The Effect of Zinc, Selenium, and Their Combined Supplementation on Androgen Receptor Protein Expression in the Prostate Lobes and Serum Steroid Hormone Concentrations of Wistar Rats
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Animals
- -
- zinc gluconate in conversion to Zn: 5 mg Zn/kg body weight (b.w.)/day (n = 21);
- -
- selenomethionine in conversion to Se: 2.8 µg Se/kg b.w./day (n = 21);
- -
- zinc gluconate and selenomethionine combined at the above doses (n = 21).
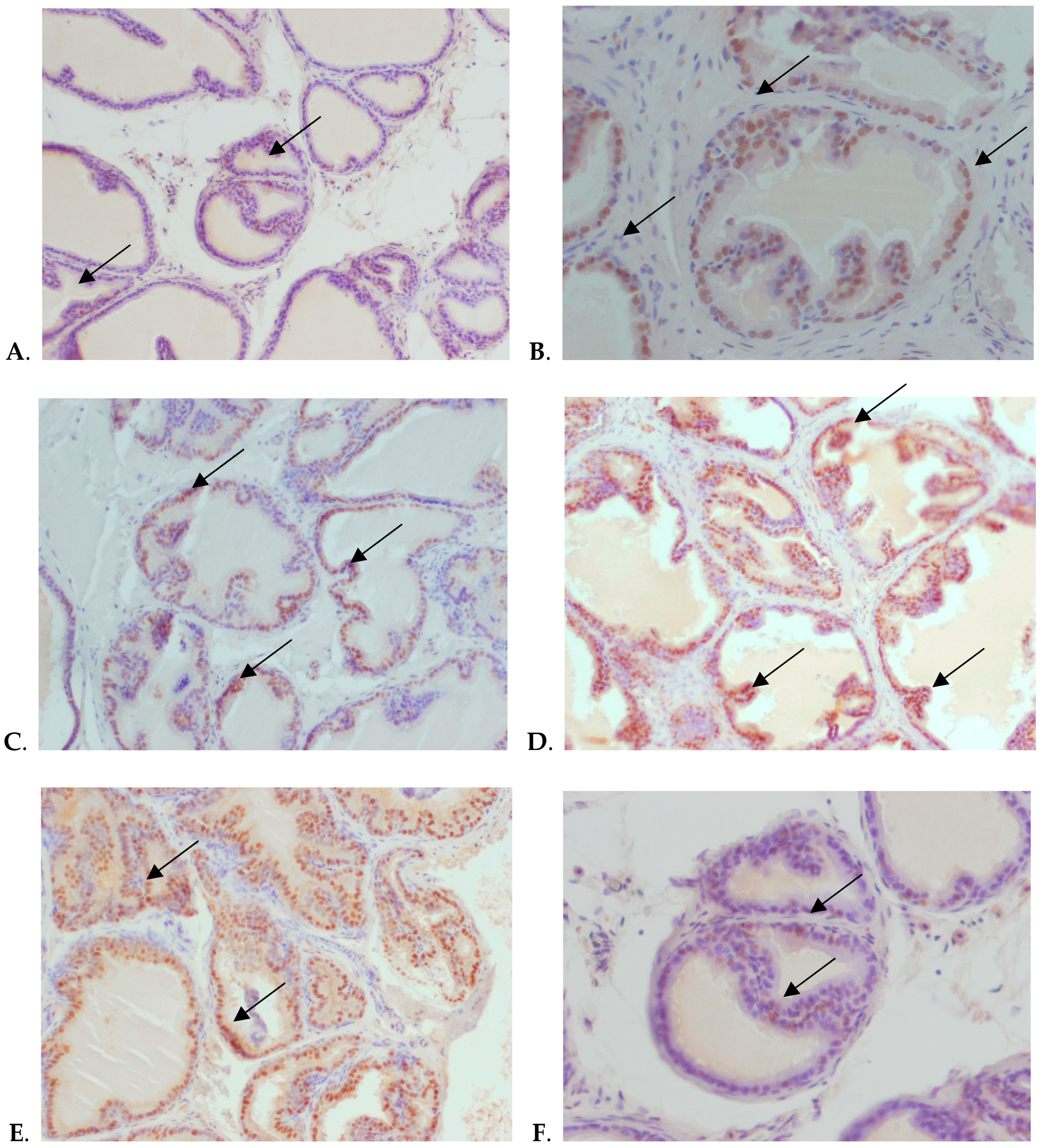
2.3. Immunohistochemical Staining and Scoring of AR
2.4. Hormone Determination
2.5. Zinc and Selenium Determinations in Blood
2.6. Statistical Analysis
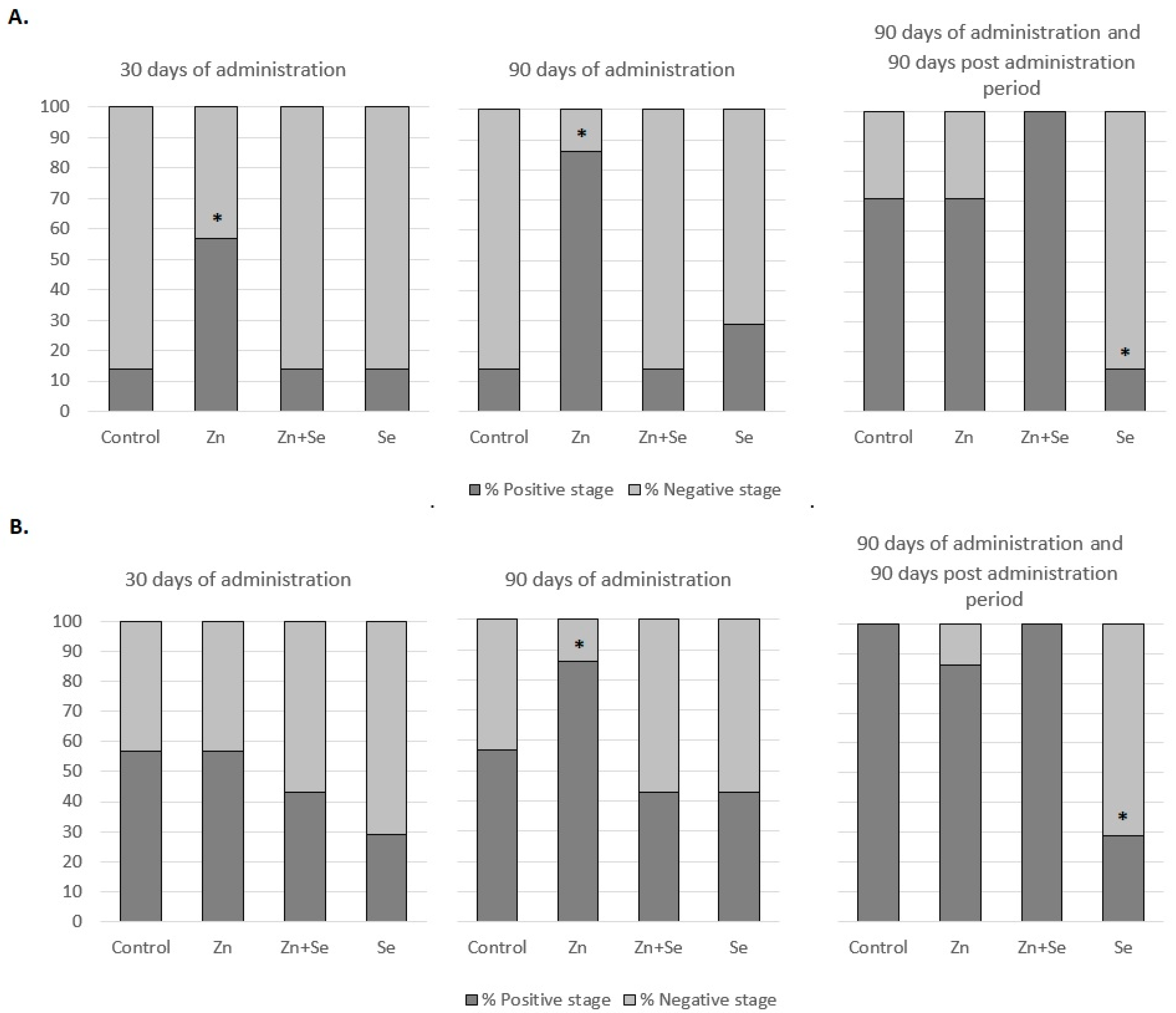
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyer, F.; Galan, P.; Douville, P.; Bairati, I.; Kegle, P.; Bertrais, S.; Czernichow, S.; Hercberg, S. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int. J. Cancer 2005, 116, 182–186. [Google Scholar] [CrossRef]
- Kulbacka, J.; Saczko, J.; Chwiłkowska, A. Oxidative stress in cells damage processes. Pol. Merkur. Lekarski 2009, 27, 44–47. (In Polish) [Google Scholar]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Helzlsouer, K.J. Selenium, zinc, and prostate cancer. Epidemiol. Rev. 2001, 23, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; O’Neill, J.S. The significance of steroid metabolism in human cancer. J. Steroid Biochem. Mol. Biol. 1990, 37, 317–325. [Google Scholar] [CrossRef]
- Habib, F.K. Steroid hormones and cancer: IV. Prostate cancer. Eur. J. Surg. Oncol. 1997, 23, 264–268. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Fujita, K.; Nonomura, N. Role of androgen receptor in prostate cancer: A review. World J. Men’s Health 2019, 37, 288–295. [Google Scholar] [CrossRef]
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011, 10, 20. [Google Scholar]
- Husain, I.; Shukla, S.; Soni, P.; Husain, N. Role of androgen receptor in prostatic neoplasia versus hyperplasia. J. Cancer Res. Ther. 2016, 12, 112–116. [Google Scholar]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941, 1, 293–297. [Google Scholar]
- Oliveira, A.G.; Coelho, P.H.; Guedes, F.D.; Mahecha, G.A.; Hess, R.A.; Oliveira, C.A. 5alpha-Androstane-3beta,17beta-diol (3beta-diol), an estrogenic metabolite of 5alpha-dihydrotestosterone, is a potent modulator of estrogen receptor ERbeta expression in the ventral prostrate of adult rats. Steroids 2007, 72, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Testosterone, prolactin, and oncogenic regulation of the prostate gland. A new concept: Testosterone-independent malignancy is the development of prolactin-dependent malignancy! Oncol. Rev. 2018, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Arnold, K.B.; Schenk, J.M.; Neuhouser, M.L.; Goodman, P.; Penson, D.F.; Thompson, I.M. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: Results from the prostate cancer prevention trial. Am. J. Epidemiol. 2008, 167, 925–934. [Google Scholar] [CrossRef]
- Gutiérrez-González, E.; Castelló, A.; Fernández-Navarro, P.; Castaño-Vinyals, G.; Llorca, J.; Salas, D.; Salcedo-Bellido, I.; Aragonés, N.; Fernández-Tardón, G.; Alguacil, J.; et al. Dietary zinc and risk of prostate cancer in Spain: MCC-Spain study. Nutrients 2018, 11, 18. [Google Scholar] [CrossRef]
- Franklin, R.B.; Costello, L.C. The important role of the apoptotic effects of zinc in the development of cancers. J. Cell Biochem. 2009, 106, 750–757. [Google Scholar] [CrossRef]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the interaction between Selenium and Zinc on DNA repair in association with cancer prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef]
- Christensen, M.J. Selenium and prostate cancer prevention: What next-if anything? Cancer Prev. Res. 2014, 7, 781–785. [Google Scholar] [CrossRef]
- Shalini, S.; Bansal, M.P. Role of selenium in regulation of spermatogenesis: Involvement of activator protein 1. Biofactors 2005, 23, 151–162. [Google Scholar] [CrossRef]
- Maret, W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp. Gerontol. 2008, 43, 363–369. [Google Scholar] [CrossRef]
- Bell, S.G.; Vallee, B.L. The metallothionein/thionein system: An oxidoreductive metabolic zinc link. Chembiochem 2009, 10, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Daragó, A.; Sapota, A.; Nasiadek, M.; Klimczak, M.; Kilanowicz, A. The effect of Zinc and Selenium supplementation mode on their bioavailability in the rat prostate. Should administration be joint or separate? Nutrients 2016, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R.; Zirkin, B.R. Androgen action in prostate function and disease. Am. J. Clin. Exp. Urol. 2018, 6, 62–77. [Google Scholar] [PubMed]
- Nicastro, H.L.; Dunn, B.K. Selenium and prostate cancer prevention: Insights from the selenium and vitamin E cancer prevention trial (SELECT). Nutrients 2013, 5, 1122–1148. [Google Scholar] [CrossRef]
- Milne, G.W.A. Gardner’s Commercially Important Chemicals: Synonyms, Trade Names, and Properties; Ashgate Publishing: Farnham, UK, 2005. [Google Scholar]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Danch, A.; Drozdz, M. A simplified technique of fluorometric selenium assay in biological material. Diagn. Lab. 1996, 32, 529–534. [Google Scholar]
- Feng, Q.; He, B. Androgen receptor signaling in the development of castration-resistant prostate cancer. Front. Oncol. 2019, 9, 858. [Google Scholar] [CrossRef]
- Raynaud, J.P. Prostate cancer risk in testosterone-treated men. J. Steroid Biochem. Mol. Biol. 2006, 102, 261–266. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: Connecting the dots. Mol. Cancer 2006, 5, 17. [Google Scholar] [CrossRef][Green Version]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2019, in press. [Google Scholar] [CrossRef]
- Chung, K.W.; Kim, S.Y.; Chan, W.Y.; Rennert, O.M. Androgen receptors in ventral prostate glands of zinc deficient rats. Life Sci. 1986, 38, 351–356. [Google Scholar] [CrossRef]
- Om, A.S.; Chung, K.W. Dietary zinc deficiency alters 5 alpha-reduction and aromatization of testosterone and androgen and estrogen receptors in rat liver. J. Nutr. 1996, 126, 842–848. [Google Scholar] [CrossRef] [PubMed]
- To, P.K.; Do, M.H.; Cho, Y.S.; Kwon, S.Y.; Kim, M.S.; Jung, C. Zinc inhibits expression of androgen receptor to suppress growth of prostate cancer cells. Int. J. Mol. Sci. 2018, 19, 3062. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.W.; Hoeschele, J.D.; Turner, J.E.; Jacobson, K.B.; Christie, N.T.; Paton, C.L.; Smith, L.H.; Witschi, H.R.; Lee, E.H. Chemical softness and acute metal toxicity in mice and Drosophila. Toxicol. Appl. Pharmacol. 1982, 63, 461–469. [Google Scholar] [CrossRef]
- Dong, Y.; Lee, S.O.; Zhang, H.; Marshall, J.; Gao, A.C.; Ip, C. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004, 64, 19–22. [Google Scholar] [CrossRef]
- Chun, J.Y.; Nadiminty, N.; Lee, S.O.; Onate, S.A.; Lou, W.; Gao, A.C. Mechanisms of selenium down-regulation of androgen receptor signaling in prostate cancer. Mol. Cancer Ther. 2006, 5, 913–918. [Google Scholar] [CrossRef]
- Corcoran, N.M.; Najdovska, M.; Costello, A.J. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J. Urol. 2004, 171, 907–910. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, H.; Hawthorn, L.; Ganther, H.E.; Ip, C. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Res. 2003, 63, 52–59. [Google Scholar]
- Zhao, H.; Whitfield, M.L.; Xu, T.; Botstein, D.; Brooks, J.D. Diverse effects of methylseleninic acid on the transcriptional program of human prostate cancer cells. Mol. Biol. Cell 2004, 15, 506–519. [Google Scholar] [CrossRef]
- Legg, R.L.; Tolman, J.R.; Lovinger, C.T.; Lephart, E.D.; Setchell, K.D.; Christensen, M.J. Diets high in selenium and isoflavones decrease androgen-regulated gene expression in healthy rat dorsolateral prostate. Reprod. Biol. Endocrinol. 2008, 6, 57. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, H.; Gao, A.C.; Marshall, J.R.; Ip, C. Androgen receptor signaling intensity is a key factor in determining the sensitivity of prostate cancer cells to selenium inhibition of growth and cancer-specific biomarkers. Mol. Cancer Ther. 2005, 4, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Husbeck, B.; Bhattacharyya, R.S.; Feldman, D.; Knox, S.J. Inhibition of androgen receptor signaling by selenite and methylseleninic acid in prostate cancer cells: Two distinct mechanisms of action. Mol. Cancer Ther. 2006, 5, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
| Analytical Range | Sensitivity | |
|---|---|---|
| LH | 0.052–10.4 ng/mL | 2.6 pg/mL |
| FSH | 0.052–8.9 ng/mL | 5.2 pg/mL |
| E2 | 20–200 pg/mL | 15 pg/mL |
| T | 0.0125–15.0 ng/mL | 0.12 ng/mL |
| DHT | 0.3–72 pg/mL | 0.224 pg/mL |
| A. | ||||
| Group | 30 Days of Administration Mean ± SEM (Median) | 90 Days of Administration Mean ± SEM (Median) | 90 Days of Administration and 90-Day Post Administration Period Mean ± SEM (Median) | Comparing Means between Time Points in the Study Groups (p-Value) |
| Control | 1.57 ± 0.43 (2) | 1.57 ± 0.43 (2) | 2.86 ± 0.26 (3) | 0.490 |
| Zn | 2.14 ± 0.59 (3) | 3.14 ± 0.55 (4) | 4.43 ± 0.78 (5) | 0.201 |
| Zn + Se | 0.71 ± 0.47 (0) | 0.71 ± 0.47 (0) | 5.14 ± 0.59 (5) | 0.002 |
| Se | 0.57 ± 0.57 (0) | 2.14 ± 0.67 (2) | 1.28 ± 0.47 (2) | 0.336 |
| Comparing groups (mean) at the same time points (p-value) | 0.105 | 0.026 | 0.015 | Interaction test: p = 0.217 |
| B. | ||||
| Group | 30 Days of Administration Mean ± SEM (Median) | 90 Days of Administration Mean ± SEM (Median) | 90 Days of Administration and 90 Day Post Administration Period Mean ± SEM (Median) | Comparing Means between Time Points in the Study Groups (p-Value) |
| Control | 2.29 ± 0.64 (3) | 2.86 ± 0.59 (4) | 4.86 ± 0.26 (5) | 0.015 |
| Zn | 2.57 ± 0.78 (3) | 4.14 ± 0.59 (4) | 4.86 ± 0.98 (5) | 0.023 |
| Zn + Se | 1.86 ± 0.70 (2) | 2.57 ± 0.30 (2) | 6.14 ± 0.26 (6) | <0.005 |
| Se | 1.86 ± 0.99 (0) | 2.57 ± 0.75 (3) | 1.57 ± 1.02 (0) | 0.365 |
| Comparing groups (mean) at the same time points (p-value) | 0.545 | 0.036 | <0.005 | Interaction test: p = 0.061 |
| LH [ng/mL] | FSH [ng/mL] | E2 [pg/mL] | T [ng/mL] | DHT [pg/mL] | DHT/T [* 103] | E/T [* 103] | ||
|---|---|---|---|---|---|---|---|---|
| 30 days of administration | Control | 0.68 ± 0.28 | 2.2 ± 0.55 | 25 ± 5.4 | 3.6 ± 0.75 | 5.50 ± 1.3 | 1.4 ± 0.19 | 7.0 ± 2.3 |
| Zn | 0.54 ± 0.41 | 2.0 ± 0.43 | 28 ± 7.8 | 4.8 ± 0.49 * | 4.5 ± 0.70 | 1.0 ± 0.11 * | 5.8 ± 2.5 | |
| Se | 0.64 ± 0.28 | 2.5 ± 0.49 | 34 ± 5.3 | 4.5 ± 1.5 | 5.3 ± 1.7 | 1.1 ± 0.34 | 7.5 ± 2.2 | |
| Zn + Se | 0.73 ± 0.14 | 2.7 ± 0.54 | 31 ± 5.4 | 3.1 ± 1.6 | 4.6 ± 0.80 | 1.5 ± 0.24 | 10.2 ± 3.1 | |
| 90 days of administration | Control | 0.73 ± 0.21 | 2.3 ± 0.48 | 37 ± 6.6 | 5.7 ± 0.81 | 5.4 ± 0.75 | 1.0 ± 0.14 | 6.8 ± 2.1 |
| Zn | 0.35 ± 0.43 | 2.9 ± 0.55 | 33 ± 7.1 | 13 ± 6.4 * | 4.8 ± 0.89 | 0.4 ± 0.4 * | 2.4 ± 2.2 * | |
| Se | 0.81 ± 0.31 | 2.0 ± 0.15 | 30 ± 11 | 6.4 ± 1.2 | 5.4 ± 1.0 | 0.8 ± 0.21 | 4.7 ± 2.8 | |
| Zn + Se | 0.82 ± 0.32 | 2.8 ± 0.33 | 30 ± 6.8 | 3.1 ± 1.0 | 4.9 ± 1.6 | 1.4 ± 0.28 | 9.8 ± 2.8 | |
| 90 days of administration and 90 day post administration period | Control | 0.77 ± 0.21 | 2.2 ± 0.41 | 47 ± 9.1 | 2.2 ± 1.8 | 5.1 ± 0.69 | 2.3 ± 0.36 | 21.6 ± 3.7 |
| Zn | 0.72 ± 0.15 | 2.1 ± 0.31 | 44 ± 18 | 3.7 ± 2.1 | 5.2 ± 0.75 | 1.6 ± 0.38 | 11.8 ± 4.8 * | |
| Se | 0.68 ± 0.13 | 2.4 ± 0.31 | 38 ± 11 | 2.7 ± 1.6 | 4.5 ± 1.6 | 1.8 ± 0.31 | 14.0 ± 4.2 | |
| Zn + Se | 0.74 ± 0.19 | 2.2 ± 0.21 | 40 ± 5.4 | 2.6 ± 0.46 | 5.3 ± 1.8 | 2.1 ± 0.39 | 15.5 ± 3.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daragó, A.; Klimczak, M.; Stragierowicz, J.; Stasikowska-Kanicka, O.; Kilanowicz, A. The Effect of Zinc, Selenium, and Their Combined Supplementation on Androgen Receptor Protein Expression in the Prostate Lobes and Serum Steroid Hormone Concentrations of Wistar Rats. Nutrients 2020, 12, 153. https://doi.org/10.3390/nu12010153
Daragó A, Klimczak M, Stragierowicz J, Stasikowska-Kanicka O, Kilanowicz A. The Effect of Zinc, Selenium, and Their Combined Supplementation on Androgen Receptor Protein Expression in the Prostate Lobes and Serum Steroid Hormone Concentrations of Wistar Rats. Nutrients. 2020; 12(1):153. https://doi.org/10.3390/nu12010153
Chicago/Turabian StyleDaragó, Adam, Michał Klimczak, Joanna Stragierowicz, Olga Stasikowska-Kanicka, and Anna Kilanowicz. 2020. "The Effect of Zinc, Selenium, and Their Combined Supplementation on Androgen Receptor Protein Expression in the Prostate Lobes and Serum Steroid Hormone Concentrations of Wistar Rats" Nutrients 12, no. 1: 153. https://doi.org/10.3390/nu12010153
APA StyleDaragó, A., Klimczak, M., Stragierowicz, J., Stasikowska-Kanicka, O., & Kilanowicz, A. (2020). The Effect of Zinc, Selenium, and Their Combined Supplementation on Androgen Receptor Protein Expression in the Prostate Lobes and Serum Steroid Hormone Concentrations of Wistar Rats. Nutrients, 12(1), 153. https://doi.org/10.3390/nu12010153






