Biological Activity of New Cichoric Acid–Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis
2.1.1. Sample Preparation
2.1.2. FTIR (Fourier Transform Infrared) Study
2.1.3. Calculations
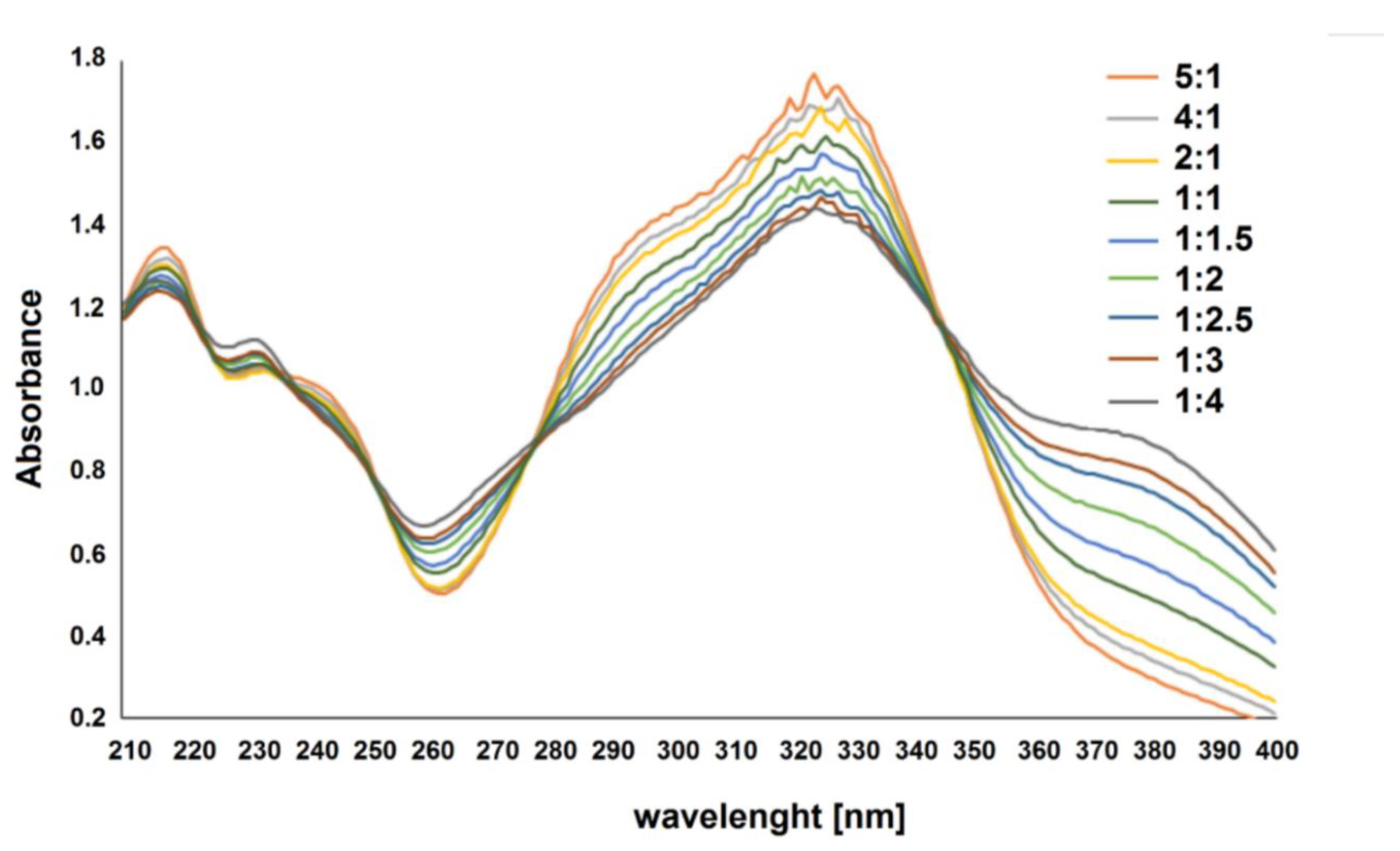
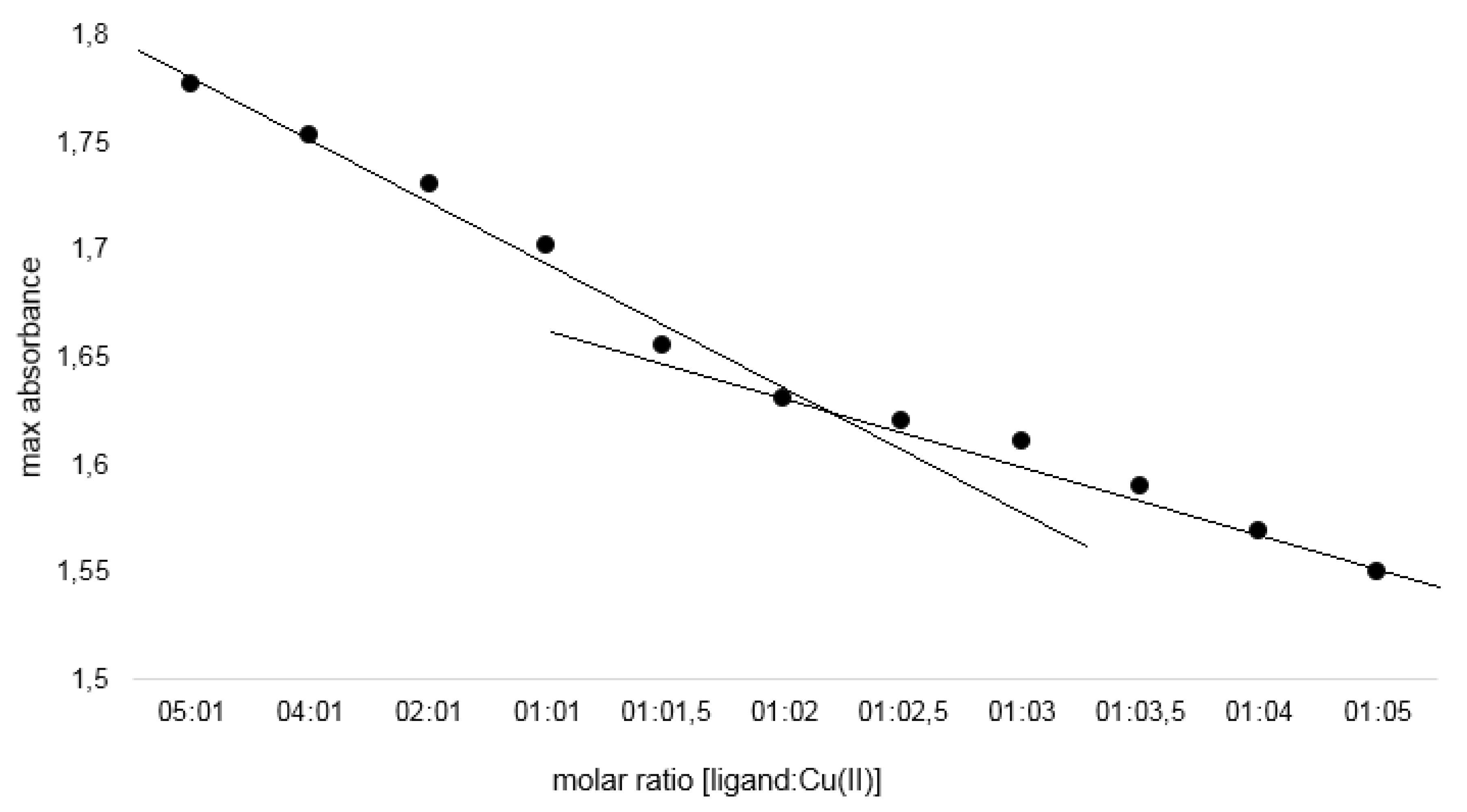
2.1.4. Job’s Study
2.2. Toxicological Studies
2.2.1. Reagents
2.2.2. Microbial Strains
2.2.3. Cell Culture
2.2.4. CA and Its Metal Complexes Antimicrobial Activity
2.2.5. Estimation of CA and Its Metal Complexes Cytotoxicity
2.2.6. Statistical Analysis
3. Results
3.1. Chemical Synthesis Results
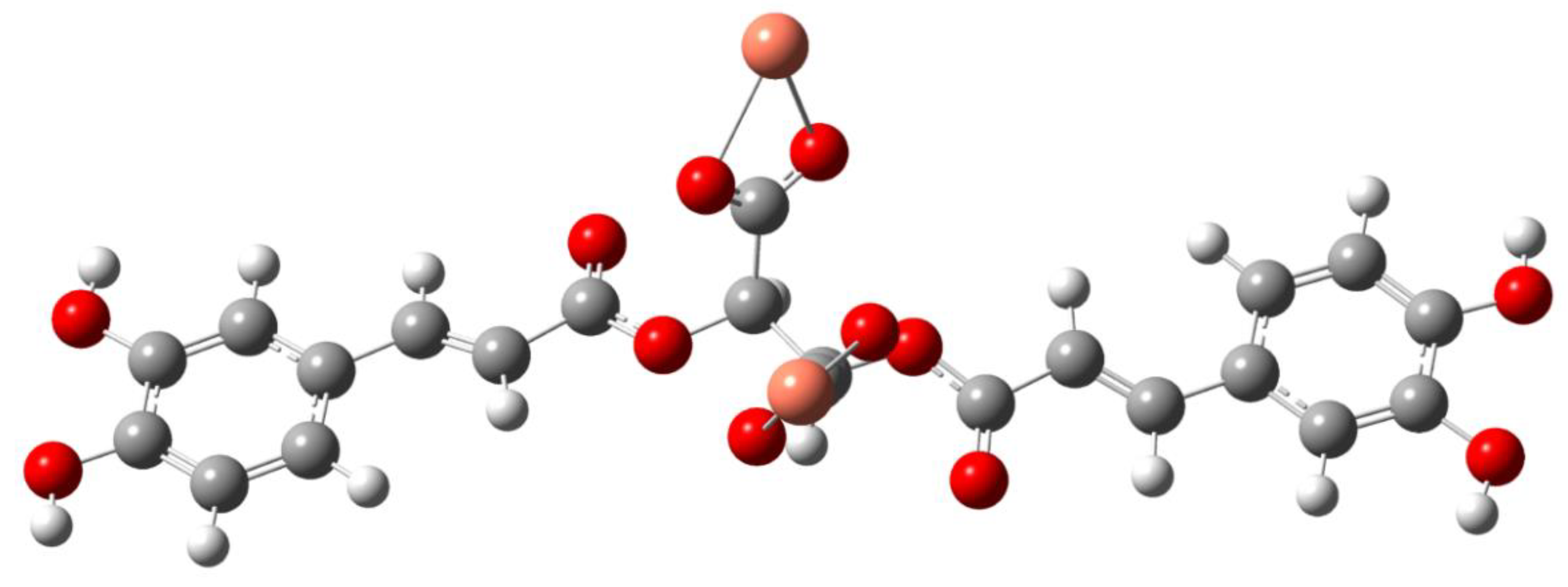
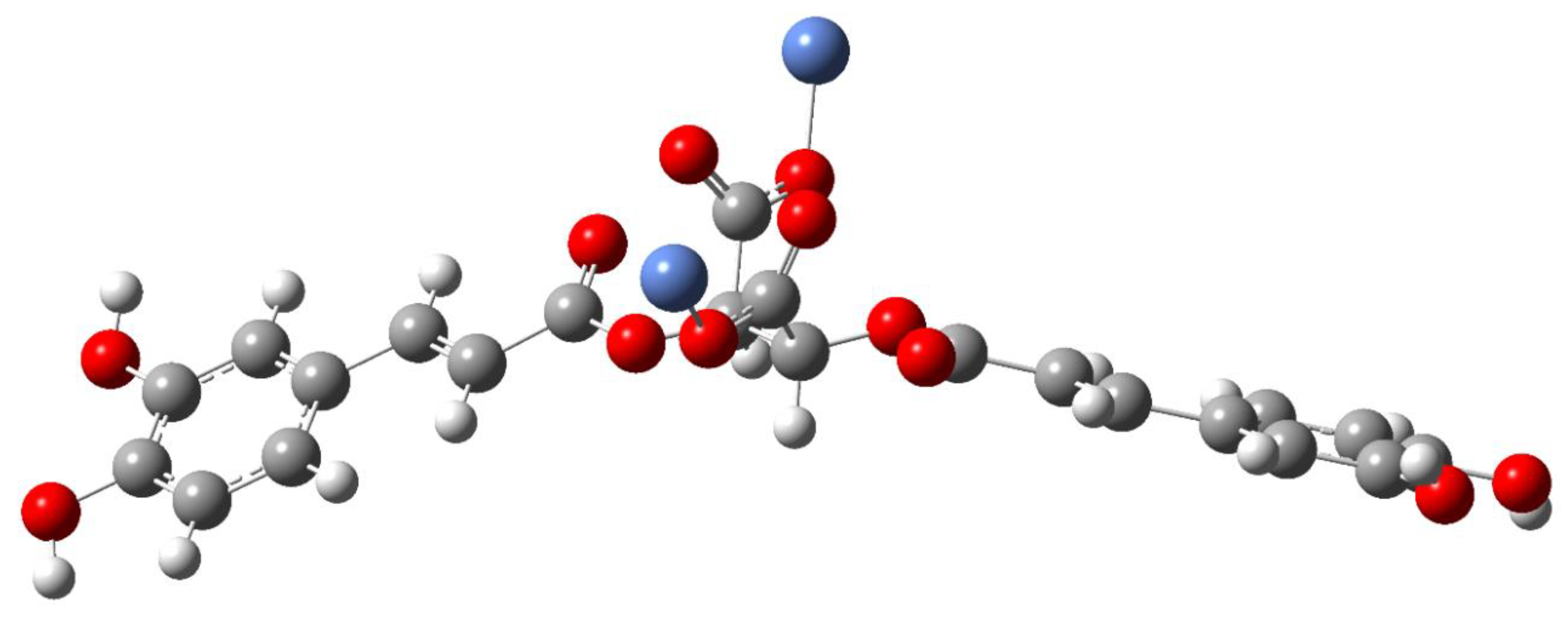
Composition and Structure of Examined Complexes
3.2. Toxicological Studies Results
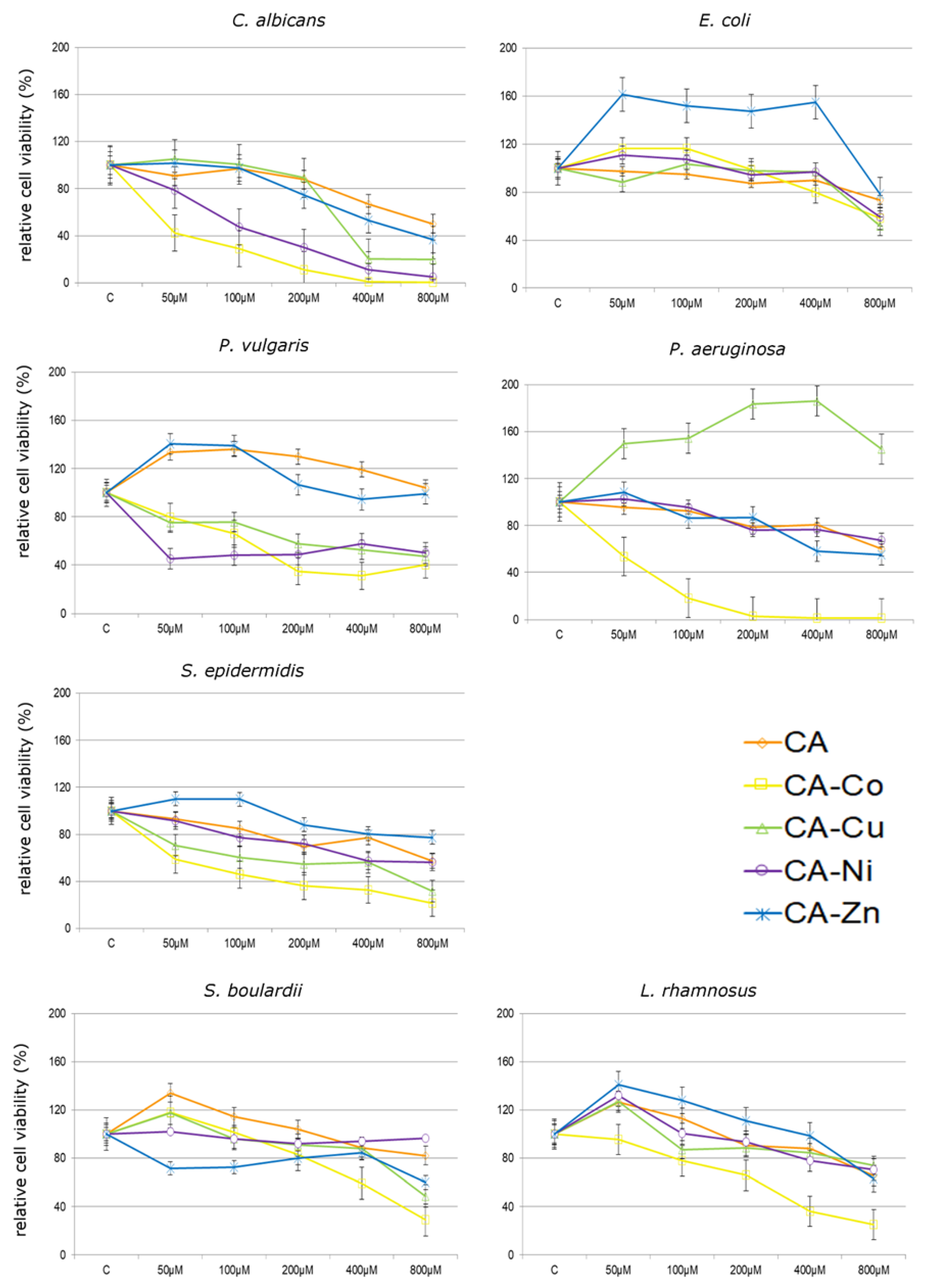
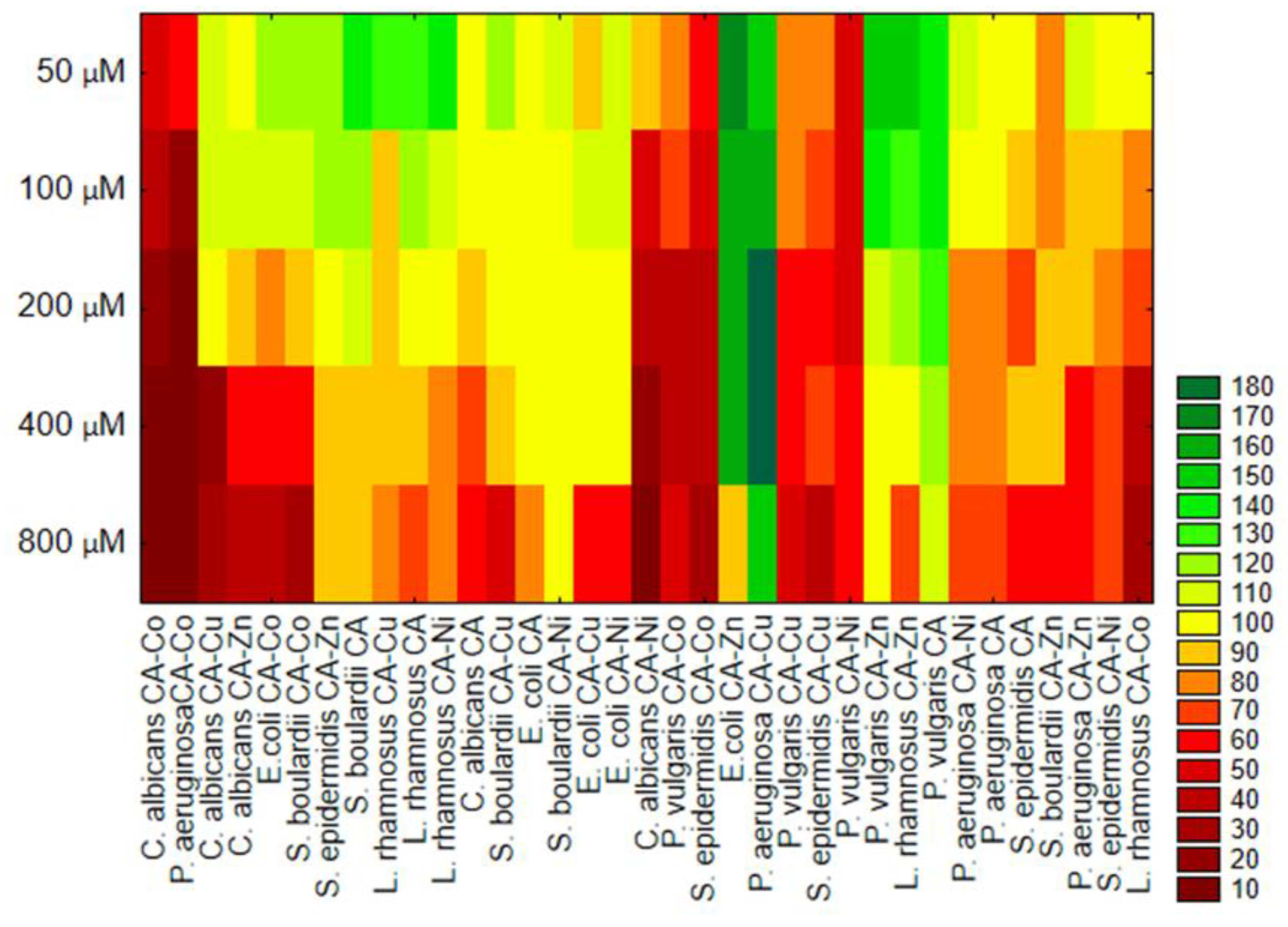
3.2.1. Antibacterial and Antifungal Activity of CA and Its Metal Complexes
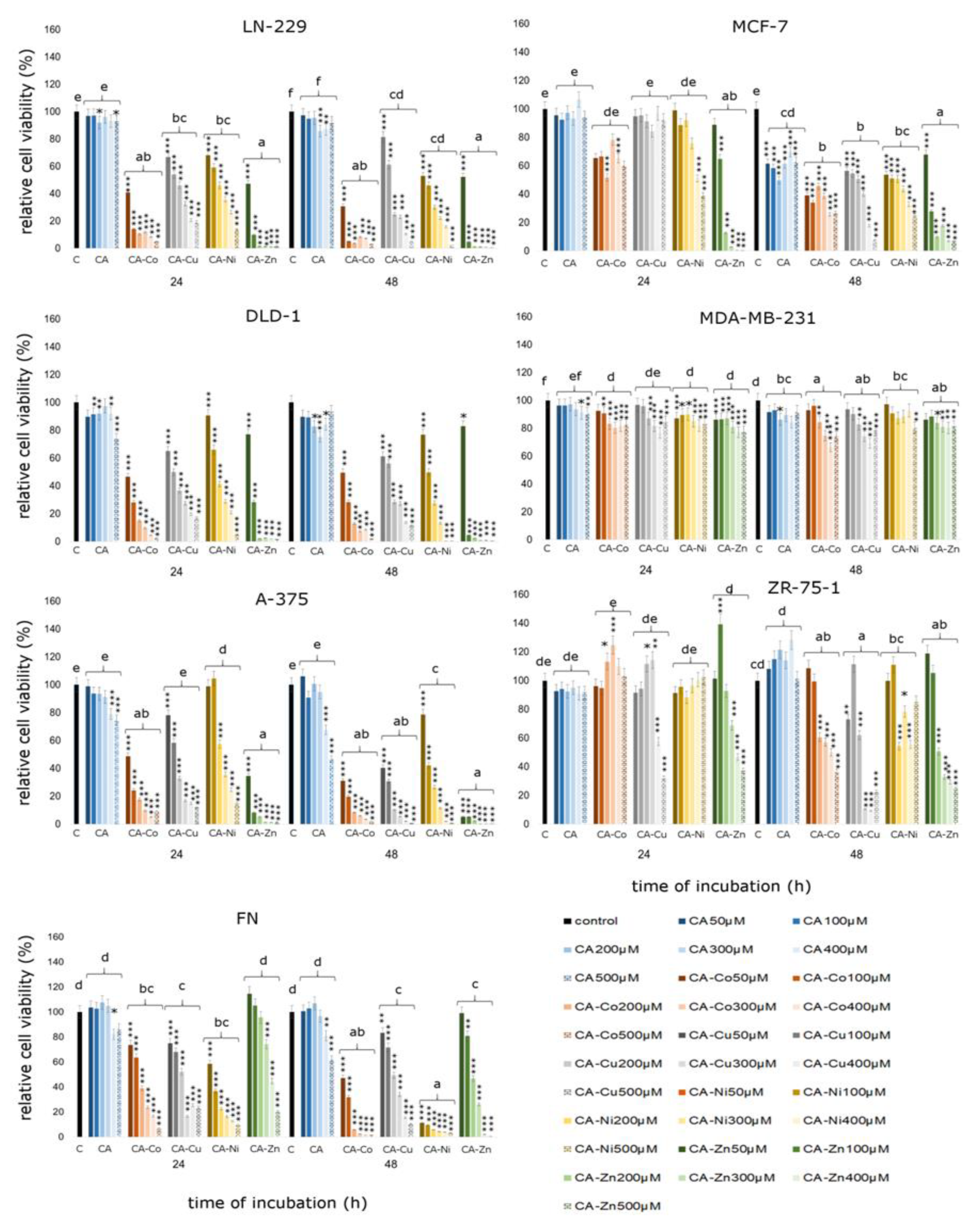
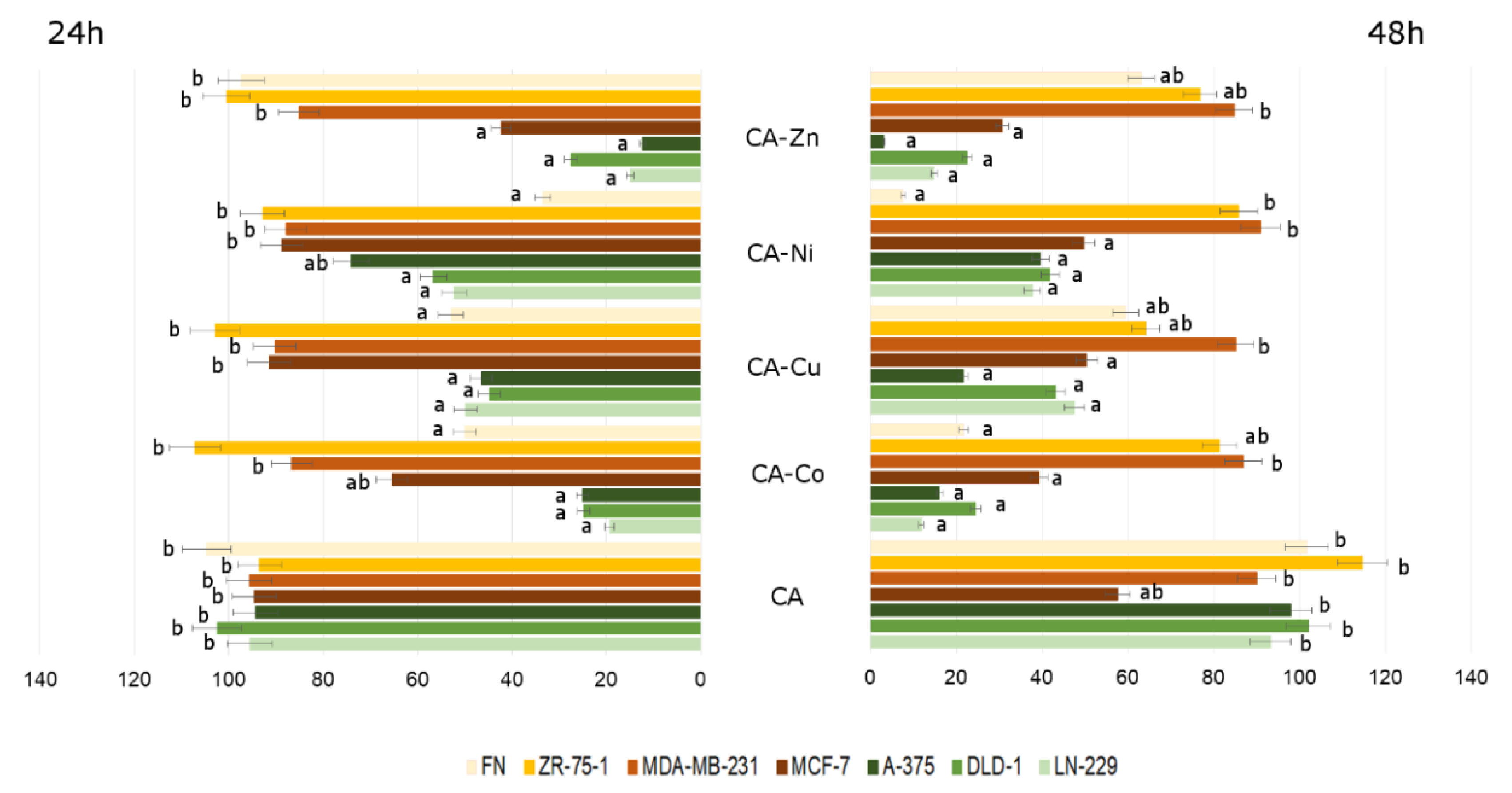
3.2.2. Cytotoxicity of Analyzed Compounds in Human Cancerous and Non-Cancerous Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.; Scagel, C. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Satoh, A. Antimicrobial and nematicidal substances from the root of chicory (Cichorium intybus). Allelochem. Biol. Control Plant Pathog. Dis. 2006, 2, 177–180. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, H.; Jung, C.H.; Lee, S.J.; Ha, T.Y.; Ahn, J. Chicoric acid mitigates impaired insulin sensitivity by improving mitochondrial function. Biosci. Biotechnol. Biochem. 2018, 82, 1197–1206. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, X.; Niu, Y.; Diao, Z.; Ren, B.; Li, X.; Liu, Z.; Liu, X. Cichoric acid improved hyperglycaemia and restored muscle injury via activating antioxidant response in MLD-STZ-induced diabetic mice. Food Chem. Toxicol. 2017, 107, 138–149. [Google Scholar] [CrossRef]
- Tsai, K.L.; Kao, C.L.; Hung, C.H.; Cheng, Y.H.; Lin, H.C.; Chu, P.M. Chicoric acid is a potent anti-atherosclerotic ingredient by anti-oxidant action and anti-inflammation capacity. Oncotarget 2017, 8, 29600–29612. [Google Scholar] [CrossRef]
- Mokhtar, M.; Ginestra, G.; Youcefi, F.; Filocamo, A.; Bisignano, C.; Riazi, A. Antimicrobial Activity of Selected Polyphenols and Capsaicinoids Identified in Pepper (Capsicum annuum L.) and Their Possible Mode of Interaction. Curr. Microbiol. 2017, 74, 1253–1260. [Google Scholar] [CrossRef]
- Smith, N.A.; Sadler, P.J. Photoactivatable Metal Complexes: From Theory to Applications in Biotechnology and Medicine. Philos. Trans. R. Soc. A 2013, 371, 20120519. [Google Scholar] [CrossRef]
- Sanna, D.; Ugone, V.; Lubinu, G.; Micera, G.; Garribba, E. Behavior of the potential antitumor V(IV)O complexes formed by flavonoid ligands. 1. Coordination modes and geometry in solution and at the physiological pH. J. Inorg. Biochem. 2014, 140, 173–184. [Google Scholar] [CrossRef]
- Sanna, D.; Ugone, V.; Pisano, L.; Serra, M.; Micera, G.; Garribba, E. Behavior of the potential antitumor V(IV)O complexes formed by flavonoid ligands. 2. Characterization of sulfonate derivatives of quercetin and morin, interaction with the bioligands of the plasma and preliminary biotransformation studies. J. Inorg. Biochem. 2015, 153, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Ugone, V.; Fadda, A.; Micera, G.; Garribba, E. Behavior of the potential antitumor V(IV)O complexes formed by flavonoid ligands. 3. Antioxidant properties and radical production capability. J. Inorg. Biochem. 2016, 161, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Mercer, J.F.B. Copper Transport and Its Disorders, Molecular and Cellular Aspects; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; Volume 448, ISBN 978-1-4613-7204-2. [Google Scholar]
- Łodyga-Chruscińska, E.; Pilo, M.; Zucca, A.; Garribba, E.; Klewicka, E.; Rowińska-Żyrek, M.; Symonowicz, M.; Chrusciński, L.; Cheshchevik, V.T. Physicochemical, antioxidant, DNA cleaving properties and antimicrobial activity of fisetin-copper chelates. J. Inorg. Biochem. 2018, 180, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.M.; Ullah, M.F.; Azmi, A.S.; Ahmad, A.; Shamim, U.; Zubair, H.; Khan, H.Y. Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for chemoprevention of cancer. Pharm. Res. 2010, 27, 979–988. [Google Scholar] [CrossRef]
- Capo, C.R.; Pedersen, J.Z.; Falconi, M.; Rossi, L. Oleuropein shows copper complexing properties and noxious effect on cultured SH-SY5Y neuroblastoma cells depending on cell copper content. J. Trace Elem. Med. Biol. 2017, 44, 225–232. [Google Scholar] [CrossRef]
- Dias, K.; Nikolaou, S. Does the combination of resveratrol with Al (III) and Zn (II) improve its antioxidant activity? Nat. Prod. Commun. 2011, 6, 1673–1676. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, L.L.; Yang, J.; Han, R.M.; Zhang, J.P.; Skibsted, L.H. Kaempferol Binding to Zinc(II), Efficient Radical Scavenging through Increased Phenol Acidity. J. Phys. Chem. B 2018, 122, 10108–10117. [Google Scholar] [CrossRef]
- Chen, X.; Tang, L.J.; Sun, Y.N.; Qiu, P.H.; Liang, G.J. Syntheses, characterization and antitumor activities of transition metal complexes with isoflavones. J. Inorg. Biochem. 2010, 104, 379–384. [Google Scholar] [CrossRef]
- Bravo, A.; Anacona, J.R. Metal complexes of the flavonoid quercetin: Antibacterial properties. Transit. Met. Chem. 2001, 26, 20–23. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Wang, J.; Tang, N. Synthesis, characterization, antioxidative and antitumor activities of solid quercetin rare earth(III) complexes. J. Inorg. Biochem. 2001, 83, 41–48. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Krętowski, R.; Kalinowska, M.; Świderski, G.; Cechowska-Pasko, M.; Lewandowski, W. Possible Mechanisms of the Prevention of Doxorubicin Toxicity by Cichoric Acid—Antioxidant Nutrient. Nutrients 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Świsłocka, R.; Regulska, E.; Karpińska, J.; Świderski, G.; Lewandowski, W. Molecular Structure and Antioxidant Properties of Alkali Metal Salts of Rosmarinic Acid. Experimental and DFT Studies. Molecules 2019, 24, 2645. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision A. 02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Kuban-Jankowska, A.; Sahu, K.K.; Gorska, M.; Tuszynski, J.A.; Wozniak, M. Chicoric acid binds to two sites and decreases the activity of the YopH bacterial virulence factor. Oncotarget 2016, 7, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Couto, J.A.; Hogg, T.A. Influence of phenolic acids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii. J. Appl. Microbiol. 2003, 94, 167–174. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial Activity and Membrane-Disruptive Mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic Acid, a Novel Phenolic Compound from Pine Needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef]
- Qin, F.; Yao, L.; Lu, C.; Li, C.; Zhou, Y.; Su, C.; Chen, B.; Shen, Y. Phenolic composition, antioxidant and antibacterial properties, and in vitro anti-HepG2 cell activities of wild apricot (Armeniaca Sibirica L. Lam) kernel skins. Food Chem. Toxicol. 2019, 129, 354–364. [Google Scholar] [CrossRef]
- Faheim, A.A.; Abdou, S.N.; El-Wahab, Z.H.A. Synthesis and characterization of binary and ternary complexes of Co(II), Ni(II), Cu(II) and Zn(II) ions based on 4-aminotoluene-3-sulfonic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 109–124. [Google Scholar] [CrossRef]
- Kavak, D.D.; Kecec, S. Extraction of phenolic antioxidants from Pyrus elaeagrifolia Pallas: Process optimization, investigation of the bioactivity and β-glucuronidase inhibitory potential. J. Food Meas. Charact. 2019, 13, 2894–2902. [Google Scholar] [CrossRef]
- EL Moussaoui, A.; Zahra Jawhari, F.; Almehdi, A.M.; Elmsellem, H.; Fikri Benbrahim, K.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens.L. Bioorg. Chem. 2019, 93, 103337. [Google Scholar] [CrossRef]
- Cueva, C.; Mingo, S.; Munoz-Gonzalez, I.; Bustos, I.; Requena, T.; del Campo, R.; Martın-Alvarez, P.J.; Bartolome, B.; Moreno-Arribas, M.V. Antibacterial activity of wine phenolic compounds and oenological extracts against potential respiratory pathogens. Lett. Appl. Microb. 2012, 54, 557–563. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Ying, Z.; Qiu, T.; Pan, S.; Xu, X.A. structure–activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Prasad, V.G.N.V.; Krishna, B.V.; Swamy, P.L.; Rao, T.S.; Rao, G.S. Antibacterial synergy between quercetin and polyphenolic acids against bacterial pathogens of fish. Asian Pac. J. Trop. Dis. 2014, 4, 326–329. [Google Scholar] [CrossRef]
- Anh, H.T.P.; Huang, C.; Huang, C. Intelligent Metal-Phenolic Metallogels as Dressings for Infected Wounds. Sci. Rep. 2019, 9, 11562. [Google Scholar] [CrossRef] [PubMed]
- Gałczyńska, K.; Ciepluch, K.; Madej, Ł.; Kurdziel, K.; Maciejewska, B.; Drulis-Kawa, Z.; Węgierek-Ciuk, A.; Lankoff, A.; Arabski, M. Selective cytotoxicity and antifungal properties of copper(II) and cobalt(II) complexes with imidazole-4-acetate anion or 1-allylimidazole. Sci. Rep. 2019, 9, 9777. [Google Scholar] [CrossRef] [PubMed]
- Castillo, K.F.; Bello-Vieda, N.J.; Nuñez-Dallos, N.G.; Pastrana, H.F.; Celis, A.M.; Restrepo, S.; Hurtado, J.J.; Ávila, A.G. Metal complex derivatives of azole: A study on their synthesis, characterization, and antibacterial and antifungal activities. J. Braz. Chem. Soc. 2016, 27, 2334–2347. [Google Scholar] [CrossRef]
- Lv, Q.; Yan, L.; Jiang, Y. The synthesis, regulation, and functions of sterols in Candida albicans: Well-known but still lots to learn. Virulence 2016, 7, 649–659. [Google Scholar] [CrossRef]
- Murcia, R.A.; Leal, S.M.; Roa, M.V.; Nagles, E.; Muñoz-Castro, A.; Hurtado, J.J. Development of Antibacterial and Antifungal Triazole Chromium(III) and Cobalt(II) Complexes: Synthesis and Biological Activity Evaluations. Molecules 2018, 13, 23. [Google Scholar] [CrossRef]
- Saitta, M.; Curto, S.L.; Salvo, F.; Di Bella, G.; Dugo, G. Gas chromatographic–tandem mass spectrometric identification of phenolic compounds in Sicilian olive oils. Anal. Chim. Acta 2002, 466, 335–344. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Aguilera, C.M.; Gil, A. A systematic review of the efficacy of bioactive compounds in cardiovascular disease: Phenolic compounds. Nutrients 2015, 29, 5177–5216. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Pezzuto, J.M. Flavonoids in cancer prevention. Anti-Cancer Agents Med. Chem. 2012, 12, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Azmi, A.S.; Bhat, S.H.; Hadi, S. Resveratrol–Cu (II) induced DNA breakage in human peripheral lymphocytes: Implications for anticancer properties. FEBS Lett. 2005, 579, 3131–3135. [Google Scholar] [CrossRef]
- Ahmadi, M.; Pup, M.; Olariu, L.; Vermean, H.; Prejbeanu, R. Manganese and zinc overdose-risk of oxidative stress appearance. Rev. Chim. 2008, 59, 982–985. [Google Scholar]
- Borovansky, J.; Blasko, M.; Siracky, J.; Schothorst, A.A.; Smit, N.P.M.; Pavel, S. Cytotoxic interactions of Zn2+ in vitro: Melanoma cells are more susceptible than melanocytes. Melanoma Res. 1997, 7, 449–453. [Google Scholar] [CrossRef]
- Farmer, P.J.; Gidanian, S.; Shahandeh, B.; Di Bilio, A.J.; Tohidian, N.; Meyskens, F.L., Jr. Melanin as a target for melanoma chemotherapy: Pro-oxidant effect of oxygen and metals on melanoma viability. Pigment Cell Res. 2003, 16, 273–279. [Google Scholar] [CrossRef]
- Song, Y.; Yang, P.; Yang, M.; Kang, J.; Qin, S.; Lü, B.; Wang, L. Spectroscopic and voltammetric studies of the cobalt (II) complex of Morin bound to calf thymus DNA. Transit. Met. Chem. 2003, 28, 712–716. [Google Scholar] [CrossRef]
- Baile, M.B.; Kolhe, N.S.; Deotarse, P.P.; Jain, A.S.; Kulkarni, A.A. Metal Ion Complex -Potential Anticancer Drug- A Review. IJPRR 2015, 4, 59–66. [Google Scholar]
- Miller, S.C. Echinacea: A miracle herb against aging and cancer? Evidence in vivo in mice. Evid. Based Complement. Altern. Med. 2005, 2, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cha, K.H.; Kim, C.Y.; Nho, C.W.; Pan, C.H. Bioavailability of hydroxycinnamic acids from Crepidiastrum denticulatum using simulated digestion and Caco-2 intestinal cells. J. Agric. Food Chem. 2014, 23, 5290–5295. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Sadler, P. Using coordination chemistry to design new Medicines. Coord. Chem. Rev. 2007, 251, 1633–1648. [Google Scholar] [CrossRef]
- Pereira, R.M.; Andrades, N.E.; Paulino, N.; Sawaya, A.C.; Eberlin, M.N.; Marcucci, M.C.; Favero, G.M.; Novak, E.M.; Bydlowski, S.P. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
| Empirical Formula | Yield | %H (Theoret) | %C (Theoret) | %H (Exp) | %C (Exp) |
|---|---|---|---|---|---|
| [C22O12H16Zn2] ·4H2O | 70–75% | 3.60 | 39.59 | 3.69 | 39.80 |
| [C22O12H16Ni2] ·4H2O | 70–80% | 3.67 | 40.41 | 3.56 | 39.76 |
| [C22O12H16Cu2] ·4H2O | 70% | 3.62 | 39.81 | 3.57 | 39.13 |
| [C22O12H16Co2] ·4H2O | 70–75% | 3.67 | 40.38 | 3.59 | 40.05 |
| Cichoric Acid | Sodium Salt | Complexes | Assignments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Copper | Nickel | Zinc | Cobalt | |||||||||||
| Experimental | Theoretical | Experimental | Theoretical | Exp | Theoret | Exp | Exp | Exp | ||||||
| IR | Raman | HF | Int | IR | Raman | HF | Int | IR | HF | Int | IR | IR | IR | |
| 1746 m | 1809 | 331.95 | νasCOOHtart | |||||||||||
| 1716 s | 1797 | 512.19 | νasCOOHtart | |||||||||||
| 1682 vs | 1681 s | 1744 | 18.96 | 1699 s | 1703 w | 1735 | 7.47 | 1721 s | 1736 | 9.23 | 1698 s | 1692 m | νC = Ocaff. νC = Calcaff | |
| 1624 s | 1627 vs | 1742 | 697.97 | 1732 | 686.64 | 1731 | 669.21 | νC = Ocaff. νC = Calcaff | ||||||
| 1626 vs | 1580 | 1255.35 | 1626 s | 1570 | 1105.71 | 1630 s | 1629 vs | 1605 s | νasCOO- | |||||
| 1385 s | 1381 vw | 1447 | 204.62 | 1384 m | 1446 | 185.54 | 1385 m | 1384 s | 1385 m | νsCOO- | ||||
| 853 w | 860 vw | 870 | 18.44 | 868 w | 871 | 10.97 | 851 w | 853 w | 851 m | βsCOO- | ||||
| 869 | 8.1 | 869 | 20.35 | βsCOO- | ||||||||||
| 698 vw | 699 vw | 739 | 2.12 | 746 | 3.41 | 745 | 4.05 | 698 w | 695 m | γCOOH | ||||
| 680 w | 685 vw | 727 | 4.36 | 688 w | 727 | 1.11 | 726 | 2.16 | 691 m | 685 m | defring. γCOOH | |||
| 521 w | 539 | 40.51 | 521 m | 536 | 39.39 | 520 m | 520 m | 521 m | βasCOO- | |||||
| 472 | 39.33 | 471 | 46.44 | βasCOO- | ||||||||||
| 504 w | 504 vw | 517 | 7.63 | γO-Hcaff | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Świderski, G.; Lewandowski, W. Biological Activity of New Cichoric Acid–Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro. Nutrients 2020, 12, 154. https://doi.org/10.3390/nu12010154
Jabłońska-Trypuć A, Wydro U, Wołejko E, Świderski G, Lewandowski W. Biological Activity of New Cichoric Acid–Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro. Nutrients. 2020; 12(1):154. https://doi.org/10.3390/nu12010154
Chicago/Turabian StyleJabłońska-Trypuć, Agata, Urszula Wydro, Elżbieta Wołejko, Grzegorz Świderski, and Włodzimierz Lewandowski. 2020. "Biological Activity of New Cichoric Acid–Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro" Nutrients 12, no. 1: 154. https://doi.org/10.3390/nu12010154
APA StyleJabłońska-Trypuć, A., Wydro, U., Wołejko, E., Świderski, G., & Lewandowski, W. (2020). Biological Activity of New Cichoric Acid–Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro. Nutrients, 12(1), 154. https://doi.org/10.3390/nu12010154









