Free Amino Acid Content in Human Milk Is Associated with Infant Gender and Weight Gain during the First Four Months of Lactation
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects, Material and Methods
2.2. Breast Milk Collection and Amino Acids Measurements
2.3. Anthropometric Measurements
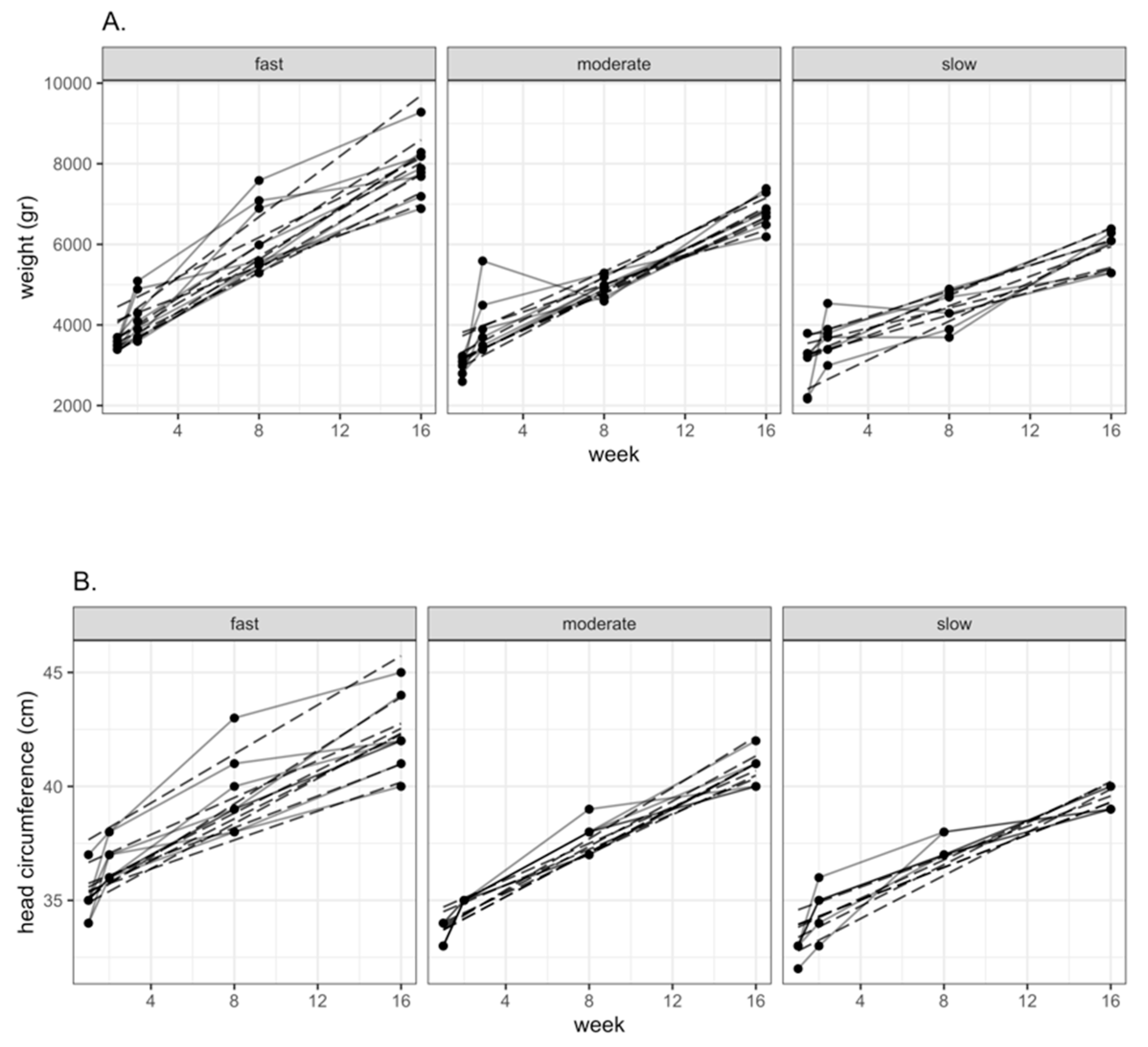
2.4. Ranking of Infants according to Growth Rate
2.5. Statistical Analyses
Regression Models
2.6. Ethics
3. Results
Characteristics of Study Population
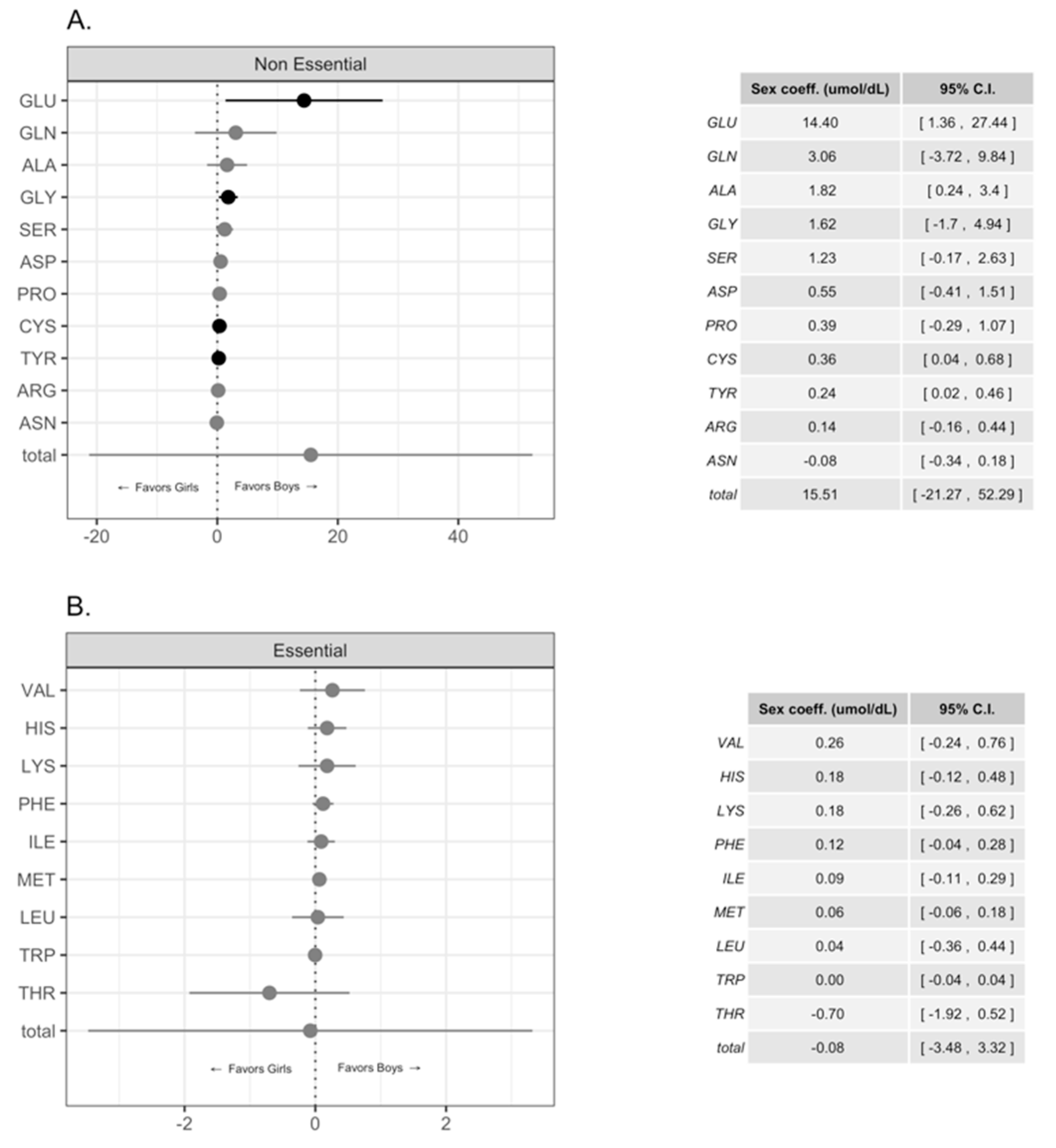
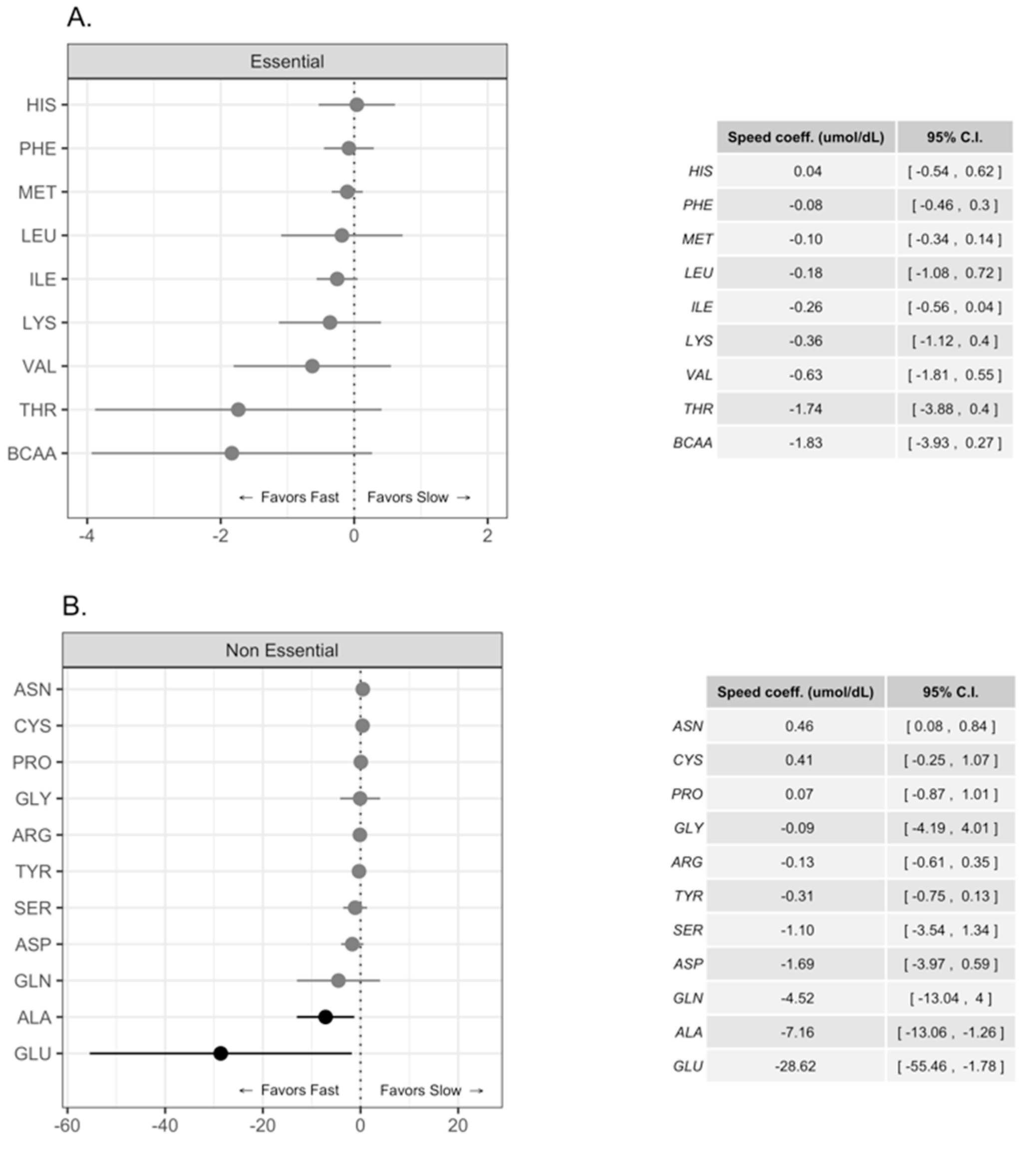
Differences in Free Amino Acid Concentrations in Human Milk for Female and Male Infants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodenas, C.; Affolter, M.; Vinyes-Pares, G.; De Castro, C.; Karagounis, L.; Zhang, Y.; Wang, P.; Thakkar, S. Amino Acid Composition of Breast Milk from Urban Chinese Mothers. Nutrients 2016, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Rito, A.I.; Buoncristiano, M.; Spinelli, A.; Salanave, B.; Kunešová, M.; Hejgaard, T.; García Solano, M.; Fijałkowska, A.; Sturua, L.; Hyska, J.; et al. Association between Characteristics at Birth, Breastfeeding and Obesity in 22 Countries: The WHO European Childhood Obesity Surveillance Initiative—COSI 2015/2017. Obes. Facts 2019, 12, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.; Chaaya, N.; Beecher, K.; Ali, S.A.; Belmer, A.; Bartlett, S. The Impact of Sugar Consumption on Stress Driven, Emotional and Addictive Behaviors. Neurosci. Biobehav. Rev. 2019, 103, 178–199. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kohut, T.; Robbins, J.; Panganiban, J. Update on childhood/adolescent obesity and its sequela. Curr. Opin. Pediatr. 2019, 30, 1–9. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- van Sadelhoff, J.H.J.; Mastorakou, D.; Weenen, H.; Stahl, B.; Garssen, J.; Hartog, A. Short Communication: Differences in Levels of Free Amino Acids and Total Protein in Human Foremilk and Hindmilk. Nutrients 2018, 10, 1828. [Google Scholar] [CrossRef]
- Alexandre-Gouabau, M.-C.; Moyon, T.; David-Sochard, A.; Fenaille, F.; Cholet, S.; Royer, A.-L.; Guitton, Y.; Billard, H.; Darmaun, D.; Rozé, J.-C.; et al. Comprehensive Preterm Breast Milk Metabotype Associated with Optimal Infant Early Growth Pattern. Nutrients 2019, 11, 528. [Google Scholar] [CrossRef]
- Krishna Rao, R.; Samak, G. Role of Glutamine in Protection of Intestinal Epithelial Tight Junctions. J. Epithel. Biol. Pharmacol. 2012, 5, 47–54. [Google Scholar] [CrossRef]
- Jiao, N.; Wu, Z.; Ji, Y.; Wang, B.; Dai, Z.; Wu, G. L-Glutamate Enhances Barrier and Antioxidative Functions in Intestinal Porcine Epithelial Cells. J. Nutr. 2015, 145, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013, 4, 27. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- van Sadelhoff, J.H.J.; van de Heijning, B.J.M.; Stahl, B.; Amodio, S.; Rings, E.H.H.M.; Mearin, M.L.; Garssen, J.; Hartog, A. Longitudinal Variation of Amino Acid Levels in Human Milk and Their Associations with Infant Gender. Nutrients 2018, 10, 1233. [Google Scholar] [CrossRef] [PubMed]
- Baldeón, M.E.; Mennella, J.A.; Flores, N.; Fornasini, M.; San Gabriel, A. Free amino acid content in breast milk of adolescent and adult mothers in Ecuador. Springerplus 2014, 3, 104. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Zhang, Q.-W.; Sugimoto, T.; Furuhata, Y.; Sakai, R.; Mori, M.; Takahashi, M.; Kimura, T. Network analysis of plasma and tissue amino acids and the generation of an amino index for potential diagnostic use. Am. J. Clin. Nutr. 2006, 83, 513S–519S. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 25 June 2019).
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0. Available online: https://www.ibm.com/analytics/spss-statistics-software (accessed on 25 June 2019).
- Keane, K.; Newsholme, P. Metabolic Regulation of Insulin Secretion. Vitam. Horm. 2014, 95, 1–33. [Google Scholar]
- Ganor, Y.; Levite, M. The neurotransmitter glutamate and human T cells: Glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J. Neural Transm. 2014, 121, 983–1006. [Google Scholar] [CrossRef]
- San Gabriel, A.; Uneyama, H. Amino acid sensing in the gastrointestinal tract. Amino Acids 2013, 45, 451–461. [Google Scholar] [CrossRef]
- Zhang, Z.; Adelman, A.S.; Rai, D.; Boettcher, J.; Lőnnerdal, B. Amino acid profiles in term and preterm human milk through lactation: A systematic review. Nutrients 2013, 5, 4800–4821. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.L.; Gill, H.S. Immunomodulatory properties of milk. Br. J. Nutr. 2000, 84, S81–S89. [Google Scholar] [CrossRef] [PubMed]
- Larnkjær, A.; Bruun, S.; Pedersen, D.; Zachariassen, G.; Barkholt, V.; Agostoni, C.; Molgaard, C.; Husby, S.; Michaelsen, K.F. Free Amino Acids in Human Milk and Associations with Maternal Anthropometry and Infant Growth. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.H.; Saddler, N.I.; Devries, M.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: A parallel-group crossover study. Am. J. Clin. Nutr. 2016, 104, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Anthony, T.G.; Rasmussen, B.B.; Adams, S.H.; Lynch, C.J.; Brinkworth, G.D.; Davis, T.A. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am. J. Clin. Nutr. 2015, 101, 1330S–1338S. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.; Ashmeade, T.; Duffy, A.; Morse, S.; Zaritt, J. Changes in the Immune Components of Preterm Human Milk and Associations with Maternal and Infant Characteristics. J. Obstet. Gynecol. Neonatal Nurs. 2016, 45, 639–648. [Google Scholar] [CrossRef]


| Week (Type of Milk) | Number of Observations Used |
|---|---|
| 1 (colostrum, S1) | 61 |
| 2 (transition milk, S2) | 47 |
| 8 (mature milk, S3) | 38 |
| 16 (mature milk, S4) | 37 |
| Anthropometric Characteristics | Weight (kg) | pValue | Height (m) | pValue | |
| Adolescent mothers (16.5 ± 1.0 years) | (n = 32) | 59.5 ± 8.1 | 0.815 | 1.6 ± 0.1 | 0.533 |
| Adult mothers (21.7 ± 2.6 years) | (n = 18) | 60.1 ± 9.7 | 1.6 ± 0.1 | ||
| Infants Anthropometric Characteristics | Weight (kg) | Mean Difference | Head Circumference (cm) | Mean Difference | |
| Female | Week 1 | 3.45 ± 0.78 (n = 18) | 3.41 | 33.53 ± 0.87 (n = 17) | 6.02 |
| Week 16 | 6.86 ± 1.50 (n = 12) | 39.55 ± 1.37 (n = 11) | |||
| Male | Week 1 | 3.16 ± 0.43 (n = 32) | 4.08 | 33.87 ± 1.36 (n = 32) | 7.24 |
| Week 16 | 7.24 ± 1.09 (n = 17) | 41.11 ± 1.57 (n = 18) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldeón, M.E.; Zertuche, F.; Flores, N.; Fornasini, M. Free Amino Acid Content in Human Milk Is Associated with Infant Gender and Weight Gain during the First Four Months of Lactation. Nutrients 2019, 11, 2239. https://doi.org/10.3390/nu11092239
Baldeón ME, Zertuche F, Flores N, Fornasini M. Free Amino Acid Content in Human Milk Is Associated with Infant Gender and Weight Gain during the First Four Months of Lactation. Nutrients. 2019; 11(9):2239. https://doi.org/10.3390/nu11092239
Chicago/Turabian StyleBaldeón, Manuel E., Federico Zertuche, Nancy Flores, and Marco Fornasini. 2019. "Free Amino Acid Content in Human Milk Is Associated with Infant Gender and Weight Gain during the First Four Months of Lactation" Nutrients 11, no. 9: 2239. https://doi.org/10.3390/nu11092239
APA StyleBaldeón, M. E., Zertuche, F., Flores, N., & Fornasini, M. (2019). Free Amino Acid Content in Human Milk Is Associated with Infant Gender and Weight Gain during the First Four Months of Lactation. Nutrients, 11(9), 2239. https://doi.org/10.3390/nu11092239




