W.; Hernández-Alcaraz, C.; Hernández-Ramírez, R.U.; Jay-Gandolfi, A.; Franco-Marina, F.; Cebrián, M.E. Dietary micronutrient intake and its relationship with arsenic metabolism in Mexican women. Environ. Res. 2016, 151, 445–450. [Google Scholar] [CrossRef]
- Mitra, S.R.; Guha-Mazumder, D.N.; Basu, A.; Block, G.; Haque, R.; Samanta, S.; Ghosh, N.; Hira-Smith, M.M.; Von Ehrenstein, O.S.; Smith, A.H. Nutritional factors and susceptibility to arsenic-caused skin lesions in west Bengal, India. Environ. Health Perspect. 2004, 112, 1104–1109. [Google Scholar] [CrossRef]
- Misbahuddin, M.; Islam, A.Z.; Khandker, S.; Al-Mahmud, I.; Islam, N.; Anjumanara. Efficacy of spirulina extract plus zinc in patients of chronic arsenic poisoning: A randomized placebo-controlled study. Clin. Toxicol. (Phila) 2006, 44, 135–141. [Google Scholar] [CrossRef]
- Pilsner, J.R.; Hall, M.N.; Liu, X.; Ahsan, H.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Graziano, J.H.; Gamble, M.V. Associations of plasma selenium with arsenic and genomic methylation of leukocyte DNA in Bangladesh. Environ. Health Perspect. 2011, 119, 113–118. [Google Scholar] [CrossRef]
- Berry, J.P.; Galle, P. Selenium-arsenic interaction in renal cells: Role of lysosomes. Electron microprobe study. J. Submicrosc. Cytol. Pathol. 1994, 26, 203–210. [Google Scholar]
- Clemente, M.J.; Devesa, V.; Velez, D. In vitro reduction of arsenic bioavailability using dietary strategies. J. Agric. Food Chem. 2017, 65, 3956–3964. [Google Scholar] [CrossRef]
- Sun, G.X.; Van de Wiele, T.; Alava, P.; Tack, F.; Du Laing, G. Arsenic in cooked rice: Effect of chemical, enzymatic and microbial processes on bioaccessibility and speciation in the human gastrointestinal tract. Environ. Pollut. 2012, 162, 241–246. [Google Scholar] [CrossRef]
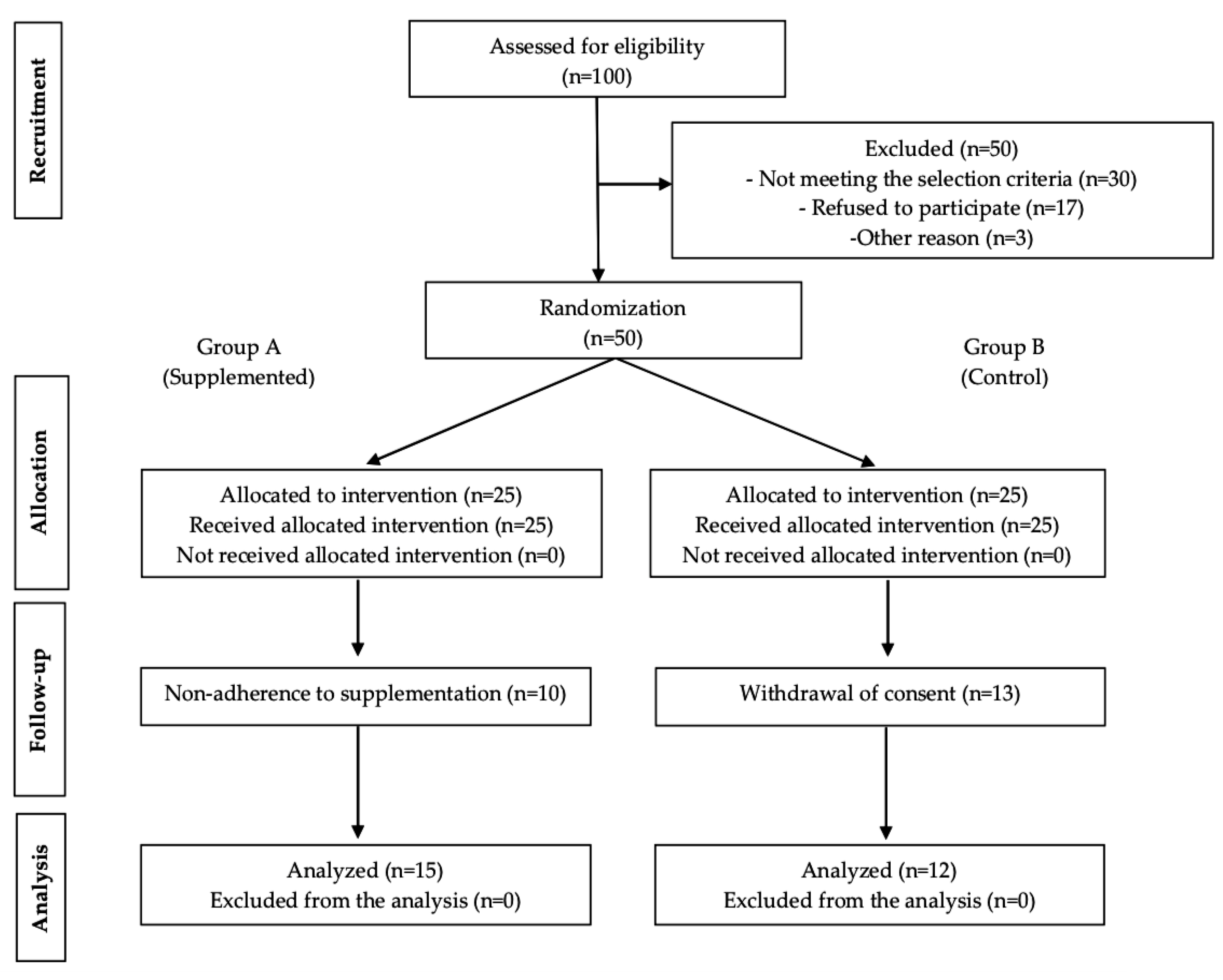

| Baseline Variables | Supplemented n = 15 | Control n = 12 | p-Value |
|---|---|---|---|
| Age (years) | 11.80 ± 0.41 | 12 ± 0.36 | 3 NS |
| Weight (kg) | 47.31 ± 10.54 | 48.95 ± 11.33 | NS |
| Height (cm) | 150.31 ± 6.74 | 153.30 ± 7.21 | NS |
| 1 H/A interpretation (Z-Score) | NS | ||
| Normal height | 15 ± 82.19 | 12 ± 91.42 | |
| 2 BMI interpretation (Z-Score) | NS | ||
| Obesity | 2 ± 10.14 | 2 ± 16.05 | |
| Overweight | 5 ± 33.67 | 3 ± 19.36 | |
| Normal weight | 8 ± 31.94 | 7 ± 35.02 | |
| Fat mass (%) | 21.92 ± 14.71 | 23.02 ± 14.07 | NS |
| Muscular mass (%) | 33.46 ± 11.08 | 32.12 ± 8.95 | NS |
| Waist circumference (cm) | 71.19 ± 12.05 | 71.48 ± 9.44 | NS |
| Abdominal circumference (cm) | 76.28 ± 11.58 | 79.33 ± 10.27 | NS |
| Baseline Variables | Total Population n = 27 | Men n = 13 | Women n = 14 | p-Value |
|---|---|---|---|---|
| Age (years) | 11.88 ± 0.32 | 11.84 ± 0.37 | 11.92 ± 0.26 | 3 NS |
| Weight (kg) | 48.13 ± 10.72 | 48.07 ± 10.89 | 48.19 ± 10.98 | NS |
| Height (cm) | 151.78 ± 7.01 | 152.56 ± 7.28 | 151.00 ± 6.64 | NS |
| 1 H/A interpretation (Z-Score) | NS | |||
| Normal height | 27 ± 98.42 | 13 ± 86.24 | 14 ± 93.37 | |
| 2 BMI interpretation (Z-Score) | NS | |||
| Obesity | 4 ± 31.26 | 1 ± 9.32 | 3 ± 17.21 | |
| Overweight | 8 ± 35.74 | 5 ± 35.11 | 3 ± 21.17 | |
| Normal weight | 15 ± 42.31 | 8 ± 32.29 | 7 ± 35.18 | |
| Fat mass (%) | 22.46 ± 14.16 | 13.98 ± 12.64 | 30.32 ± 10.74 | <0.001 |
| Muscular mass (%) | 32.80 ± 10.03 | 40.47 ± 9.25 | 25.67 ± 2.93 | <0.001 |
| Waist circumference (cm) | 71.34 ± 10.77 | 71.20 ± 11.77 | 71.47 ± 10.20 | NS |
| Abdominal circumference (cm) | 77.81 ± 10.96 | 75.62 ± 10.90 | 79.84 ± 11.01 | NS |
| Nutrients | Supplemented n = 15 | Control n = 12 | p-Value |
|---|---|---|---|
| Energy (kcal) | 2006.04 ± 5.07 | 2000.12 ± 3.49 | <0.002 |
| Protein (g) | 70.05 ± 27.52 | 69.96 ± 27.96 | 1 NS |
| Fat (g) | 70.02 ± 33.71 | 66.15 ± 29.66 | NS |
| Cholesterol (mg) | 284.21 ± 169.46 | 282.59 ± 180.64 | NS |
| Carbohydrates (g) | 290.38 ± 96.12 | 273.41 ± 51.34 | NS |
| Sugar (g) | 32.36 ± 31.67 | 30.02 ± 36.32 | NS |
| Fiber (g) | 21.09 ± 12.20 | 20.17 ± 8.91 | NS |
| Vitamin A-Retinol (µg) | 767.04 ± 182.09 | 753.62 ± 173.48 | NS |
| Vitamin B1(mg) | 1.15 ± 0.50 | 1.09 ± 0.38 | NS |
| Vitamin B2 (mg) | 1.23 ± 0.57 | 1.21 ± 0.45 | NS |
| Vitamin B6 (mg) | 0.94 ± 0.55 | 0.81 ± 0.46 | NS |
| Vitamin B12 (µg) | 1.93 ± 1.12 | 1.91 ± 1.27 | NS |
| Vitamin C (mg) | 52.47 ± 45.74 | 34.03 ± 29.79 | NS |
| Folic acid (µg) | 186.39 ± 174.18 | 136.25 ± 88.08 | NS |
| Niacin (mg) | 12.28 ± 6.60 | 9.08 ± 5.67 | NS |
| Vitamin E (mg) | 2.67 ± 2.01 | 1.97 ± 3.73 | NS |
| Ca (mg) | 947.59 ± 361.72 | 937.66 ± 363.81 | NS |
| Fe (mg) | 14.82 ± 7.30 | 13.29 ± 4.43 | NS |
| K (mg) | 1605.14 ± 654.99 | 1094.35 ± 566.09 | <0.05 |
| Mg (mg) | 303.39 ± 220.35 | 240.26 ± 184.69 | NS |
| Na (mg) | 2501.90 ± 811.04 | 1949.78 ± 721.42 | NS |
| P (mg) | 631.40 ± 304.63 | 595.08 ± 264.43 | NS |
| Se (µg) | 51.95 ± 30.00 | 50.06 ± 23.06 | NS |
| Zn (mg) | 4.68 ± 2.60 | 4.26 ± 2.00 | NS |
| Nutrients | Total Population n = 27 | Men n = 13 | Women n = 14 | p-Value |
|---|---|---|---|---|
| Energy (kcal) | 2003.07 ± 1.24 | 2345.11 ± 1.35 | 1490 ± 1.11 | <0.001 |
| Protein (g) | 70.24 ± 32.01 | 87.84 ± 27.96 | 43.85 ± 19.88 | <0.001 |
| Fat (g) | 68.19 ± 29.36 | 81.03 ± 26.83 | 48.93 ± 8.62 | <0.001 |
| Cholesterol (mg) | 283.63 ± 127.38 | 398.28 ± 142.17 | 111.67 ± 101.48 | <0.001 |
| Carbohydrates (g) | 281.85 ± 82.19 | 321.01 ± 72.98 | 223.13 ± 27.13 | <0.001 |
| Sugar (g) | 31.07 ± 29.70 | 38.01 ± 36.21 | 20.65 ± 14.98 | 1 NS |
| Fiber (g) | 20.64 ± 8.56 | 22.81 ± 10.68 | 17.39 ± 3.81 | NS |
| Vitamin A-Retinol (µg) | 759.60 ± 128.11 | 877.67 ± 143.62 | 582.50 ± 105.69 | <0.001 |
| Vitamin B1(mg) | 1.13 ± 0.54 | 1.41 ± 0.52 | 0.69 ± 0.11 | <0.001 |
| Vitamin B2 (mg) | 1.22 ± 0.65 | 1.60 ± 0.55 | 0.64 ± 0.30 | <0.001 |
| Vitamin B6 (mg) | 0.87 ± 0.56 | 1.11 ± 0.50 | 0.50 ± 0.34 | <0.001 |
| Vitamin B12 (µg) | 1.92 ± 1.10 | 2.46 ± 1.01 | 1.11 ± 0.88 | <0.001 |
| Vitamin C (mg) | 43.30 ± 44.13 | 52.41 ± 50.86 | 29.65 ± 11.81 | NS |
| Folic acid (µg) | 161.33 ± 198.05 | 235.89 ± 219.80 | 49.49 ± 21.16 | <0.002 |
| Niacin (mg) | 10.69 ± 6.75 | 13.97 ± 6.61 | 5.76 ± 1.50 | <0.001 |
| Vitamin E (mg) | 2.33 ± 1.80 | 2.67 ± 1.80 | 1.82 ± 1.87 | NS |
| Ca (mg) | 942.63 ± 270.27 | 1133.50 ± 332.51 | 656.33 ± 255.79 | <0.001 |
| Fe (mg) | 14.10 ± 9.59 | 18.31 ± 9.57 | 7.79 ± 2.49 | <0.001 |
| K (mg) | 1349.47 ± 436.12 | 1597.94 ± 445.58 | 976.75 ± 407.24 | <0.001 |
| Mg (mg) | 271.83 ± 222.07 | 328.83 ± 238.09 | 186.33 ± 164.75 | NS |
| Na (mg) | 2226.77 ± 658.33 | 2993.61 ± 776.12 | 1076.50 ± 501.16 | <0.001 |
| P (mg) | 613.67 ± 307.02 | 754 ± 220.09 | 403.17 ± 225.73 | <0.001 |
| Se (µg) | 51.33 ± 29.33 | 67.56 ± 19.44 | 27 ± 14.33 | <0.001 |
| Zn (mg) | 4.46 ± 2.94 | 5.99 ± 2.49 | 2.15 ± 1.37 | <0.001 |
| Supplement Total Participants n = 15 | Men n = 9 | Women n = 6 | Control Total Participants n = 12 | Men n = 4 | Women n = 8 | |||
|---|---|---|---|---|---|---|---|---|
| Weeks | Urinary MDA concentrations (µM/g creatinine) | |||||||
| p-value | p-value | |||||||
| Baseline | 3.01 ± 0.85 | 3.26 ± 0.97 | 2.63 ± 0.48 | NS | 2.88 ± 0.61 | 3.44 ± 0.35 | 2.59 ± 0.51 | <0.014 |
| 2 | 1.59 ± 0.89 | 1.84 ± 1.06 | 1.21 ± 0.36 | NS | 2.90 ± 0.56 | 3.47 ± 0.39 | 2.61 ± 0.38 | <0.004 |
| 4 | 0.98 ± 0.80 | 1.13 ± 1.01 | 0.75 ± 0.28 | NS | 2.94 ± 0.57 | 3.51 ± 0.26 | 2.66 ± 0.44 | <0.006 |
| Men Supplemented n = 9 | Control n = 4 | Women Supplemented n = 6 | Control n = 8 | |||
|---|---|---|---|---|---|---|
| Weeks | Urinary MDA concentrations (µM/g creatinine) | |||||
| p-value | p-value | |||||
| Baseline | 3.26 ± 0.97 | 3.44 ± 0.35 | NS | 2.63 ± 0.48 | 2.59 ± 0.51 | NS |
| 2 | 1.84 ± 1.06 | 3.47 ± 0.39 | <0.014 | 1.21 ± 0.36 | 2.61 ± 0.38 | <0.001 |
| 4 | 1.13 ± 1.01 | 3.51 ± 0.26 | <0.001 | 0.75 ± 0.28 | 2.66 ± 0.44 | <0.001 |
| Supplemented Total Participants n = 15 | Men n = 9 | Women n = 6 | Control Total Participants n = 12 | Men n = 4 | Women n = 8 | |||
|---|---|---|---|---|---|---|---|---|
| Weeks | Urinary As concentrations (µg/g creatinine) | |||||||
| p-value | p-value | |||||||
| 0 | 56.85 | 57.42 | 52.09 | NS | 51.34 | 53.98 | 50.81 | NS |
| 1 | 53.60 | 53.90 | 53.48 | NS | 52.57 | 53.13 | 52.19 | NS |
| 2 | 2.02 | 3.95 | 0.45 | NS | 56.49 | 58.62 | 54.02 | NS |
| 3 | 0.29 | 0.27 | 0.30 | NS | 50.97 | 54.53 | 49.76 | <0.05 |
| 4 | 0.29 | 0.27 | 0.30 | NS | 54.60 | 54.78 | 54.45 | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Saenz, Y.O.; Monroy-Torres, R.; Rocha-Amador, D.O.; Hernández-Fuentes, A.D. Effect of a Supplementation with Two Quelites on Urinary Excretion of Arsenic in Adolescents Exposed to Water Contaminated with the Metalloid in a Community in the State of Guanajuato, Mexico. Nutrients 2020, 12, 98. https://doi.org/10.3390/nu12010098
Santiago-Saenz YO, Monroy-Torres R, Rocha-Amador DO, Hernández-Fuentes AD. Effect of a Supplementation with Two Quelites on Urinary Excretion of Arsenic in Adolescents Exposed to Water Contaminated with the Metalloid in a Community in the State of Guanajuato, Mexico. Nutrients. 2020; 12(1):98. https://doi.org/10.3390/nu12010098
Chicago/Turabian StyleSantiago-Saenz, Yair Olovaldo, Rebeca Monroy-Torres, Diana Olivia Rocha-Amador, and Alma Delia Hernández-Fuentes. 2020. "Effect of a Supplementation with Two Quelites on Urinary Excretion of Arsenic in Adolescents Exposed to Water Contaminated with the Metalloid in a Community in the State of Guanajuato, Mexico" Nutrients 12, no. 1: 98. https://doi.org/10.3390/nu12010098
APA StyleSantiago-Saenz, Y. O., Monroy-Torres, R., Rocha-Amador, D. O., & Hernández-Fuentes, A. D. (2020). Effect of a Supplementation with Two Quelites on Urinary Excretion of Arsenic in Adolescents Exposed to Water Contaminated with the Metalloid in a Community in the State of Guanajuato, Mexico. Nutrients, 12(1), 98. https://doi.org/10.3390/nu12010098






