Abstract
Diabetes and frailty are highly prevalent conditions that impact the health status of older adults. Perturbations in protein/amino acid metabolism are associated with both functional impairment and type 2 diabetes mellitus (T2DM). In the present study, we compared the concentrations of a panel of circulating 37 amino acids and derivatives between frail/pre-frail older adults with T2DM and robust non-diabetic controls. Sixty-six functionally impaired older persons aged 70+ with T2DM and 30 age and sex-matched controls were included in the analysis. We applied a partial least squares-discriminant analysis (PLS-DA)-based analytical strategy to characterize the metabotype of study participants. The optimal complexity of the PLS-DA model was found to be two latent variables. The proportion of correct classification was 94.1 ± 1.9% for frail/pre-frail persons with T2DM and 100% for control participants. Functionally impaired older persons with T2DM showed higher levels of 3-methyl histidine, alanine, arginine, glutamic acid, ethanolamine sarcosine, and tryptophan. Control participants had higher levels of ornithine and taurine. These findings indicate that a specific profile of amino acids and derivatives characterizes pre-frail/frail older persons with T2DM. The dissection of these pathways may provide novel insights into the metabolic perturbations involved in the disabling cascade in older persons with T2DM.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a chronic condition frequently occurring in old age [1,2]. T2DM is associated with higher risk of negative outcomes, including disability and mortality [3]. T2DM-related complications are especially prevalent in older adults and account for the increasing costs of T2DM [4]. Frailty defines a geriatric syndrome characterized by reduced ability to cope with life stressors and increased risk of adverse events (e.g., falls, delirium, loss of independence, mortality) [5,6]. T2DM and frailty are intimately related and share common features, including complex pathophysiology and heterogeneous phenotypes [7], that challenge their management [5,8].
Muscle failure, both in its metabolic and functional manifestations, is a hallmark of T2DM and frailty [7,9]. The progressive and generalized loss of muscle mass, strength, and function with age, termed sarcopenia, fuels a self-reinforcing cycle in which structural, metabolic, and endocrine perturbations in muscle exacerbate T2DM-related signs and symptoms [10]. T2DM further promotes the decline in muscle mass and function [11,12] which, in turn, aggravates functional impairment [13].
The central role of muscle wasting in frailty and T2DM may guide the identification of novel biomarkers and possibly new treatment targets for the two conditions [14,15,16]. In this context, circulating amino acids are promising candidates given their sensor-transducer-effector role in systemic metabolism, muscle homeostasis, and physical function [17,18,19,20]. Moreover, amino acids are involved in processes critical to the development and progression of frailty and T2DM, such as inflammation, glucose homeostasis, and redox regulation [21,22,23].
Targeted metabolomics allowed identifying specific amino acid profiles that were associated with insulin resistance and risk of developing T2DM in independent cohorts across US, Europe, and China [24,25,26,27]. A plasma amino acid signature, together with specific circulating lipid species, was linked to glucose dyshomeostasis and impaired insulin sensitivity in older adults from the Baltimore Longitudinal Study of Aging (BLSA) [28]. In addition, distinct patterns of circulating amino acids were associated with frailty and/or muscle-related parameters (mass, turnover, performance) in older individuals at risk for frailty [29,30,31,32]. Finally, within the “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (BIOSPHERE) study, a combination of serum amino acids and derivatives was identified that characterized the metabotype of older adults with physical frailty and sarcopenia (PF&S) [17].
Here, we sought to define the circulating amino acid profile of frail/pre-frail older adults with T2DM (F-T2DM). Our approach, described in the context of the “Metabolic biomarkers of frailty in older people with type 2 diabetes mellitus” (MetaboFrail) study, coupled targeted metabolomics with a chemometric modeling strategy [16]. Through this innovative biomarker discovery strategy, we identified a specific profile of serum amino acids in F-T2DM older people. These findings may offer new insights into the metabolic perturbations associated with the disabling cascade in older persons with T2DM.
2. Materials and Methods
2.1. Study Population
MetaboFrail was developed as an ancillary study of the “Multi-modal Intervention in Diabetes in Frailty” (MID-Frail) project [16,33,34]. The latter was a cluster-randomized multicenter clinical trial that evaluated the effectiveness of a multicomponent intervention (mainly based on resistance exercise and lifestyle counseling) on improving physical performance compared with usual care in F-T2DM older adults from seven European countries (ClinicalTrials.gov identifier: NCT01654341) [16,33]. For the present study, a subgroup of MID-Frail participants recruited in Spanish and French study centers were enrolled. The main eligibility criteria were: (a) age at screening 70 years or older; (b) T2DM diagnosis from at least two years; and (c) being pre-frail or frail according to Fried’s criteria [35]. The main exclusion criteria were: (a) poor cognition operationalized as a Mini Mental State Examination score <20 [36]; (b) severe disability defined as a Barthel index score <60 [37]; critical conditions and/or major illnesses with a life expectancy <6 months; (c) inability or unwillingness to provide informed consent. Control participants were enrolled at the Università Cattolica del Sacro Cuore (Rome, Italy) and had the following characteristics: 70+ years of age, no T2DM, and no functional impairment. The study protocol was approved by local ethics committees according to both national and international laws. Prior to enrolment, all participants provided written informed consent. The study was conducted in agreement with legal requirements and international norms (Declaration of Helsinki, 1964).
2.2. Blood Collection and Determination of Serum Concentrations of Amino Acids and Derivatives
Blood samples were collected after overnight fasting. For serum separation, blood samples were kept on ice for about 30 min until clotting and were subsequently centrifuged at 1000× g for 10 min at 4 °C. Serum samples were eventually aliquoted and stored at −80 °C until analysis.
Concentrations of 37 amino acids and derivatives were determined in serum by ultraperformance liquid chromatography/mass spectrometry (UPLC/MS), as described previously [17]. Briefly, 50 μL of sample was added to 100 μL 10% (w/v) sulfosalicylic acid containing an internal standard mix (50 μM; Cambridge Isotope Laboratories, Inc., Tewksbury, MA, USA) and centrifuged at 1000× g for 15 min. Ten μL of the resulting supernatant were mixed with 70 μL of borate buffer and 20 μL of AccQ Tag reagents (Waters Corporation, Milford, MA, USA) and heated at 55 °C for 10 min. Samples were eventually loaded onto a CORTECS UPLC C18 column 1.6 μm 2.1 × 150 mm (Waters Corporation) for chromatographic separation (ACQUITY H-Class, Waters Corporation, Milford, MA, USA). Elution was performed at 500 μL/min flow rate with a linear gradient (9 min) from 99:1 to 1:99 water 0.1% formic acid/acetonitrile 0.1% formic acid. Analytes were detected on an ACQUITY QDa single quadrupole mass spectrometer equipped with electrospray source operating in positive mode (Waters Corporation, Milford, MA, USA). Amino acid controls (MCA laboratory of the Queen Beatrix Hospital, Winterswijk, The Netherlands) were used to monitor the analytic process.
2.3. Statistical Analysis
The normal distribution of data was ascertained through the Kolmogorov-Smirnov test. Comparisons between F-T2DM and control participants for normally distributed continuous variables were performed by t-test statistics. The non-parametric test Mann-Whitney U was applied to assess differences for non-normally distributed continuous data. Differences in categorical variables between groups were determined via χ2 statistics. Descriptive analyses were performed using the GraphPrism 5.03 software (GraphPad Software, Inc., San Diego, CA), with statistical significance set at p < 0.05.
2.4. Partial Least Squares-Discriminant Analysis and Double Cross-Validation Procedures
In order to unveil possible differences in circulating amino acid patterns between F-T2DM and control participants, a multivariate classification strategy based on partial least squares-discriminant analysis (PLS-DA) modeling was adopted [38]. PLS-DA is a classification method particularly suited for dealing with highly correlated predictors, as it is based on projecting the predictors (measured variables) onto a reduced subspace of latent variables (LVs; directions in space) of highest covariance with the responses, i.e., providing the maximum separation between classes. In order to validate the results of PLS-DA modeling and rule out the possibility that good results were obtained because of chance correlation, a procedure based on repeated double cross-validation (DCV) and permutation tests was used [39,40]. DCV consists of spitting the samples to obtain two cross-validation loops, an internal loop for model building/model selection and an outer loop that mimics external (test set) validation. The DCV procedure is repeated a sufficient number of times such that estimates do to depend on one specific sample splitting. This allows evaluating the consistency of model parameters and the confidence intervals for model predictions. To assess the statistical significance of the obtained predictions, the figures of merit which summarize the classification accuracy in repeated DCV [i.e., number of misclassifications (NMC), area under the receiver operating characteristic curve (AUROC), and discriminant (DQ2)] are compared with their distribution under the null hypothesis, which is estimated non-parametrically through permutation tests with 1000 randomizations. A more detailed description of the procedure can be found elsewhere [41]. PLS-DA and DCV were run under Matlab R2015b environment by means of in-house written functions (freely downloadable at: https://www.chem.uniroma1.it/romechemometrics/research/algorithms/plsda/).
3. Results
3.1. Study Population
The present investigation included 66 F-T2DM older adults and 30 age and sex-matched robust, non-diabetic controls. The main characteristics of the two groups are reported in Table 1. F-T2DM and control participants were comparable for age, sex distribution, and number of diseases. F-T2DM older adults showed higher body mass index relative to controls. As expected, a significant difference was found in physical functional between groups, as indicated by the scores on the short physical performance battery (SPPB).

Table 1.
Main characteristics of study participants.
3.2. Identification of Circulating Amino Acid Profiles
In the present study, we aimed at identifying profiles of circulating amino acids that discriminate older persons with F-T2DM from functionally intact non-diabetic peers. Among the available statistical options, we selected a PLS-DA-based strategy for its ability to handle multiple interdependent variables. The best PLS-DA model was built using two LVs. As indicated by the stringent DCV applied, the classification performance of the model was almost perfect. Indeed, the proportion of correct classification of participants was 96.6 ± 1.5% over the calibration sets (95.0 ± 2.2% for cases and 100.0 ± 0.0% for controls), 96.6 ± 1.5% (95.0 ± 2.2% for cases and 100.0 ± 0.0% for controls) in the internal DCV loop (i.e., the one used for model selection), and 95.9 ± 1.3% (94.1 ± 1.9% for cases and 100.0 ± 0.0% for controls) in the outer DCV loop, which accounts for the results of repeated external validation.
The remarkable classification performance of the PLS-DA model can be appreciated by inspecting the projection of study participants over the space spanned by the two LVs (Figure 1).
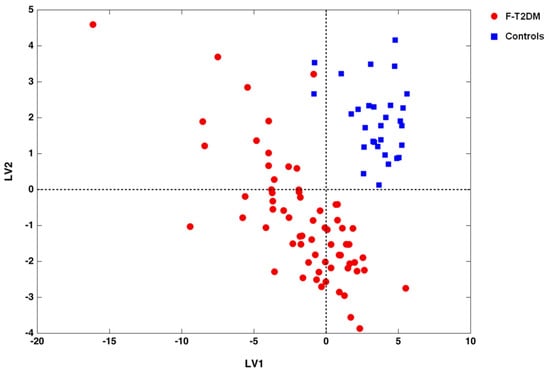
Figure 1.
Scores plot showing the separation of frail/pre-frail older adults with type 2 diabetes mellitus (F-T2DM) from control participants according to the serum concentrations of amino acids and derivatives in the space spanned by the two latent variables (LV1 and LV2), as determined by partial least squares-discriminant analysis.
A sharp separation between F-T2DM participants and controls is evident. To ensure the reliability of our findings against the possibility of chance correlations, DCV results of the PLS-DA model were compared with the distributions of specific figures of merit under the null hypothesis. As depicted in Figure 2, for all of the figures considered (i.e., NMC, AUROC, and DQ2), values obtained from the unpermuted dataset fell outside the corresponding null hypothesis distribution, indicating a p value <0.05.
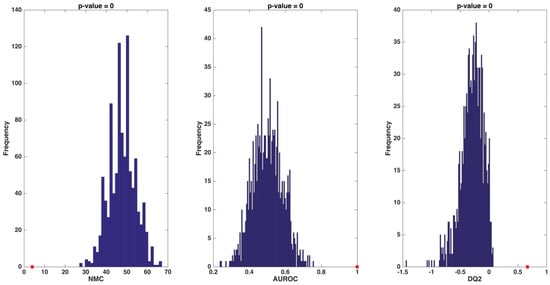
Figure 2.
Distribution of values of number of misclassifications (NMC), area under the receiver operating characteristic curve (AUROC), and discriminant Q2 (DQ2) under their respective null hypothesis as estimated by permutation tests (blue histograms) and the corresponding values obtained by the partial least squares-discriminant analysis model on unpermuted data (red circles). Values obtained on the real dataset (red circles) fall outside of the corresponding null hypothesis distribution (blue histograms), corresponding to a p < 0.05.
The identification of the metabolites with the greatest discriminating power was accomplished by inspecting variable importance in projection (VIP) indices. Table 2 reports variables with a VIP value higher than 1.

Table 2.
Serum concentrations of discriminant biomolecules as resulted from partial least squares-discriminant analysis.
F-T2DM participants showed higher serum levels of 3-methyl histidine, alanine, arginine, ethanolamine, glutamic acid, sarcosine, and tryptophan. Instead, controls were characterized by higher circulating levels of ornithine and taurine. Serum concentrations of non-discriminant analytes in the two participant groups are listed in Table S1.
4. Discussion
Over the last few years, analytical platforms have been developed together with sophisticated computational algorithms to allow the study of complex diseases in unprecedented detail [42]. This new healthcare paradigm, called personalized or precision medicine, incorporates “omics” technologies to unveil the inner biological properties of morbid conditions [42] and devise innovative diagnostics and interventions tailored to the needs of single individuals [42,43]. Metabolomics, by virtue of its privileged position at the interface between biological pathways and clinical manifestations of healthy/disease conditions [44], are improving our understanding of “normal” physiology and the pathophysiology of many disorders, including frailty and T2DM [15,45,46]. These premises led to the design of the MetaboFrail study [16].
In the present investigation, we applied targeted metabolomics to characterize the circulating amino acid profile of functionally impaired older persons with T2DM. Our major finding was that a specific pattern of amino acids discriminated F-T2DM older people from age- and sex-matched controls. The amino acid signature of F-T2DM participants included higher circulating levels of 3-methyl histidine, alanine, arginine, ethanolamine, glutamic acid, sarcosine, and tryptophan.
The presence of 3-methyl histidine among the most relevant predictors supports the involvement of muscle wasting in frailty [30]. Indeed, 3-methyl histidine derives from the post-translational methylation of histidine moieties of actin and myosin [47,48]. Hence, 3-methyl histidine has been proposed as a biomarker of myofibrillar proteolysis and skeletal muscle loss [49]. Interestingly, 3-methyl histidine is also a marker of increased protein catabolism in T2DM [50].
Alanine and glutamic acid are crucial intermediates of muscle energy metabolism and liver-muscle metabolic interchange under both physiologic and pathologic conditions [51,52,53]. Perturbations in alanine and glutamate circulating pool may be indicative of skeletal muscle dysfunction, and are commonly encountered in age-related chronic conditions and models of muscle atrophy [54,55]. Notably, both alanine and glutamic acid levels were positively associated with insulin resistance and risk of T2DM in several independent study cohorts [56,57,58].
Arginine metabolism involves the cooperation of various organs, including kidneys, muscles, gut, and liver [59]. Major pathways in arginine metabolism include muscle protein breakdown and its de novo synthesis from citrulline [59]. Arginine is involved in nitric oxide as well as urea and polyamine synthesis, and research focus has recently been directed towards the determination of the pathophysiological role of arginine and its metabolites in aging and age-related chronic diseases [60]. Noticeably, our findings mirror the higher levels of arginine and lower concentrations of its urea-cycle companion ornithine found in Chinese adults with T2DM [61]. Higher concentrations of another intermediate of urea-cycle, i.e., citrulline, defined the serum amino acid profile of older people with PF&S [17]. Further investigations on arginine/nitrogen interorgan networks are needed to explain the results found in F-T2DM older adults.
Sarcosine, the N-methylated derivative of glycine, is an important intermediate of one-carbon metabolism [62]. Recent studies have investigated the association between sarcosine levels and age-related conditions in humans with conflicting results [63,64,65,66]. In a comparative metabolomics analysis, circulating sarcosine was found to be reduced with aging and increased by dietary restriction in both rodents and humans [67]. Conversely, elevated urine sarcosine levels were associated with incident T2DM [68], and higher serum concentrations of sarcosine characterized the metabotype of older adults with PF&S [17]. Collectively, these findings suggest that perturbations in folate/one-carbon metabolism may play a role in frailty and T2DM as well as in other age-related conditions.
Ethanolamine is a crucial intermediate of the CDP-ethanolamine pathway, the main route of phosphatidylethanolamine synthesis, and modulates lipid metabolism and the turnover of biological membranes [69]. Recently, a critical role for skeletal muscle phospholipid metabolism has been described in the regulation of both insulin sensitivity and contractile function [70]. Moreover, the ethanolamine/phosphatidylethanolamine synthesis dyad is directly involved in autophagy regulation, thereby modulating anti-aging properties of this cellular process [71]. Interestingly, perturbations in the CDP-ethanolamine pathway were associated with altered mitochondrial biogenesis in mouse models of muscle atrophy [71], while higher circulating levels of ethanolamine were found in older adults with PF&S [17].
Tryptophan is an essential amino acid that exerts multiple roles in growth, mood, behavior, and immune responses [72]. Tryptophan is metabolized via two major pathways, the tryptophan-kynurenine and the tryptophan-methoxyndole pathways, that lead to the production of NAD, serotonin, and melatonin [72]. Alterations in tryptophan metabolism have been described in the context of frailty and T2DM [73,74]. In particular, tryptophan and its associated metabolites have been associated with insulin resistance and incident T2DM in independent cohorts [74,75]. Moreover, higher circulating levels of tryptophan were associated with low muscle quality in a large cohort of men and women enrolled in the BLSA [32].
Non-frail non-T2DM controls were characterized by higher serum levels of taurine. Taurine is the most abundant free amino acid in several organs, including the skeletal muscle that contains approximately 70% of total body taurine [76]. Taurine has multiple regulatory effects and its role in osmoregulation, modulation of inflammatory response, protection against oxidative stress, and stimulation of cellular quality control processes is widely acknowledged [76,77]. Taurine deficiency is commonly encountered in people with T2DM [78,79], and its supplementation has been proposed as a strategy against T2DM complications, including retinopathy, nephropathy, neuropathy, atherosclerosis, and cardiomyopathy [80]. Recently, taurine supplementation has also been proposed as a possible remedy against sarcopenia [81]. The causes of taurine depletion in F-T2DM older adults are multifaceted and may include decreased dietary intake, reduced intestinal absorption, renal wasting, and inflammation [77].
Unexpectedly, branched-chain amino acids (BCAAs) were not found to be discriminant by the PLS-DA classification model. BCAAs are metabolic rheostats that modulate whole-body and tissular metabolism [20]. Recent evidence suggests that BCAAs may have a Janus-like behavior in F-T2DM older adults. Indeed, while BCAA-induced activation of the mammalian target of rapamycin (mTOR) in skeletal myocytes may contrast sarcopenia and functional decline in advanced age [20,31], other studies found an association between higher circulating levels of BCAAs and insulin resistance or T2DM in older adults [24,25,26,27]. The low discriminant power of BCAAs in MetaboFrail might therefore reflect the dual effect of BCAAs in F-T2DM older people.
Although innovative, the present investigation has some limitations that should be mentioned. The study sample was quite small while the dataset comprised numerous variables. To cope with this issue, we adopted a PLS-DA-based strategy which is particularly suited for handling matrices populated by highly correlated variables. The MetaboFrail study enrolled older adults from three European countries; thus, a validation study in other ethnic groups is warranted. Eating habits may affect circulating amino acid levels, but diet was not objectively assessed in our study sample. However, it has recently been reported that differences in blood amino acid concentrations do not necessarily mirror those of amino acid intakes [82]. As commonly occurs in biomarker discovery studies, although a large number of candidates were investigated, we could not evaluate all possible mediators involved in frailty and T2DM.
In conclusion, in the present investigation, we showed the existence of a specific amino acid signature in F-T2DM older persons. Our novel approach enabled us to obtain new insights into the pathophysiology of the two conditions. In particular, a relevant role for perturbations in muscle metabolism and muscle-liver interorgan communication was highlighted. The longitudinal implementation of our analytical strategy could allow validating novel sets of biomarkers and identifying new targets for interventions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/1/199/s1; Table S1: Serum concentrations of non-discriminant amino acids and derivatives in frail/pre-frail participants with type 2 diabetes mellitus (F-T2DM) and controls.
Author Contributions
Conceptualization, A.P. (Anna Picca), E.M., and R.C.; methodology, A.P. (Aniello Primiano), G.C., and J.G.; software, A.B. and F.M.; validation, A.P. (Anna Picca), E.M., and R.C.; formal analysis, A.B. and F.M.; investigation, A.P. (Anna Picca), E.M., and R.C.; resources, A.J.S., G.G., L.R.-M., and R.B.; data curation, A.P. (Anna Picca) and R.C.; writing—original draft preparation, E.M. and R.C.; writing—review and editing, A.P. (Anna Picca), F.M., I.B.-M., L.P., O.L., R.C., and S.C.R.; supervision, A.J.S., G.G., L.R.-M., and R.B.; funding acquisition, A.J.S., G.G., L.R.-M., and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the European Commission - EU 7th Framework Programme (Contract N° 278803), and Innovative Medicine Initiative-Joint Undertaking (IMI-JU #115621). The work was also partly supported by the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon”. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Acknowledgments
The authors thank Luca Mariotti for his precious technical and administrative support.
Conflicts of Interest
A.J.S., E.M., L.R.-M., R.B., and R.C are partners of the SPRINTT Consortium, which is partly funded by the European Federation of Pharmaceutical Industries and Associations (EFPIA). R.C. served as a consultant for Abbott, Novartis, and Nutricia. E.M. served as a consultant for Abbott, Biophytis, Nestlè, Novartis, and Nutricia.
References
- Sinclair, A.; Dunning, T.; Rodriguez-Mañas, L. Diabetes in older people: New insights and remaining challenges. Lancet Diabetes Endocrinol. 2015, 3, 275–285. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators; James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Golden, S.H.; Cefalu, W.T. Diabetes and Aging: Unique Considerations and Goals of Care. Diabetes Care 2017, 40, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in Older Persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- El Assar, M.; Laosa, O.; Rodríguez Mañas, L. Diabetes and frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 52–57. [Google Scholar] [CrossRef]
- LeRoith, D.; Biessels, G.J.; Braithwaite, S.S.; Casanueva, F.F.; Draznin, B.; Halter, J.B.; Hirsch, I.B.; McDonnell, M.E.; Molitch, M.E.; Murad, M.H.; et al. Treatment of Diabetes in Older Adults: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1520–1574. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Rodriguez-Mañas, L. Diabetes and Frailty: Two Converging Conditions? Can. J. Diabetes 2016, 40, 77–83. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y., II; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Guerrero, N.; Bunout, D.; Hirsch, S.; Barrera, G.; Leiva, L.; Henríquez, S.; De la Maza, M.P. Premature loss of muscle mass and function in type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 117, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated Loss of Skeletal Muscle Strength in Older Adults With Type 2 Diabetes: The Health, Aging, and Body Composition Study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia as the Biological Substrate of Physical Frailty. Clin. Geriatr. Med. 2015, 31, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Marini, F.; Cesari, M.; Tosato, M.; Anker, S.D.; von Haehling, S.; Miller, R.R.; Bernabei, R.; Landi, F.; Marzetti, E.; et al. Biomarkers for physical frailty and sarcopenia: State of the science and future developments. J. Cachexia. Sarcopenia Muscle 2015, 6, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Coelho-Junior, H.J.; Cesari, M.; Marini, F.; Miccheli, A.; Gervasoni, J.; Bossola, M.; Landi, F.; Bernabei, R.; Marzetti, E.; et al. The metabolomics side of frailty: Toward personalized medicine for the aged. Exp. Gerontol. 2019, 126, 110692. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Rodriguez-Mañas, L.; Picca, A.; Marini, F.; Biancolillo, A.; Laosa, O.; Pedraza, L.; Gervasoni, J.; Primiano, A.; Miccheli, A.; et al. The “Metabolic biomarkers of frailty in older people with type 2 diabetes mellitus” (MetaboFrail) study: Rationale, design and methods. Exp. Gerontol. 2020, 129, 110782. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Junior, H.; Bossola, M.; Urbani, A.; et al. A Distinct Pattern of Circulating Amino Acids Characterizes Older Persons with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients 2018, 10, 1691. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F.S. Protein-Amino Acid Metabolism Disarrangements: The Hidden Enemy of Chronic Age-Related Conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef]
- Lu, Y.; Karagounis, L.G.; Ng, T.P.; Carre, C.; Narang, V.; Wong, G.; Tan, C.T.Y.; Zin Nyunt, M.S.; Gao, Q.; Abel, B.; et al. Systemic and Metabolic Signature of Sarcopenia in Community-Dwelling Older Adults. J. Gerontol. Ser. A 2019. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef]
- Semba, R.D.; Gonzalez-Freire, M.; Moaddel, R.; Sun, K.; Fabbri, E.; Zhang, P.; Carlson, O.D.; Khadeer, M.; Chia, C.W.; Salem, N.; et al. Altered Plasma Amino Acids and Lipids Associated With Abnormal Glucose Metabolism and Insulin Resistance in Older Adults. J. Clin. Endocrinol. Metab. 2018, 103, 3331–3339. [Google Scholar] [CrossRef]
- Adachi, Y.; Ono, N.; Imaizumi, A.; Muramatsu, T.; Andou, T.; Shimodaira, Y.; Nagao, K.; Kageyama, Y.; Mori, M.; Noguchi, Y.; et al. Plasma Amino Acid Profile in Severely Frail Elderly Patients in Japan. Int. J. Gerontol. 2018, 12, 290–293. [Google Scholar] [CrossRef]
- Kochlik, B.; Stuetz, W.; Pérès, K.; Féart, C.; Tegner, J.; Rodriguez-Mañas, L.; Grune, T.; Weber, D. Associations of Plasma 3-Methylhistidine with Frailty Status in French Cohorts of the FRAILOMIC Initiative. J. Clin. Med. 2019, 8, 1010. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Price, L.L.; Chale, A.; Phillips, E.M.; Fielding, R.A. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Moaddel, R.; Fabbri, E.; Khadeer, M.A.; Carlson, O.D.; Gonzalez-Freire, M.; Zhang, P.; Semba, R.D.; Ferrucci, L. Plasma Biomarkers of Poor Muscle Quality in Older Men and Women from the Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mañas, L.; Bayer, A.J.; Kelly, M.; Zeyfang, A.; Izquierdo, M.; Laosa, O.; Hardman, T.C.; Sinclair, A.J.; Moreira, S.; Cook, J.; et al. An evaluation of the effectiveness of a multi-modal intervention in frail and pre-frail older people with type 2 diabetes--the MID-Frail study: Study protocol for a randomised controlled trial. Trials 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mañas, L.; Laosa, O.; Vellas, B.; Paolisso, G.; Topinkova, E.; Oliva-Moreno, J.; Bourdel-Marchasson, I.; Izquierdo, M.; Hood, K.; Zeyfang, A.; et al. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J. Cachexia. Sarcopenia Muscle 2019. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Ståhle, L.; Wold, S. Partial least squares analysis with cross-validation for the two-class problem: A Monte Carlo study. J. Chemom. 1987, 1, 185–196. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Hoefsloot, H.C.J.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.J.; van Duijnhoven, J.P.M.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Smit, S.; van Breemen, M.J.; Hoefsloot, H.C.J.; Smilde, A.K.; Aerts, J.M.F.G.; de Koster, C.G. Assessing the statistical validity of proteomics based biomarkers. Anal. Chim. Acta 2007, 592, 210–217. [Google Scholar] [CrossRef]
- Marzetti, E.; Landi, F.; Marini, F.; Cesari, M.; Buford, T.W.; Manini, T.M.; Onder, G.; Pahor, M.; Bernabei, R.; Leeuwenburgh, C.; et al. Patterns of Circulating Inflammatory Biomarkers in Older Persons with Varying Levels of Physical Performance: A Partial Least Squares-Discriminant Analysis Approach. Front. Med. 2014, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Snyder, M. Promise of Personalized Omics to Precision Medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J.; Barabasi, A.-L. Systems biology and the future of medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Perry, S.V. Biological activity and the 3-methylhistidine content of actin and myosin. Biochem. J. 1970, 119, 293–298. [Google Scholar] [CrossRef]
- Asatoor, A.M.; Armstrong, M.D. 3-methylhistidine, a component of actin. Biochem. Biophys. Res. Commun. 1967, 26, 168–174. [Google Scholar] [CrossRef]
- Sheffield-Moore, M.; Dillon, E.L.; Randolph, K.M.; Casperson, S.L.; White, G.R.; Jennings, K.; Rathmacher, J.; Schuette, S.; Janghorbani, M.; Urban, R.J.; et al. Isotopic decay of urinary or plasma 3-methylhistidine as a potential biomarker of pathologic skeletal muscle loss. J. Cachexia. Sarcopenia Muscle 2014, 5, 19–25. [Google Scholar] [CrossRef]
- Marchesini, G.; Forlani, G.; Zoli, M.; Vannini, P.; Pisi, E. Muscle protein breakdown in uncontrolled diabetes as assessed by urinary 3-methylhistidine excretion. Diabetologia 1982, 23, 456–458. [Google Scholar] [CrossRef][Green Version]
- Wagenmakers, A.J. Protein and amino acid metabolism in human muscle. Adv. Exp. Med. Biol. 1998, 441, 307–319. [Google Scholar] [PubMed]
- Jang, C.; Hui, S.; Zeng, X.; Cowan, A.J.; Wang, L.; Chen, L.; Morscher, R.J.; Reyes, J.; Frezza, C.; Hwang, H.Y.; et al. Metabolite Exchange between Mammalian Organs Quantified in Pigs. Cell Metab. 2019, 30, 594–606.e3. [Google Scholar] [CrossRef] [PubMed]
- Gancheva, S.; Jelenik, T.; Álvarez-Hernández, E.; Roden, M. Interorgan Metabolic Crosstalk in Human Insulin Resistance. Physiol. Rev. 2018, 98, 1371–1415. [Google Scholar] [CrossRef] [PubMed]
- Jagoe, R.T.; Engelen, M.P.K.J. Muscle wasting and changes in muscle protein metabolism in chronic obstructive pulmonary disease. Eur. Respir. J. 2003, 22, 52s–63s. [Google Scholar] [CrossRef] [PubMed]
- Ilaiwy, A.; Quintana, M.T.; Bain, J.R.; Muehlbauer, M.J.; Brown, D.I.; Stansfield, W.E.; Willis, M.S. Cessation of biomechanical stretch model of C2C12 cells models myocyte atrophy and anaplerotic changes in metabolism using non-targeted metabolomics analysis. Int. J. Biochem. Cell Biol. 2016, 79, 80–92. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Liang, X.; Zou, L.; Ong, C.N.; Yuan, J.M.; Koh, W.P.; Pan, A. Serum amino acids in association with prevalent and incident type 2 diabetes in a Chinese population. Metabolites 2019, 9, 14. [Google Scholar] [CrossRef]
- Seibert, R.; Abbasi, F.; Hantash, F.M.; Caulfield, M.P.; Reaven, G.; Kim, S.H. Relationship between insulin resistance and amino acids in women and men. Physiol. Rep. 2015, 3, e12392. [Google Scholar] [CrossRef]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Rodionov, R.N.; Mcevoy, M.; Zinellu, A.; Carru, C.; Sotgia, S. New horizons in arginine metabolism, ageing and chronic disease states. Age Ageing 2019, 48, 776–782. [Google Scholar] [CrossRef]
- Cao, Y.-F.; Li, J.; Zhang, Z.; Liu, J.; Sun, X.-Y.; Feng, X.-F.; Luo, H.-H.; Yang, W.; Li, S.-N.; Yang, X.; et al. Plasma Levels of Amino Acids Related to Urea Cycle and Risk of Type 2 Diabetes Mellitus in Chinese Adults. Front. Endocrinol. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Koutros, S.; Meyer, T.E.; Fox, S.D.; Issaq, H.J.; Veenstra, T.D.; Huang, W.Y.; Yu, K.; Albanes, D.; Chu, L.W.; Andriole, G.; et al. Prospective evaluation of serum sarcosine and risk of prostate cancer in the prostate, lung, colorectal and ovarian cancer screening trial. Carcinogenesis 2013, 34, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- De Vogel, S.; Ulvik, A.; Meyer, K.; Ueland, P.M.; Nygård, O.; Vollset, S.E.; Tell, G.S.; Gregory, J.F.; Tretli, S.; Bjørge, T. Sarcosine and other metabolites along the choline oxidation pathway in relation to prostate cancer—A large nested case-control study within the JANUS cohort in Norway. Int. J. Cancer 2014, 134, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Hasokawa, M.; Shinohara, M.; Tsugawa, H.; Bamba, T.; Fukusaki, E.; Nishiumi, S.; Nishimura, K.; Yoshida, M.; Ishida, T.; Hirata, K.; et al. Identification of biomarkers of stent restenosis with serum metabolomic profiling using gas chromatography/mass spectrometry. Circ. J. 2012, 76, 1864–1873. [Google Scholar] [CrossRef]
- Tsai, C.H.; Huang, H.C.; Liu, B.L.; Li, C.I.; Lu, M.K.; Chen, X.; Tsai, M.C.; Yang, Y.W.; Lane, H.Y. Activation of N-methyl-D-aspartate receptor glycine site temporally ameliorates neuropsychiatric symptoms of Parkinson’s disease with dementia. Psychiatry Clin. Neurosci. 2014, 68, 692–700. [Google Scholar] [CrossRef]
- Walters, R.O.; Arias, E.; Diaz, A.; Burgos, E.S.; Guan, F.; Tiano, S.; Mao, K.; Green, C.L.; Qiu, Y.; Shah, H.; et al. Sarcosine Is Uniquely Modulated by Aging and Dietary Restriction in Rodents and Humans. Cell Rep. 2018, 25, 663–676. [Google Scholar] [CrossRef]
- Svingen, G.F.T.; Schartum-Hansen, H.; Pedersen, E.R.; Ueland, P.M.; Tell, G.S.; Mellgren, G.; Njølstad, P.R.; Seifert, R.; Strand, E.; Karlsson, T.; et al. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin. Chem. 2016, 62, 755–765. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Funai, K.; Lodhi, I.J.; Spears, L.D.; Yin, L.; Song, H.; Klein, S.; Semenkovich, C.F. Skeletal muscle phospholipid metabolism regulates insulin sensitivity and contractile function. Diabetes 2016, 65, 358–370. [Google Scholar] [CrossRef]
- Rockenfeller, P.; Koska, M.; Pietrocola, F.; Minois, N.; Knittelfelder, O.; Sica, V.; Franz, J.; Carmona-Gutierrez, D.; Kroemer, G.; Madeo, F. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ. 2015, 22, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Pérez, D.; Sánchez-Flores, M.; Maseda, A.; Lorenzo-López, L.; Millán-Calenti, J.C.; Strasser, B.; Gostner, J.M.; Fuchs, D.; Pásaro, E.; Valdiglesias, V.; et al. Frailty Status in Older Adults Is Related to Alterations in Indoleamine 2,3-Dioxygenase 1 and Guanosine Triphosphate Cyclohydrolase I Enzymatic Pathways. J. Am. Med. Dir. Assoc. 2017, 18, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zheng, X.; Ma, X.; Bao, Y.; Ni, Y.; Hu, C.; Rajani, C.; Huang, F.; Zhao, A.; Jiia, W.; et al. Tryptophan Predicts the Risk for Future Type 2 Diabetes. PLoS ONE 2016, 11, e0162192. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Papandreou, C.; Ruiz-Canela, M.; Guasch-Ferre, M.; Clish, C.B.; Dennis, C.; Liang, L.; Corella, D.; Fitó, M.; Razquin, C.; et al. Association of tryptophan metabolites with incident type 2 diabetes in the PREDIMED trial: A case–cohort study. Clin. Chem. 2018, 64, 1211–1220. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine—From organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef]
- De Luca, G.; Calpona, P.R.; Caponetti, A.; Romano, G.; Di Benedetto, A.; Cucinotta, D.; Di Giorgio, R.M. Taurine and osmoregulation: Platelet taurine content, uptake, and release in type 2 diabetic patients. Metabolism 2001, 50, 60–64. [Google Scholar] [CrossRef]
- Franconi, F.A.; Bennardini, F.R.; Giuseppe, A.; Miceli, S.M.; Ciuti, M.; Mian, M. Plasma and platelet taurine are reduced in subjects with mellitus: Effects of taurine. Am. J. Clin. Nutr. 1995, 61, 1115–1119. [Google Scholar] [CrossRef]
- Ito, T.; Schaffer, S.W.; Azuma, J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids 2012, 42, 1529–1539. [Google Scholar] [CrossRef]
- Scicchitano, B.M.; Sica, G. The Beneficial Effects of Taurine to Counteract Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).