The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review
Abstract
1. Introduction
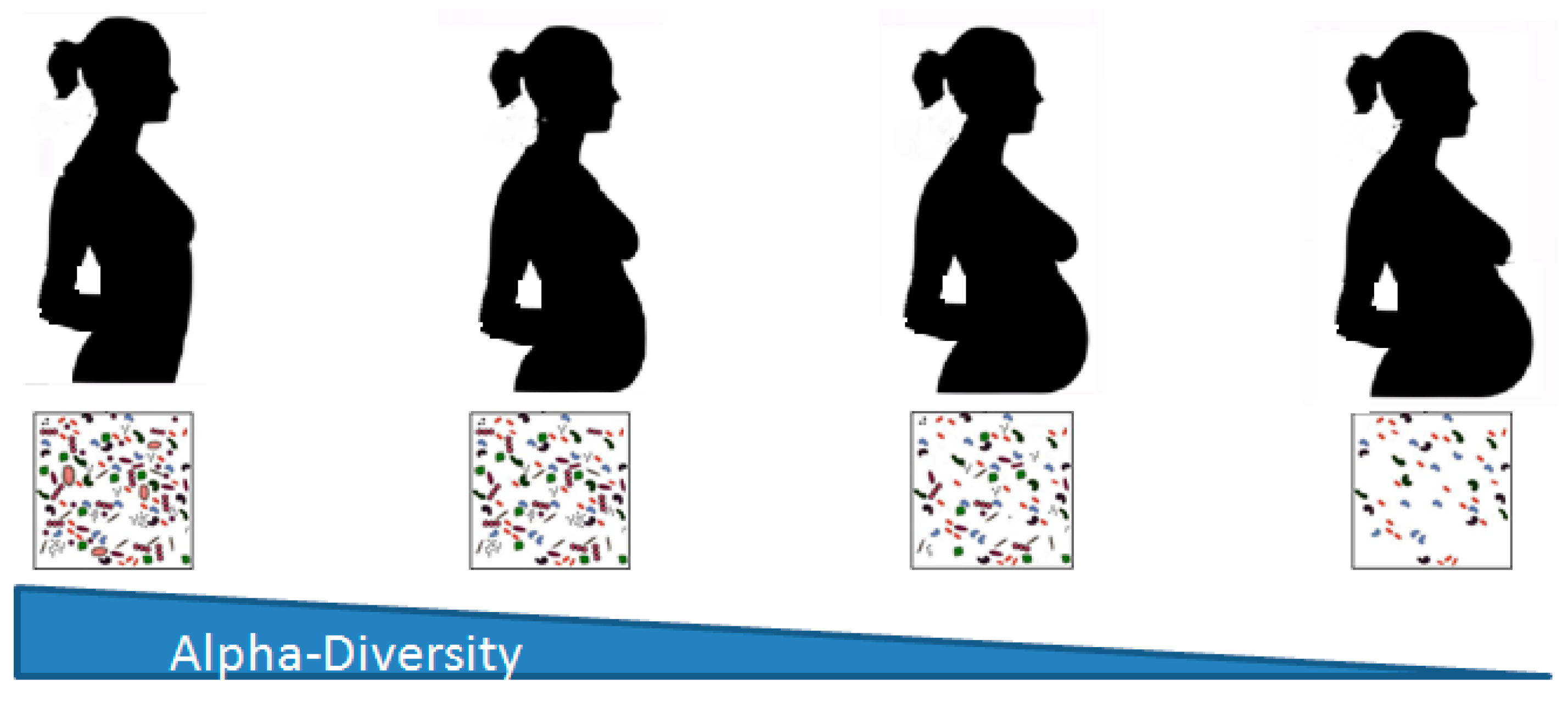
2. Changes in the Microbiome during Pregnancy
2.1. Gut Microbiota
2.2. Vaginal Microbiota
2.3. Oral Microbiota
2.4. Placental Microbiota and Fetal Colonization
3. Changes in the Microbiome Related to the Type of Delivery
4. Microbiome and the Type of Feeding
5. Microbiome in Pathological and Adverse Pregnancy Outcomes
6. Microbiome and Obese Pregnancy
7. Microbiome in Critical Ill Children
8. Microbiome and Sepsis in the Newborn
9. Microbiome and Allergic Conditions
9.1. Gut Microbiome and Atopy
9.2. Gut Microbiome and Food Allergy
9.3. Gut Microbiome and Asthma
10. Microbiome and Infection in Infants
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Newbern, D.; Freemark, M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Bale, T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocrinol. 2019, 55, 100797. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef]
- Peelen, M.J.; Luef, B.M.; Lamont, R.F.; de Milliano, I.; Jensen, J.S.; Limpens, J.; Hajenius, P.J.; Jørgensen, J.S.; Menon, R.; PREBIC Biomarker Working Group 2014–2018. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta 2019, 79, 30–39. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Piccolo, B.D.; Mercer, K.E.; Andres, A.; Thakali, K.M.; Shankar, K. Maternal high-fat diet programs offspring liver steatosis in a sexually dimorphic manner in association with changes in gut microbial ecology in mice. Sci. Rep. 2018, 8, 16502. [Google Scholar] [CrossRef]
- Olivier-Van Stichelen, S.; Rother, K.I.; Hanover, J.A. Maternal exposure to non-nutritive sweeteners impacts progeny’s metabolism and microbiome. Front. Microbiol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; Garcia-Valdes, L.; Segura, M.T.; Martin-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1230. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M.; SPRING Trial Group. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Van den Veyver, I.; Milosavljevic, A.; et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 21, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.L.; Ma, J.; Kannan, P.S.; Alvarez, M.; Gisslen, T.; Harris, R.A.; Sweeney, E.L.; Knox, C.L.; Lambers, D.S.; Jobe, A.H.; et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am. J. Obstet. Gynecol. 2016, 214, 627. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 28, 48. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Hase, K. Commensal microbiota regulates T cell fate decision in the gut. Semin. Immunopathol. 2015, 37, 17–25. [Google Scholar] [CrossRef]
- Havstad, S.; Johnson, C.C.; Kim, H.; Levin, A.M.; Zoratti, E.M.; Joseph, C.L.; Ownby, D.R.; Wegienka, G. Atopic phenotypes identified with latent class analyses at age 2 years. J. Allergy Clin. Immunol. 2014, 134, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V. Gut Microbiota and Allergic Disease. New Insights. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 1), S51–S54. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Koren, O. The Pregnancy Microbiome. Nestle Nutr. Inst. Workshop Ser. 2017, 88, 1–9. [Google Scholar] [CrossRef]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef]
- Gosalbes, M.J.; Compte, J.; Moriano-Gutierrez, S.; Vallès, Y.; Jiménez-Hernández, N.; Pons, X.; Artacho, A.; Francino, M.P. Metabolic adaptation in the human gut microbiota during pregnancy and the first year of life. EBioMedicine 2019, 39, 497–509. [Google Scholar] [CrossRef]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef]
- Nyangahu, D.D.; Jaspan, H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 2019, 23. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Stoyancheva, G.; Marzotto, M.; Dellaglio, F.; Torriani, S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch. Microbiol. 2014, 196, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.J.; Zhou, Y.; Wylie, K.M.; Tarr, P.I.; Macones, G.A.; Tuuli, M.G. Early pregnancy vaginal microbiome trends and preterm birth. Am. J. Obstet. Gynecol. 2017, 217, 356.e1–356.e18. [Google Scholar] [CrossRef]
- Haque, M.M.; Merchant, M.; Kumar, P.N.; Dutta, A.; Mande, S.S. First-trimester vaginal microbiome diversity: A potential indicator of preterm delivery risk. Sci. Rep. 2017, 7, 16145. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, W.; Hu, X.; Gao, L.; Ai, D.; Pan, H.; Niu, C.; Yuan, K.; Zhou, X.; Xu, C.; et al. Ecological Shifts of Supragingival Microbiota in Association with Pregnancy. Front. Cell. Infect. Microbiol. 2018, 8, 24. [Google Scholar] [CrossRef]
- Fujiwara, N.; Tsuruda, K.; Iwamoto, Y.; Kato, F.; Odaki, T.; Yamane, N.; Hori, Y.; Harashima, Y.; Sakoda, A.; Tagaya, A.; et al. Significant increase of oral bacteria in the early pregnancy period in Japanese women. J. Investig. Clin. Dent. 2017, 8, 1. [Google Scholar] [CrossRef]
- Kornman, K.S.; Loesche, W.J. Effects of estradiol and progesteroneon Bacteroides melaninogenicus and Bacteroides gingivalis. Infect. Immun. 1982, 35, 256–263. [Google Scholar]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Pelzer, E.; Gomez-Arango, L.F.; Barrett, H.L.; Nitert, M.D. Review: Maternal health and the placental microbiome. Placenta 2017, 54, 30–37. [Google Scholar] [CrossRef]
- D’Argenio, V. The Prenatal Microbiome: A New Player for Human Health. High. Throughput 2018, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.; Fernandez, L.; Marin, M.L.; Martin, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by C-section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Panigrahi, P. Is a foetus developing in a sterile environment? Lett. Appl. Microbiol. 2014, 59, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 22, 23129. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B.; Gervasi, M.; Romero, R.; Mazaki-Tovi, S.; Vaisbuch, E.; Kusanovic, J.P.; Seok, K.S.; Gómez, R.; Mittal, P.; Gotsch, F.; et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J. Perinat. Med. 2010, 38, 503–513. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Romero, R.; Kusanovic, J.P.; Gómez, R.; Kim, C.J.; Seok, K.S.; Gotsch, F.; Mazaki-Tovi, S.; Vaisbuch, E.; Sanders, K.; et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod. Immunol. 2010, 64, 38–57. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaemsaithong, P.; Chaiworapongsa, T.; Kusanovic, J.P.; Dong, Z.; Ahmed, A.I.; Shaman, M.; Lannaman, K.; Yoon, B.H.; et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern.-Fetal Neonatal Med. 2015, 28, 1394–1409. [Google Scholar] [CrossRef]
- McClure, E.M.; Goldenberg, R.L. Infection and stillbirth. Semin. Fetal Neonatal Med. 2009, 14, 182–189. [Google Scholar] [CrossRef]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatumadhesinFadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef]
- Jimenez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Scott, K.P.; Khan, S.; Martin, J.C.; Berry, S.H.; Stevenson, M.; Okpapi, A.; Munro, M.J.; Hold, G.L. First-Pass Meconium Samples from Healthy Term Vaginally-Delivered Neonates: An Analysis of the Microbiota. PLoS ONE 2015, 10, e0133320. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Makino, H.; Cetinyurek Yavuz, A.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef] [PubMed]
- Lauder, A.P.; Roche, A.M.; Sherrill-Mix, S.; Bailey, A.; Laughlin, A.L.; Bittinger, K.; Leite, R.; Elovitz, M.A.; Parry, S.; Bushman, F.D. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 2016, 23, 29. [Google Scholar] [CrossRef]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Stinson, L.F.; Payne, M.S.; Keelan, J.A. A Critical Review of the Bacterial Baptism Hypothesis and the Impact of C-section Delivery on the Infant Microbiome. Front. Med. 2018, 4, 135. [Google Scholar] [CrossRef]
- Montoya-Williams, D.; Lemas, D.J.; Spiryda, L.; Patel, K.; Carney, O.O.; Neu, J.; Carson, T.L. The Neonatal Microbiome and Its Partial Role in Mediating the Association between Birth by C-Section and Adverse Pediatric Outcomes. Neonatology 2018, 114, 103–111. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Biasucci, G.; Rubini, M.; Riboni, S.; Morelli, L.; Bessi, E.; Retetangos, C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010, 86 (Suppl. 1), 13–15. [Google Scholar] [CrossRef]
- Shi, Y.C.; Guo, H.; Chen, J.; Sun, G.; Ren, R.R.; Guo, M.Z.; Peng, L.H.; Yang, Y.S. Initial meconium microbiome in Chinese neonates delivered naturally or by C-section. Sci. Rep. 2018, 8, 3255. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host. Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Sakwinska, O.; Foata, F.; Berger, B.; Brüssow, H.; Combremont, S.; Mercenier, A.; Dogra, S.; Soh, S.E.; Yen, J.C.K.; Heong, G.Y.S.; et al. Does the maternal vaginal microbiota play a role in seeding the microbiota of neonatal gut and nose? Benef. Microbes 2017, 8, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.S.; Klotz, B.; Valdes, B.E.; Agudelo, G.M. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. 2014, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Seedat, F.; Stinton, C.; Patterson, J.; Geppert, J.; Tan, B.; Robinson, E.R.; McCarthy, N.D.; Uthman, O.A.; Freeman, K.; Johnson, S.A.; et al. Adverse events in women and children who have received intrapartum antibiotic prophylaxis treatment: A systematic review. BMC Pregnancy Childbirth 2017, 17, 247. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L.; et al. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef]
- Martinez, K.A.; Devlin, J.C.; Lacher, C.R.; Yin, Y.; Cai, Y.; Wang, J.; Dominguez-Bello, M.G. Increased weight gain by C-section: Functional significance of the primordial microbiome. Sci. Adv. 2017, 3, eaao1874. [Google Scholar] [CrossRef]
- Laubereau, B.; Filipiak-Pittroff, B.; von Berg, A.; Grübl, A.; Reinhardt, D.; Wichmann, H.E.; Koletzko, S.; GINI Study Group. C-section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Arch. Dis. Child. 2004, 89, 993–997. [Google Scholar] [CrossRef]
- Negele, K.; Heinrich, J.; Borte, M.; von Berg, A.; Schaaf, B.; Lehmann, I.; Wichmann, H.E.; Bolte, G.; LISA Study Group. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr. Allergy Immunol. 2004, 15, 48–54. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Mira-Pascual, L.; Mira, A.; Collado, M.C. Impact of mode of delivery on the milk microbiota composition of healthy women. J. Dev. Orig. Health. Dis. 2016, 7, 54–60. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Peroni, D.G.; Grossi, E.; Facchin, V.; Comberiati, P.; Drago, L. Impact of delivery mode on the colostrum microbiota composition. BMC Microbiol. 2017, 17, 205. [Google Scholar] [CrossRef]
- Neu, J.; Rushing, J. C-section versus vaginal delivery: Long-term infant outcomes and the hygiene hypothesis. Clin. Perinatol. 2011, 38, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Moya-Perez, A.; Luczynski, P.; Renes, I.B.; Wang, S.; Borre, Y.; Anthony Ryan, C.; Knol, J.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Intervention strategies for cesarean section-induced alterations in the microbiota-gut-brain axis. Nutr. Rev. 2017, 75, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Cristofori, F.; Tripaldi, M.E.; Indrio, F. Intervention for Dysbiosis in Children Born by C-Section. Ann. Nutr. Metab. 2018, 73 (Suppl. 3), 33–39. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Priming for health: Gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr. 2014, 103, 812–819. [Google Scholar] [CrossRef]
- Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr. Res. 2012, 72, 77–85. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e884. [Google Scholar] [CrossRef]
- Belfort, M.B.; Anderson, P.J.; Nowak, V.A.; Lee, K.J.; Molesworth, C.; Thompson, D.K.; Doyle, L.W.; Inder, T.E. Breast milk feeding, brain development, and neurocognitive outcomes: A 7-year longitudinal study in infants born at less than 30 weeks’ gestation. J. Pediatr. 2016, 177, 133–139. [Google Scholar] [CrossRef]
- Meinzen-Derr, J.; Poindexter, B.; Wrage, L.; Morrow, A.L.; Stoll, B.; Donovan, E.F. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J. Perinatol. 2009, 29, 57–62. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Parra-Llorca, A.; Gormaz, M.; Alcantara, C.; Cernada, M.; Nuñez-Ramiro, A.; Vento, M.; Collado, M.C. Preterm Gut Microbiome Depending on Feeding Type: Significance of Donor Human Milk. Front. Microbiol. 2018, 27, 1376. [Google Scholar] [CrossRef] [PubMed]
- Vento, M.; Hummler, H.; Dawson, J.A.; Escobar, J.; Kuligowski, J. Use of Oxygen in the Resuscitation of Neonates. In Perinatal and Prenatal Disorders; Dennery, P.A., Buonocore, G., Saugstad, O.D., Eds.; Humana Press: New York, NY, USA, 2014; pp. 213–244. [Google Scholar]
- Parra-Llorca, A.; Gormaz, M.; Sánchez-Illana, A.; Piñeiro-Ramos, J.D.; Collado, M.C.; Serna, E.; Cernada, M.; Nuñez-Ramiro, A.; Ramón-Beltrán, A.; Oger, C.; et al. Does pasteurized donor human milk efficiently protect preterm infants against oxidative stress? Antioxid. Redox Signal. 2019, 31, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Parra-Llorca, A.; Gormaz, M.; Lorente-Pozo, S.; Cernada, M.; García-Robles, A.; Torres-Cuevas, I.; Kuligowski, J.; Collado, M.C.; Serna, E.; Vento, M. Impact of donor human milk in the preterm very low birth weight gut transcriptome profile by use of exfoliated intestinal cells. Nutrients 2019, 11, 2677. [Google Scholar] [CrossRef]
- Bertino, E.; Giuliani, F.; Occhi, L.; Coscia, A.; Tonetto, P.; Marchino, F.; Fabris, C. Benefits of donor human milk for preterm infants: Current evidence. Early Hum. Dev. 2009, 85, S9–S10. [Google Scholar] [CrossRef] [PubMed]
- Christen, L.; Lai, C.T.; Hartmann, B.; Hartmann, P.E.; Geddes, D.T. The effect of UV-C pasteurization on bacteriostatic properties and immunological proteins of donor human milk. PLoS ONE 2013, 8, e85867. [Google Scholar] [CrossRef] [PubMed]
- Madore, L.S.; Bora, S.; Erdei, C.; Jumani, T.; Dengos, A.R.; Sen, S. Effects of donor breastmilk feeding on growth and early neurodevelopmental outcomes in preterm infants: An observational study. Clin. Ther. 2017, 39, 1210–1220. [Google Scholar] [CrossRef]
- Untalan, P.B.; Keeney, S.E.; Palkowetz, K.H.; Rivera, A.; Goldman, S. Heat susceptibility of interleukin-10 and other cytokines in donor human milk. Breastfeed. Med. 2009, 4, 137–144. [Google Scholar] [CrossRef]
- Sousa, S.G.; Santos, M.D.; Fidalgo, L.G.; Delgadillo, I.; Saraiva, J.A. Effect of thermal pasteurisation and high-pressure processing on immunoglobulin content and lysozyme and lactoperoxidase activity in human colostrum. Food Chem. 2014, 151, 79–85. [Google Scholar] [CrossRef]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: A review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef]
- Ardissone, A.N.; De La Cruz, D.M.; Davis-Richardson, A.G.; Rechcigl, K.T.; Li, N.; Drew, J.C.; Murgas-Torrazza, R.; Sharma, R.; Hudak, M.L.; Triplett, E.W.; et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS ONE 2014, 9, e90784. [Google Scholar] [CrossRef]
- Antony, K.M.; Ma, J.; Mitchell, K.B.; Racusin, D.A.; Versalovic, J.; Aagaard, K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am. J. Obstet. Gynecol. 2015, 212, 653.e1–653.e16. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, X.; Zhang, Q.; Mao, L.; Yu, M.; Xu, J. The placental microbiome varies in association with low birth weight in full-term neonates. Nutrients 2015, 7, 6924–6937. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, H.; Yin, Z.; Jiang, X.; Zhong, H.; Qiu, D.; Zhu, F.; Li, R. Remodeling of the gut microbiota and structural shifts in Preeclampsia patients in South China. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 713–719. [Google Scholar] [CrossRef]
- Chanomethaporn, A.; Chayasadom, A.; Wara-Aswapati, N.; Kongwattanakul, K.; Suwannarong, W.; Tangwanichgapong, K.; Sumanonta, G.; Matangkasombut, O.; Dasanayake, A.P.; Pitiphat, W. Association between periodontitis and spontaneous abortion: A case-control study. J. Periodontol. 2019, 90, 381–390. [Google Scholar] [CrossRef]
- Stevens, G.A.; Singh, G.M.; Lu, Y.; Danaei, G.; Lin, J.K.; Finucane, M.M.; Bahalim, A.N.; McIntire, R.K.; Gutierrez, H.R.; Cowan, M.; et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 2012, 10, 22. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabates Rep. 2016, 16, 7. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Sandboge, S.; Salonen, M.; Kajantie, E.; Osmond, C. Maternal weight in pregnancy and offspring body composition in late adulthood: Findings from the Helsinki Birth Cohort Study (HBCS). Ann. Med. 2015, 47, 94–99. [Google Scholar] [CrossRef]
- Hussen, H.I.; Persson, M.; Moradi, T. Maternal overweight and obesity are associated with increased risk of type 1 diabetes in offspring of parents without diabetes regardless of ethnicity. Diabetologia 2015, 58, 1464–1473. [Google Scholar] [CrossRef]
- Gaillard, R.; Santos, S.; Duijts, L.; Felix, J.F. Childhood Health Consequences of Maternal Obesity during Pregnancy: A Narrative Review. Ann. Nutr. Metab. 2016, 69, 171–180. [Google Scholar] [CrossRef]
- Toemen, L.; Gishti, O.; van Osch-Gevers, L.; Steegers, E.A.; Helbing, W.A.; Felix, J.F.; Reiss, I.K.; Duijts, L.; Gaillard, R.; Jaddoe, V.W. Maternal obesity, gestational weight gain and childhood cardiac outcomes: Role of childhood body mass index. Int. J. Obes. 2016, 40, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 2014, 20, 3889. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Shin, H.; Pizoni, A.; Werlang, I.C.; Matte, U.; Goldani, M.Z.; Goldani, H.A.; Dominguez-Bello, M.G. Birth mode-dependent association between pre-pregnancy maternal weight status and the neonatal intestinal microbiome. Sci. Rep. 2016, 1, 23133. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Collado, M.C. Obesity and overweight: Impact on maternal and milk microbiome and their role for infant health and nutrition. Mol. Nutr. Food Res. 2016, 60, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Taveras, E.M.; Rifas-Shiman, S.L.; Belfort, M.B.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 2009, 123, 1177–1183. [Google Scholar] [CrossRef]
- Jacobs, M.C.; Haak, B.W.; Hugenholtz, F.; Wiersinga, W.J. Gut microbiota and host defense in critical illness. Curr. Opin. Crit. Care 2017, 23, 257–263. [Google Scholar] [CrossRef]
- Williams, V.; Angurana, S.K. Probiotics do have a role to play in treating critically ill children. Acta Paediatr. 2019, 108, 180. [Google Scholar] [CrossRef]
- Kitsios, G.D.; Morowitz, M.J.; Dickson, R.P.; Huffnagle, G.B.; McVerry, B.J.; Morris, A. Dysbiosis in the intensive care unit: Microbiome science coming to the bedside. J. Crit. Care 2017, 38, 84–91. [Google Scholar] [CrossRef]
- Lyons, J.D.; Coopersmith, C.M. Pathophysiology of the Gut and the Microbiome in the Host Response. Pediatr. Crit. Care Med. 2017, 18, S46–S49. [Google Scholar] [CrossRef][Green Version]
- Wischmeyer, P.E.; McDonald, D.; Knight, R. Role of the microbiome, probiotics, and ‘dysbiosis therapy’ in critical illness. Curr. Opin. Crit. Care 2016, 22, 347–353. [Google Scholar] [CrossRef]
- Lankelma, J.M.; van Vught, L.A.; Belzer, C.; Schultz, M.J.; van der Poll, T.; de Vos, W.M.; Wiersinga, W.J. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: A pilot study. Intensive Care Med. 2017, 43, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.B.; Firek, B.; Shi, M.; Yeh, A.; Brower-Sinning, R.; Aveson, V.; Kohl, B.L.; Fabio, A.; Carcillo, J.A.; Morowitz, M.J. Disruption of the microbiota across multiple body sites in critically ill children. Microbiome 2016, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Surana, N.K.; Kasper, D.L. Deciphering the tête-à-tête between the microbiota and the immune system. J. Clin. Investig. 2014, 124, 4197–4203. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 2, 926–938. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 13, 681–689. [Google Scholar] [CrossRef]
- Wolff, N.S.; Hugenholtz, F.; Wiersinga, W.J. The emerging role of the microbiota in the ICU. Crit. Care 2018, 22, 78. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Haaka, B.W.; Levi, M.; Wiersinga, W.J. Microbiota-targeted therapies on the intensive care unit. Curr. Opin. Crit. Care 2017, 23, 167–174. [Google Scholar] [CrossRef]
- Dickson, R.P. The microbiome and critical illness. Lancet Respir. Med. 2016, 4, 59–72. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Zhou, M.J.; Cohen, M.E.; Annavajhala, M.K.; Khan, S.; Moscoso, D.I.; Brooks, C.; Whittier, S.; Chong, D.H.; Uhlemann, A.C.; et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018, 44, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Stoll, B.J. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am. J. Perinatol. 2013, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Özenci, V.; Schubert, U. Earlier and more targeted treatment of neonatal sepsis. Acta Paediatr. 2018, 108, 169–170. [Google Scholar] [CrossRef]
- Rosen, G.H.; Randis, T.M.; Desai, P.V.; Sapra, K.J.; Ma, B.; Gajer, P.; Humphrys, M.S.; Ravel, J.; Gelbe, S.E.; Ratner, A.J. Group B Streptococcus and the vaginal microbiota. J. Infect. Dis. 2017, 16, 744–751. [Google Scholar] [CrossRef]
- Kolter, J.; Henneke, P. Codevelopment of microbiota and innate immunity and the risk for Group B Streptococcal disease. Front. Immunol. 2017, 8, 1497. [Google Scholar] [CrossRef]
- Berrington, J.E.; Stewart, C.J.; Embleton, N.D.; Cummings, S.P. Gut microbiota in preterm infants: Assessment and relevance to health and disease. Arch. Dis. Child.-Fetal Neonatal Ed. 2013, 98, F286–F290. [Google Scholar] [CrossRef]
- Madan, J.C.; Salari, R.C.; Saxena, D.; Davidson, L.; O’Toole, G.A.; Moore, J.H.; Sogin, M.L.; Foster, J.A.; Edwards, W.H.; Palumbo, P.; et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch. Dis. Child.-Fetal Neonatal Ed. 2012, 97, F456–F462. [Google Scholar] [CrossRef]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.L.; Smith, D.P.; Fofanova, T.; Nelson, A. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome 2017, 5, 75. [Google Scholar] [CrossRef]
- Carl, M.A.; Ndao, I.M.; Springman, A.C.; Manning, S.D.; Johnson, J.R.; Johnston, B.D.; Burnham, C.A.D.; Weinstock, E.S.; Weinstock, G.M.; Wylie, T.N.; et al. Sepsis from the gut: The enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin. Infect. Dis. 2014, 58, 1211–1218. [Google Scholar] [CrossRef]
- Dunlop, J.H.; Keet, C.A. Epidemiology of Food Allergy. Immunol. Allergy. Clin. N. Am. 2018, 38, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Burnham, C.A.; Weinstock, E.S.; Weinstock, G.M.; Wylie, T.N.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. 86), 8–160. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Kaltaev, N.; World Health Organization. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach. Global Alliance against Chronic Respiratory Diseases: 2007. Available online: http://www.who.int/iris/handle/10665/43776 (accessed on 2 August 2019).
- Johansson, S.G.; Bieber, T.; Dahl, R.; Friedmann, P.S.; Lanier, B.Q.; Lockey, R.F.; Motala, C.; Ortega Martell, J.A.; Platts-Mills, T.A.; Ring, J.; et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Allergy Clin. Immunol. 2004, 113, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2013, 44, 842–850. [Google Scholar] [CrossRef]
- Wopereis, H.; Sim, K.; Shaw, A.; Warner, J.O.; Knol, J.; Kroll, J.S. Intestinal microbiota in infants at high risk for allergy: Effects of prebiotics and role in eczema development. J. Allergy Clin. Immunol. 2017, 141, 1334–1342. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2015, 137, 852–860. [Google Scholar] [CrossRef]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef]
- Muir, A.B.; Benitez, A.J.; Dods, K.; Spergel, J.M.; Fillon, S.A. Microbiome and its impact on gastrointestinal atopy. Allergy 2016, 71, 1256–1263. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, K.J.; Kong, M.S.; Chang, H.J.; Huang, J.L. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr. Allergy Immunol. 2015, 27, 254–262. [Google Scholar] [CrossRef]
- Berni Canani, R.; Gilbert, J.A.; Nagler, C.R. The role of the commensal microbiota in the regulation of tolerance to dietary allergens. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Tan, V.Y.; Winn, J.; Svensen, M.; Bishop, C.M.; Heckerman, D.E.; Buchan, I.; Custovic, A. Beyond atopy: Multiple patterns of sensitization in relation to asthma in a birth cohort study. Am. J. Respir. Crit. Care Med. 2010, 181, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.M.; Konya, T.; Takaro, T.K.; Brook, J.R.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; et al. Exposure to household furry pets influences the gut microbiota of infant at 3–4 months following various birth scenarios. Microbiome 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.M.; Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Ryan, C.A.; Stanton, C. Programming infant gut microbiota: Influence of dietary and environmental factors. Curr. Opin. Biotechnol. 2010, 21, 149–156. [Google Scholar] [CrossRef]
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef]
- Dogaru, C.M.; Nyffenegger, D.; Pescatore, A.M.; Spycher, B.D.; Kuehni, C.E. Breastfeeding and childhood asthma: Systematic review and meta-analysis. Am. J. Epidemiol. 2014, 179, 1153–1167. [Google Scholar] [CrossRef]
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef]
- Mennini, M.; Dahdah, L.; Artesani, M.C.; Fiocchi, A.; Martelli, A. Probiotics in Asthma and Allergy Prevention. Front. Pediatr. 2017, 5, 165. [Google Scholar] [CrossRef]
- Sakwinska, O.; Bastic Schmid, V.; Berger, B.; Bruttin, A.; Keitel, K.; Lepage, M.; Moine, D.; Ngom Bru, C.; Brüssow, H.; Gervaix, A. Nasopharyngeal microbiota in healthy children and pneumonia patients. J. Clin. Microbiol. 2014, 52, 1590–1594. [Google Scholar] [CrossRef]
- Pettigrew, M.M.; Gent, J.F.; Kong, Y.; Wade, M.; Gansebom, S.; Bramley, A.M.; Jain, S.; Arnold, S.L.; McCullers, J.A. Association of sputum microbiota profiles with severity of community-acquired pneumonia in children. BMC Infect. Dis. 2016, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Machiavelli, A.; Duarte, R.T.D.; Pires, M.M.S.; Zárate-Bladés, C.R.; Pinto, A.R. The impact of in utero HIV exposure on gut microbiota, inflammation, and microbial translocation. Gut Microbes 2019, 10, 599–614. [Google Scholar] [CrossRef]
- Kaur, U.S.; Shet, A.; Rajnala, N.; Gopalan, B.P.; Moar, P.D.H.; Singh, B.P.; Chaturvedi, R.; Tandon, R. High Abundance of genus Prevotella in the gut of perinatally HIV-infected children is associated with IP-10 levels despite therapy. Sci. Rep. 2018, 5, 17679. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Villar, S.; de Lagarde, M.; Vázquez-Castellanos, J.; Vallejo, A.; Bernadino, J.I.; Madrid, N.; Matarranz, M.; Díaz-Santiago, A.; Gutiérrez, C.; Cabello, A.; et al. Effects of Immunonutrition in Advanced Human Immunodeficiency Virus Disease: A Randomized Placebo-controlled Clinical Trial [Promaltia Study]. Clin. Infect. Dis. 2019, 1, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Prodger, J.L.; Tobian, A.A.R.; Abraham, A.G.; Kigozi, G.; Hungate, B.A.; Aziz, M.; Nalugoda, F.; Sariya, S.; Serwadda, D.; et al. Penile Anaerobic Dysbiosis as a Risk Factor for HIV Infection. MBio 2017, 25, 8. [Google Scholar] [CrossRef]
- Klatt, N.R.; Cheu, R.; Birse, K.; Zevin, A.S.; Perner, M.; Noël-Romas, L.; Grobler, A.; Westmacott, G.; Xie, I.Y.; Butler, J.; et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017, 2, 938–945. [Google Scholar] [CrossRef]
- Frank, D.N.; Manigart, O.; Leroy, V.; Meda, N.; Valéa, D.; Zhang, W.; Dabis, F.; Pace, N.R.; Van de Perre, P.; Janoff, E. Altered vaginal microbiota are associated with perinatal mother-to-child transmission of HIV in African women from Burkina Faso. J. Acquir. Immune Defic. Syndr. 2012, 1, 299–306. [Google Scholar] [CrossRef]
- Freedman, S.B.; Williamson-Urquhart, S.; Farion, K.J.; Gouin, S.; Willan, A.R.; Poonai, N.; Hurley, K.; Sherman, P.M.; Finkelstein, Y.; Lee, B.E.; et al. Multicenter Trial of a Combination Probiotic for Children with Gastroenteritis. N. Engl. J. Med. 2018, 22, 2015–2026. [Google Scholar] [CrossRef]
- Schnadower, D.; Tarr, P.I.; Casper, T.C.; Gorelick, M.H.; Dean, J.M.; O’Connell, K.J.; Mahajan, P.; Levine, A.C.; Bhatt, S.R.; Roskind, C.G.; et al. Lactobacillus rhamnosus GG versus Placebo for Acute Gastroenteritis in Children. N. Engl. J. Med. 2018, 22, 2002–2014. [Google Scholar] [CrossRef]
- Yeh, T.L.; Shih, P.C.; Liu, S.J.; Lin, C.H.; Liu, J.M.; Lei, W.T.; Lin, C.Y. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: A systematic review and meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2018, 25, 217–230. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The influence of probiotics on vaccine responses—A systematic review. Vaccine 2018, 4, 207–213. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesa, M.D.; Loureiro, B.; Iglesia, I.; Fernandez Gonzalez, S.; Llurba Olivé, E.; García Algar, O.; Solana, M.J.; Cabero Perez, M.J.; Sainz, T.; Martinez, L.; et al. The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review. Nutrients 2020, 12, 133. https://doi.org/10.3390/nu12010133
Mesa MD, Loureiro B, Iglesia I, Fernandez Gonzalez S, Llurba Olivé E, García Algar O, Solana MJ, Cabero Perez MJ, Sainz T, Martinez L, et al. The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review. Nutrients. 2020; 12(1):133. https://doi.org/10.3390/nu12010133
Chicago/Turabian StyleMesa, María Dolores, Begoña Loureiro, Iris Iglesia, Sergi Fernandez Gonzalez, Elisa Llurba Olivé, Oscar García Algar, María José Solana, Mª Jesús Cabero Perez, Talia Sainz, Leopoldo Martinez, and et al. 2020. "The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review" Nutrients 12, no. 1: 133. https://doi.org/10.3390/nu12010133
APA StyleMesa, M. D., Loureiro, B., Iglesia, I., Fernandez Gonzalez, S., Llurba Olivé, E., García Algar, O., Solana, M. J., Cabero Perez, M. J., Sainz, T., Martinez, L., Escuder-Vieco, D., Parra-Llorca, A., Sánchez-Campillo, M., Rodriguez Martinez, G., Gómez Roig, D., Perez Gruz, M., Andreu-Fernández, V., Clotet, J., Sailer, S., ... Cabañas, F. (2020). The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review. Nutrients, 12(1), 133. https://doi.org/10.3390/nu12010133










