A Lowly Digestible-Starch Diet after Weaning Enhances Exogenous Glucose Oxidation Rate in Female, but Not in Male, Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Experiment
2.2. Diet Composition
2.3. Preparation and Administration of the 13C-Labelled Starch Bolus
2.4. Extended Indirect Calorimetry (InCa)
2.5. Dissection
2.6. Amylase Activity
2.7. Elemental Analysis Isotope Ratio Mass Spectrometry (EA-IRMS)
2.8. Statistical Analysis
3. Results
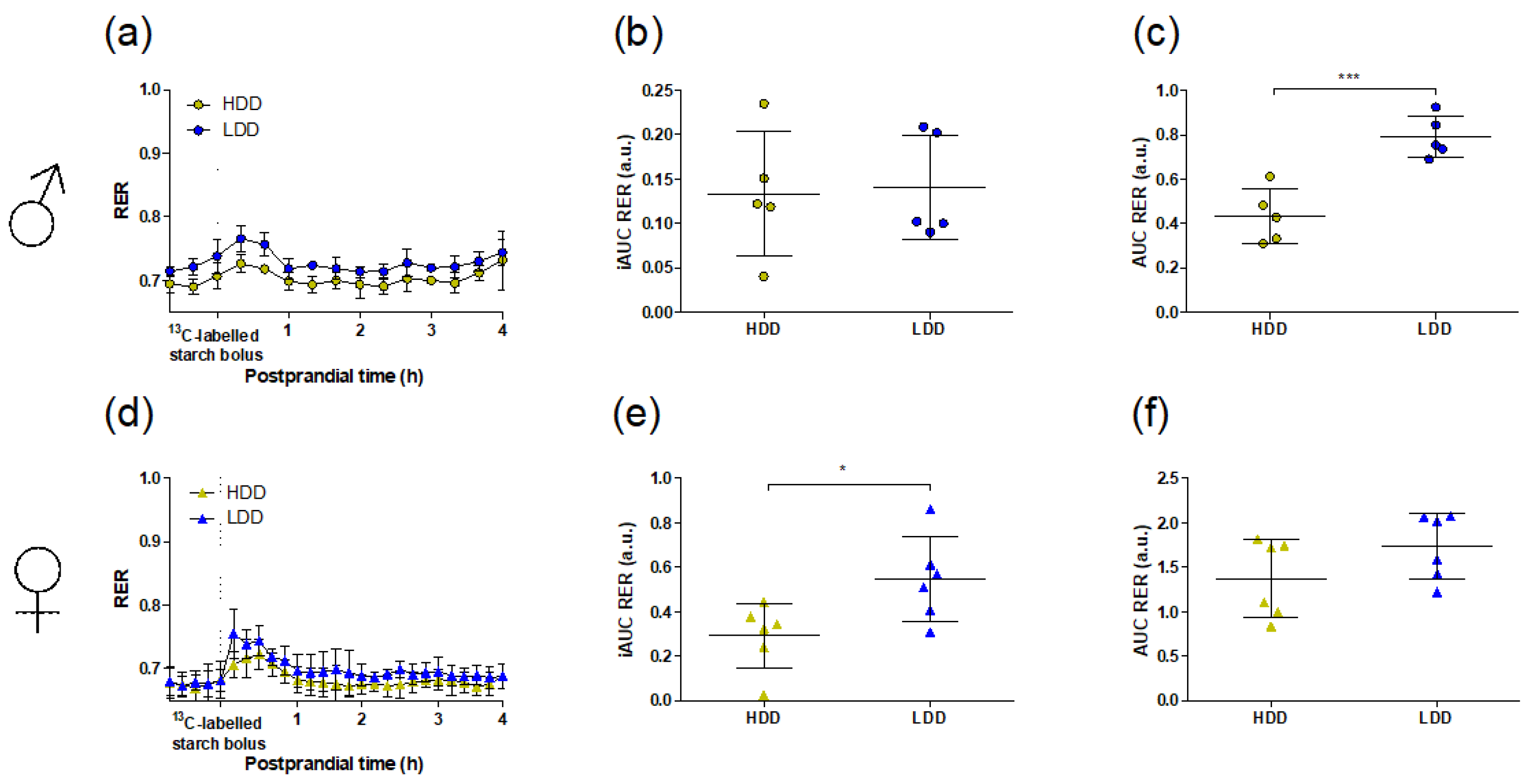
3.1. General Metabolic Phenotype
3.2. Food Restriction and Substrate Switching in Response to the 13C Starch Bolus
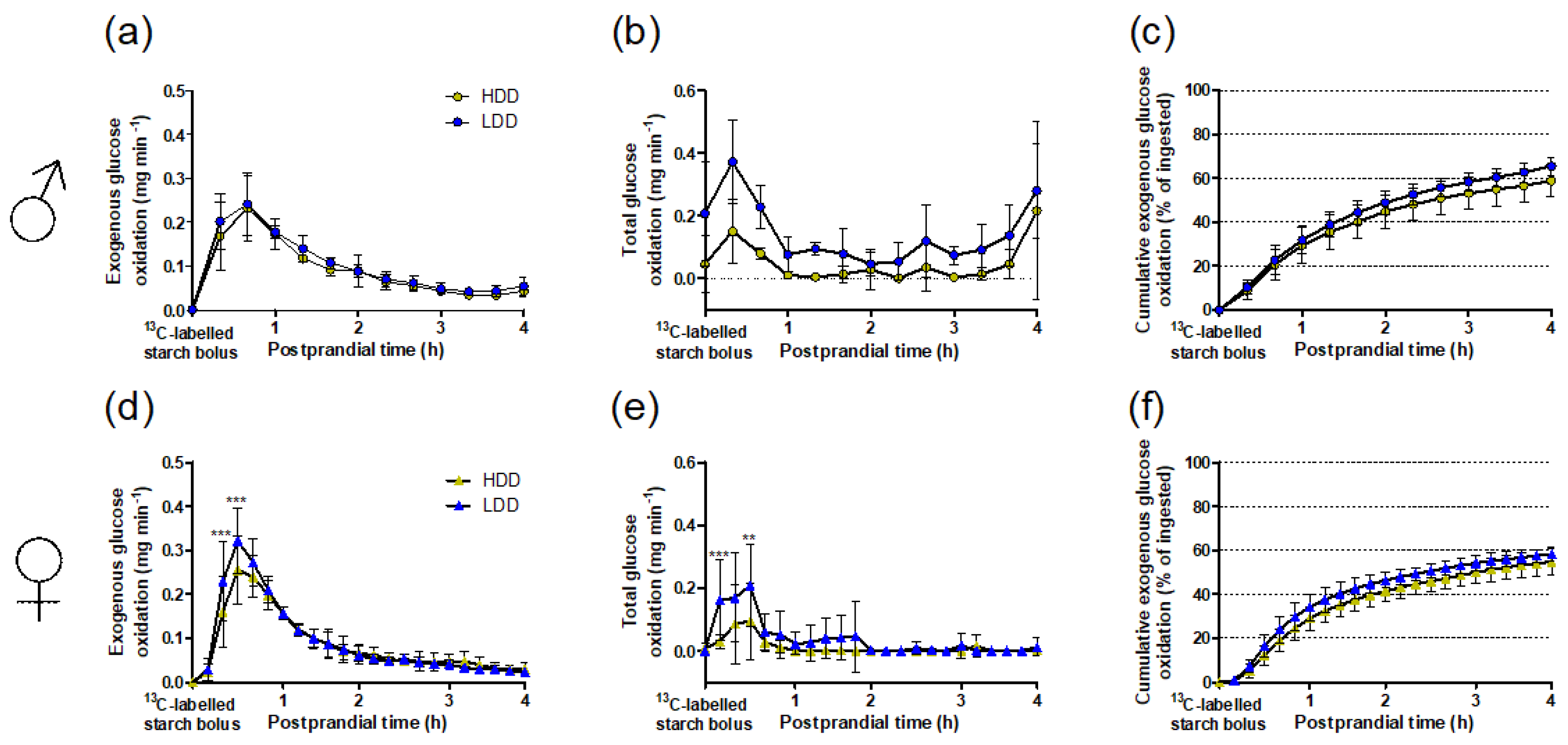
3.3. Starch-Derived and Total Glucose Oxidation in Response to the 13C Starch Bolus
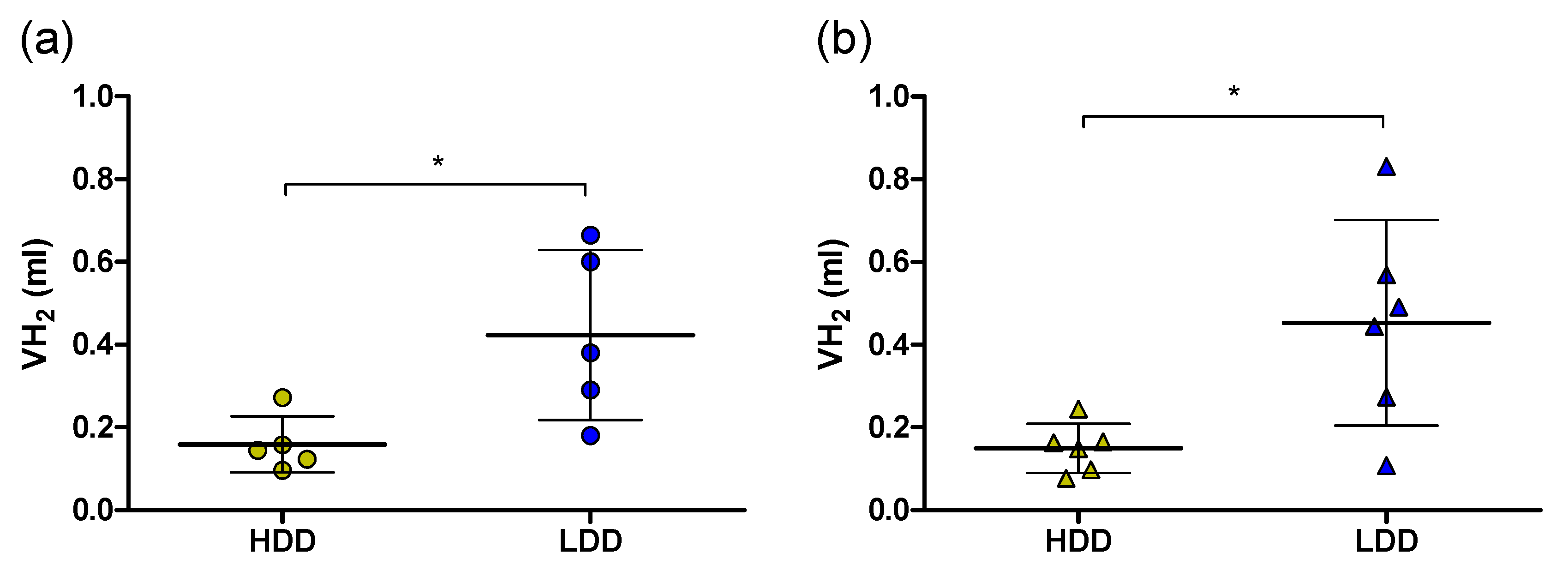
3.4. Intestinal Amylase Activity and Hepatic 13C Label Deposition in Females
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campbell, G.J.; Senior, A.M.; Bell-Anderson, K.S. Metabolic effects of high glycaemic index diets: A systematic review and meta-analysis of feeding studies in mice and rats. Nutrients 2017, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.A.; Higbee, D.R.; Donahoo, W.T.; Brown, I.L.; Bell, M.L.; Bessesen, D.H. Resistant starch consumption promotes lipid oxidation. Nutr. Metab. 2004, 1, 8. [Google Scholar] [CrossRef]
- Johnston, K.L.; Thomas, E.L.; Bell, J.D.; Frost, G.S.; Robertson, M.D. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet. Med. 2010, 27, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, Y.; Li, H.; Shen, L.; Ni, Y.; Fang, Q.; Wu, G.; Qian, L.; Xiao, Y.; Zhang, J.; et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: A randomized crossover trial. Sci. Rep. 2019, 9, 4736. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D. Dietary-resistant starch and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 362–367. [Google Scholar] [CrossRef]
- Peterson, C.M.; Beyl, R.A.; Marlatt, K.L.; Martin, C.K.; Aryana, K.J.; Marco, M.L.; Martin, R.J.; Keenan, M.J.; Ravussin, E. Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 492–501. [Google Scholar] [CrossRef]
- Robertson, M.D.; Livesey, G.; Mathers, J.C. Quantitative kinetics of glucose appearance and disposal following a 13C-labelled starch-rich meal: Comparison of male and female subjects. Brit. J. Nutr. 2007, 87, 569–577. [Google Scholar] [CrossRef]
- Fernandez-Calleja, J.M.S.; Bouwman, L.M.S.; Swarts, H.J.M.; Oosting, A.; Keijer, J.; Van Schothorst, E.M. Direct and long-term metabolic consequences of lowly versus. highly-digestible starch in the early post-weaning diet of mice. Nutrients 2018, 10, 1788. [Google Scholar]
- Isken, F.; Klaus, S.; Petzke, K.J.; Loddenkemper, C.; Pfeiffer, A.F.; Weickert, M.O. Impairment of fat oxidation under high- versus. low-glycemic index diet occurs before the development of an obese phenotype. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E287–E295. [Google Scholar]
- Hoevenaars, F.P.; Van Schothorst, E.M.; Horakova, O.; Voigt, A.; Rossmeisl, M.; Pico, C.; Caimari, A.; Kopecky, J.; Klaus, S.; Keijer, J. BIOCLAIMS standard diet (BIOsd): A reference diet for nutritional physiology. Genes Nutr. 2012, 7, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calleja, J.M.S.; Konstanti, P.; Swarts, H.J.M.; Bouwman, L.M.S.; Garcia-Campayo, V.; Billecke, N.; Oosting, A.; Smidt, H.; Keijer, J.; Van Schothorst, E.M. Non-invasive continuous real-time in vivo analysis of microbial hydrogen production shows adaptation to fermentable carbohydrates in mice. Sci. Rep. 2018, 8, 15351. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorde, L.P.; Van Schothorst, E.M.; Swarts, H.M.; Kuda, O.; Steenbergh, E.; Termeulen, S.; Kopecky, J.; Keijer, J. A difference in fatty acid composition of isocaloric high-fat diets alters metabolic flexibility in male C57BL/6JOlaHsd mice. PLoS ONE 2015, 10, e0128515. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calleja, J.M.S.; Bouwman, L.M.S.; Swarts, H.J.M.; Oosting, A.; Keijer, J.; Van Schothorst, E.M. Extended indirect calorimetry with isotopic CO2 sensors for prolonged and continuous quantification of exogenous versus. total substrate oxidation in mice. Sci. Rep. 2019, 9, 11507. [Google Scholar] [PubMed]
- Peronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar] [PubMed]
- Delarue, J.; Normand, S.; Pachiaudi, C.; Beylot, M.; Lamisse, F.; Riou, J.P. The contribution of naturally labelled 13C fructose to glucose appearance in humans. Diabetologia 1993, 36, 338–345. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.; Fernandez-Lopez, J.A.; Argiles, M.J.; Alemany, M. Role of the rat liver in the disposal of a glucose gavage. Mol. Cell. Biochem. 1992, 113, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.; Ferreira, L.M.; Breuer, M.J.; Dijkstra, J.; Pellikaan, W.F. Stable isotope labeled n-alkanes to assess digesta passage kinetics through the digestive tract of ruminants. PLoS ONE 2013, 8, e75496. [Google Scholar] [CrossRef][Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Scribner, K.B.; Pawlak, D.B.; Aubin, C.M.; Majzoub, J.A.; Ludwig, D.S. Long-term effects of dietary glycemic index on adiposity, energy metabolism, and physical activity in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1126–E1131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; Shen, L.; Lissy, E.; McCutcheon, K.; Keenan, M.J. Failure to ferment dietary resistant starch in specific mouse models of obesity results in no body fat loss. J. Agric. Food Chem. 2009, 57, 8844–8851. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F.; Lopez, B. Low-versus high-glycemic index diets in women: Effects on caloric requirement, substrate utilization and insulin sensitivity. Metab. Syndr. Relat. Disord. 2007, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Quek Yu Chin, R.; Camps, S.; Henry, C.J. The impact of a low glycaemic index (GI) diet on simultaneous measurements of blood glucose and fat oxidation: A whole body calorimetric study. J. Clin. Transl. Endocrinol. 2016, 4, 45–52. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Della Torre, S.; Mitro, N.; Meda, C.; Lolli, F.; Pedretti, S.; Barcella, M.; Ottobrini, L.; Metzger, D.; Caruso, D.; Maggi, A. Short-term fasting reveals amino acid metabolism as a major sex-discriminating factor in the liver. Cell Metab. 2018, 28, 256–267. [Google Scholar] [CrossRef]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988, 37, 287–301. [Google Scholar] [CrossRef]
- Azzout-Marniche, D.; Chaumontet, C.; Piedcoq, J.; Khodorova, N.; Fromentin, G.; Tomé, D.; Gaudichon, C.; Even, P.C. High pancreatic amylase expression promotes adiposity in obesity-prone carbohydrate-sensitive rats. J. Nutr. 2019, 149, 270–279. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Fermentations by saccharolytic intestinal bacteria. Am. J. Clin. Nutr. 1979, 32, 164–172. [Google Scholar] [CrossRef]
- Englyst, K.N.; Englyst, H.N. Carbohydrate bioavailability. Brit. J. Nutr. 2007, 94, 1. [Google Scholar] [CrossRef] [PubMed]
- Symonds, E.L.; Kritas, S.; Omari, T.I.; Butler, R.N. A combined 13CO2/H2 breath test can be used to assess starch digestion and fermentation in humans. J. Nutr. 2004, 134, 1193–1196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- So, P.W.; Yu, W.S.; Kuo, Y.T.; Wasserfall, C.; Goldstone, A.P.; Bell, J.D.; Frost, G. Impact of resistant starch on body fat patterning and central appetite regulation. PLoS ONE 2007, 2, e1309. [Google Scholar] [CrossRef] [PubMed]
- Van Schothorst, E.M.; Bunschoten, A.; Schrauwen, P.; Mensink, R.P.; Keijer, J. Effects of a high-fat, low- versus high-glycemic index diet: Retardation of insulin resistance involves adipose tissue modulation. FASEB J. 2009, 23, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Rizkalla, S.W.; Champ, M.; Luo, J.; Boillot, J.; Bruzzo, F.; Slama, G. Dietary amylose-amylopectin starch content affects glucose and lipid metabolism in adipocytes of normal and diabetic rats. J. Nutr. 1998, 128, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ingvorsen, C.; Karp, N.A.; Lelliott, C.J. The role of sex and body weight on the metabolic effects of high-fat diet in C57BL/6N mice. Nutr. Diabetes 2017, 7, e261. [Google Scholar] [CrossRef]
- Chen, X.; McClusky, R.; Itoh, Y.; Reue, K.; Arnold, A.P. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology 2013, 154, 1092–1104. [Google Scholar] [CrossRef]
- Link, J.C.; Reue, K. Genetic basis for sex differences in obesity and lipid metabolism. Annu. Rev. Nutr. 2017, 37, 225–245. [Google Scholar] [CrossRef]


| HDD | LDD | |
|---|---|---|
| Casein (g kg−1) | 212.2 | 212.0 |
| l-Cysteine (g kg−1) | 3.0 | 3.0 |
| Amylose mix (AmyloGel 03003) (g kg−1) 1 | 0.0 | 568.6 |
| Amylopectin (C*Gel 04201) (g kg−1) 2 | 568.6 | 0.0 |
| Coconut oil (g kg−1) | 21.4 | 21.4 |
| Sunflower oil (g kg−1) | 83.1 | 83.1 |
| Flaxseed oil (g kg−1) | 14.2 | 14.2 |
| Cholesterol (g kg−1) | 0.03 | 0.03 |
| Cellulose (g kg−1) | 50.0 | 50.0 |
| Mineral mix (AIN-93G-MX) (g kg−1) | 35.0 | 35.0 |
| Vitamin mix (AIN-93-VX) (g kg−1) | 10.0 | 10.0 |
| Choline bitartrate (g kg−1) | 2.5 | 2.5 |
| Calculated energy density (kJ g−1) 3 | 17.9 | 17.9 |
| Gross energy density (kJ g−1) 4 | 18.9 | 19.5 |
| Digestible energy density (kJ g−1) 5 | 17.6 | 17.0 |
| Protein (Energy%) | 20.1 | 20.1 |
| Carbohydrate (Energy%) | 54.9 | 54.9 |
| Fat (Energy%) | 25.0 | 25.0 |
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| HDD | LDD | HDD | LDD | |||||
| Mean or median | SD or range | Mean or median | SD or range | Mean or median | SD or range | Mean or median | SD or range | |
| Body weight (g) | 21.23 | 1.13 | 21.53 | 2.08 | 16.60 | (14.06, 18.54) | 16.72 | (14.01, 17.55) |
| Fat mass (g) | 2.14 | 0.43 | 1.74 | 0.52 | 1.69 | 0.60 | 1.43 | 0.33 |
| Lean mass (g) | 18.18 | 0.96 | 18.81 | 1.49 | 14.28 | 1.06 | 14.17 | 0.88 |
| Fasting blood glucose (mmol L−1) | 4.9 | 0.6 | 5.4 | 0.5 | 4.4 | 0.8 | 4.7 | 0.8 |
| Digestible energy intake (MJ) 1 | 1.15 | (1.12, 1.26) | 1.30 *** | (1.20, 1.46) | 0.88 | (0.61, 0.93) | 0.99 **** | (0.95, 1.06) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Calleja, J.M.S.; Bouwman, L.M.S.; Swarts, H.J.M.; Billecke, N.; Oosting, A.; Keijer, J.; van Schothorst, E.M. A Lowly Digestible-Starch Diet after Weaning Enhances Exogenous Glucose Oxidation Rate in Female, but Not in Male, Mice. Nutrients 2019, 11, 2242. https://doi.org/10.3390/nu11092242
Fernández-Calleja JMS, Bouwman LMS, Swarts HJM, Billecke N, Oosting A, Keijer J, van Schothorst EM. A Lowly Digestible-Starch Diet after Weaning Enhances Exogenous Glucose Oxidation Rate in Female, but Not in Male, Mice. Nutrients. 2019; 11(9):2242. https://doi.org/10.3390/nu11092242
Chicago/Turabian StyleFernández-Calleja, José M. S., Lianne M. S. Bouwman, Hans J. M. Swarts, Nils Billecke, Annemarie Oosting, Jaap Keijer, and Evert M. van Schothorst. 2019. "A Lowly Digestible-Starch Diet after Weaning Enhances Exogenous Glucose Oxidation Rate in Female, but Not in Male, Mice" Nutrients 11, no. 9: 2242. https://doi.org/10.3390/nu11092242
APA StyleFernández-Calleja, J. M. S., Bouwman, L. M. S., Swarts, H. J. M., Billecke, N., Oosting, A., Keijer, J., & van Schothorst, E. M. (2019). A Lowly Digestible-Starch Diet after Weaning Enhances Exogenous Glucose Oxidation Rate in Female, but Not in Male, Mice. Nutrients, 11(9), 2242. https://doi.org/10.3390/nu11092242






