Effect of Sleeve Gastrectomy on Proprotein Convertase Subtilisin/Kexin Type 9 (Pcsk9) Content and Lipid Metabolism in the Blood Plasma and Liver of Obese Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
- * Control, n = 10;
- * HFD, n = 12;
- * Bariatric surgery (BS), n = 12.
2.2. Preparation of Homogenates
2.3. Assessment of Total Protein Concentration
2.4. Surgical Procedure
2.5. Plasma Lipid Concentrations
2.6. Statistical Analyses
3. Results
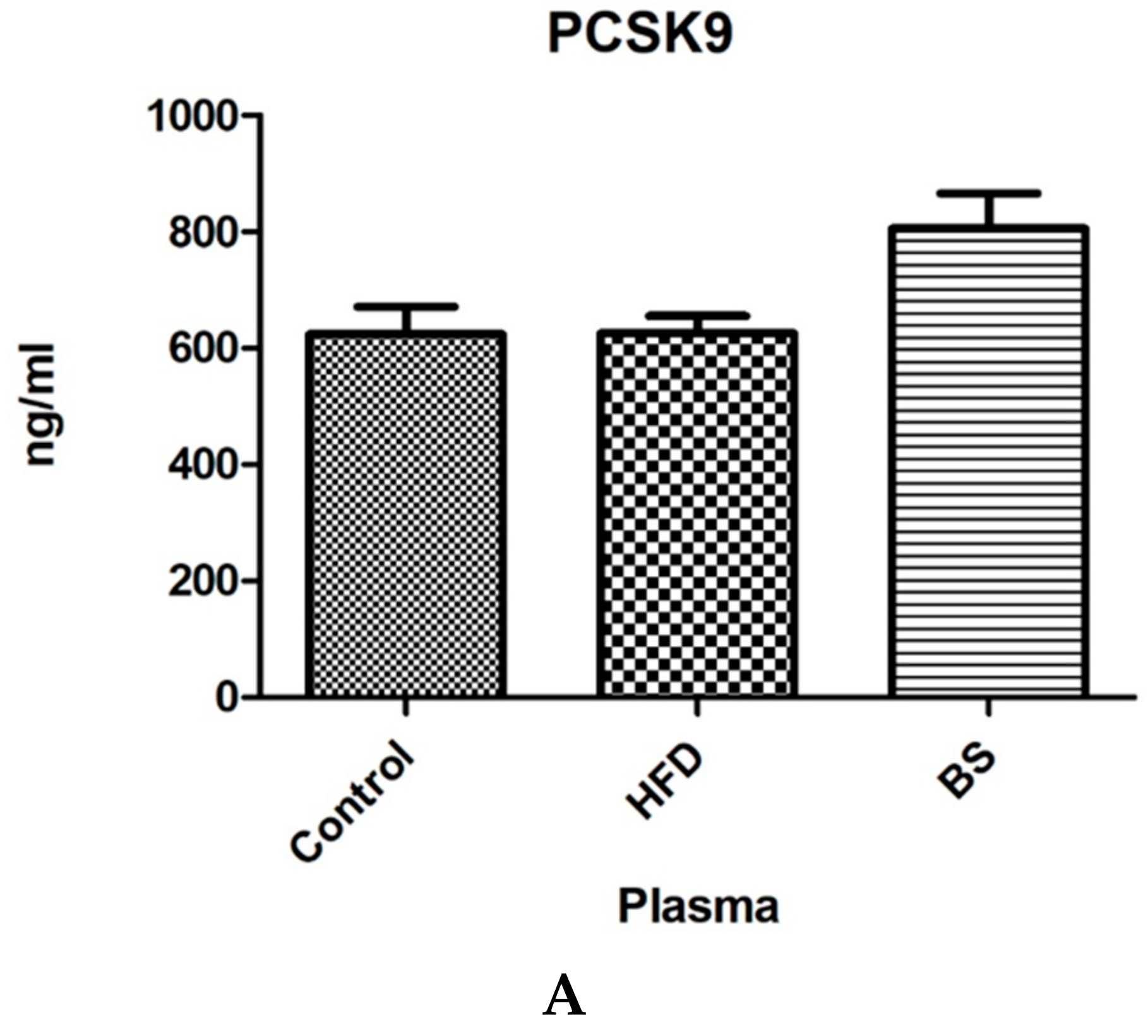
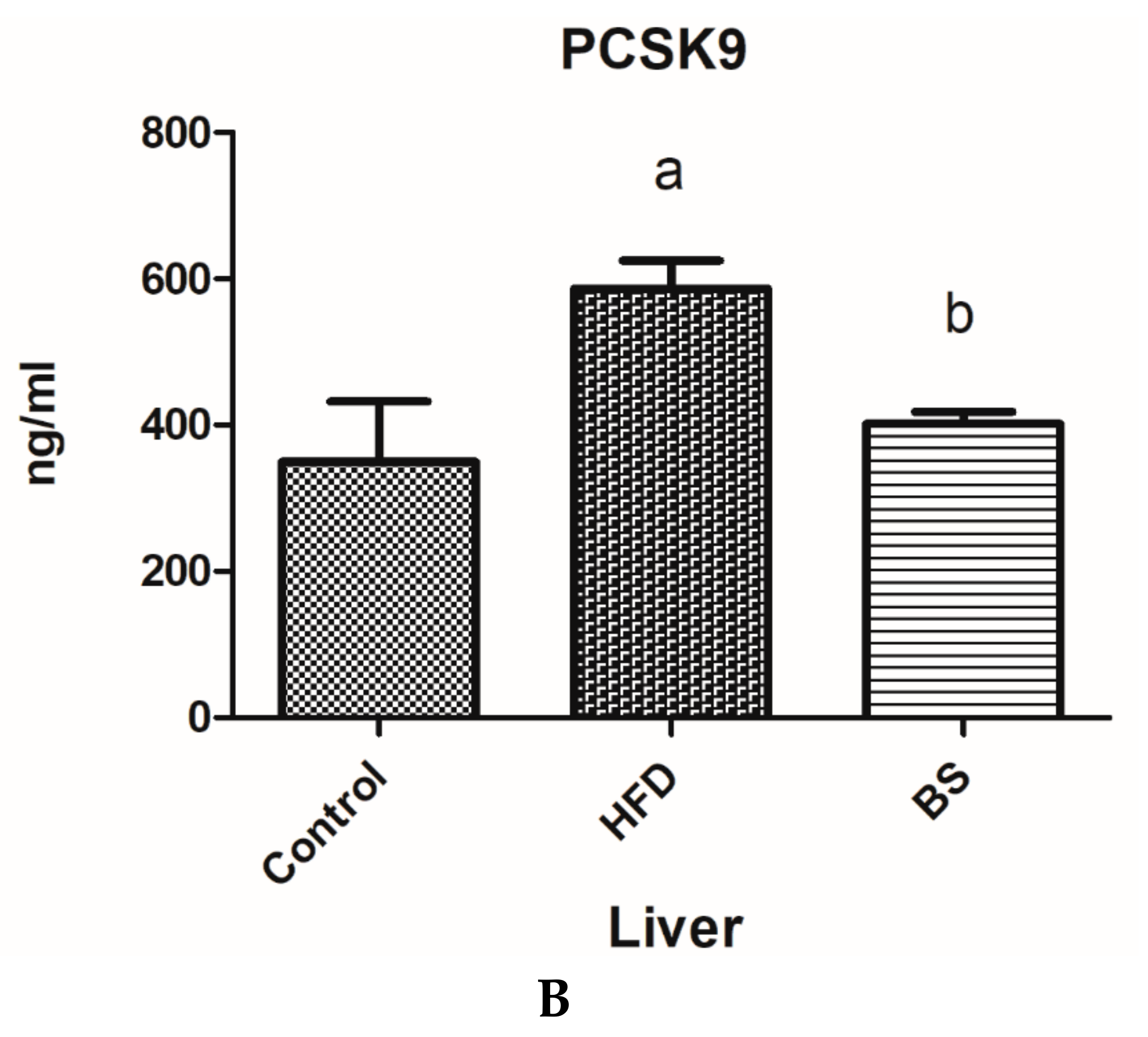
3.1. Plasma and Liver PCSK9 Concentrations.
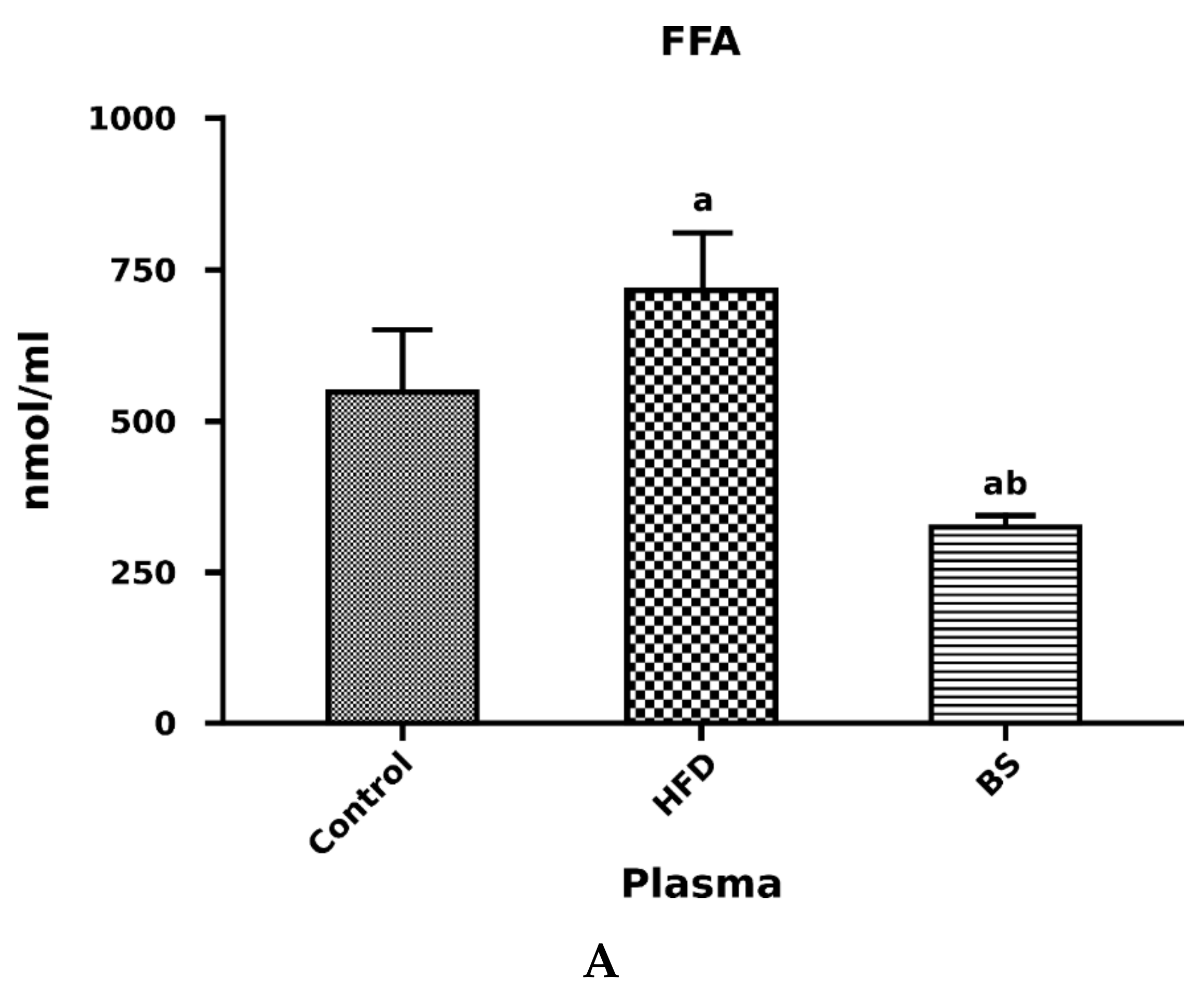
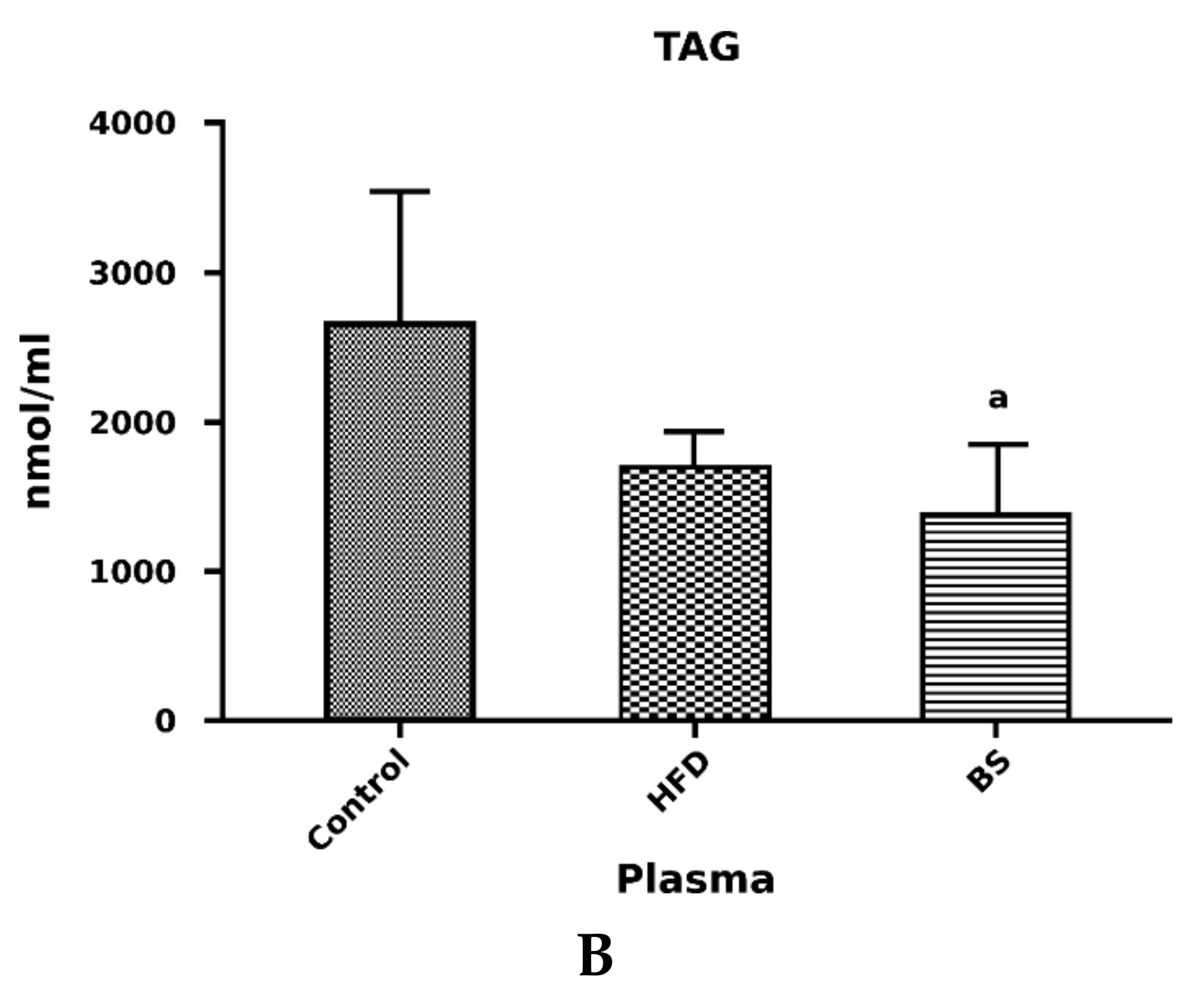
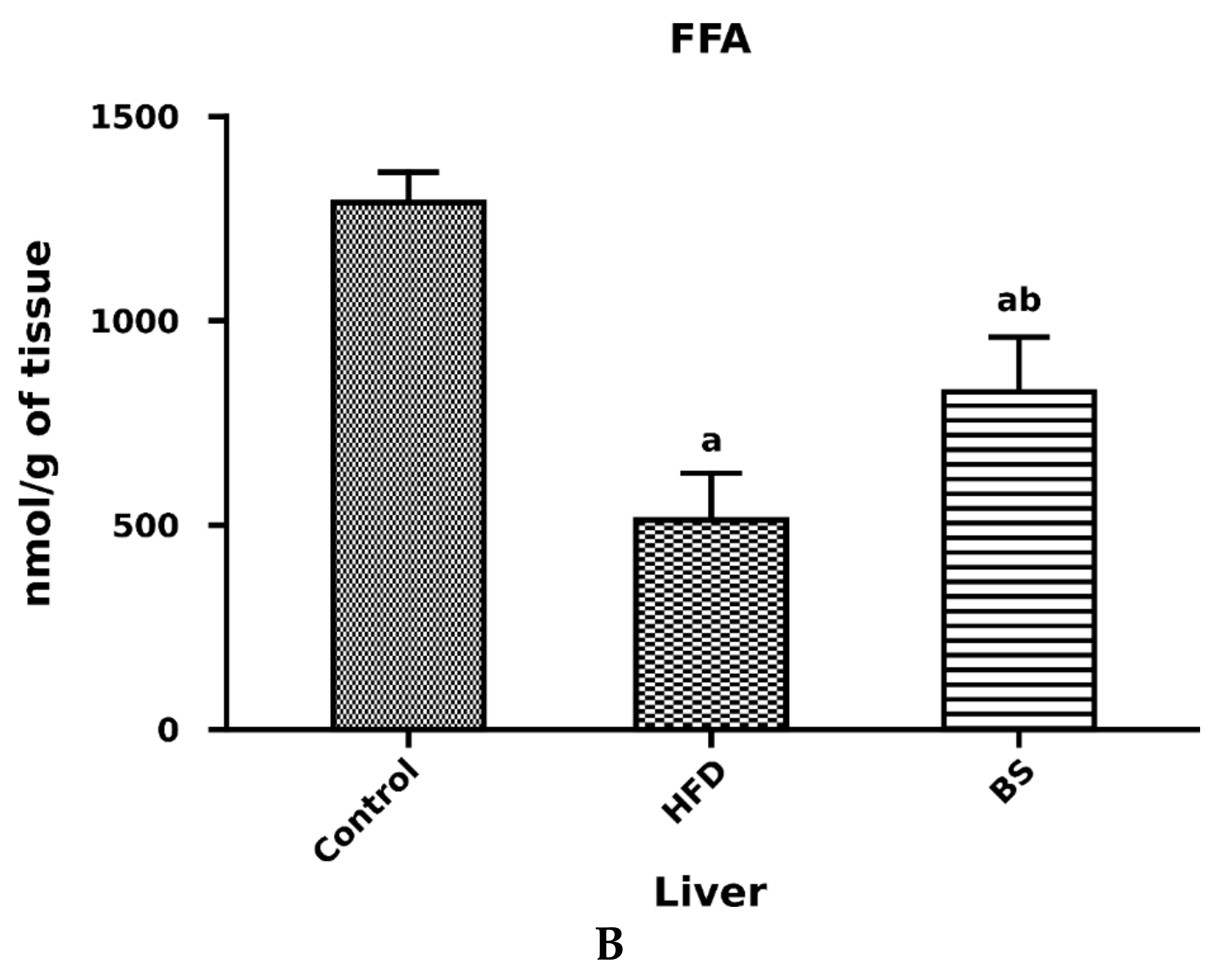
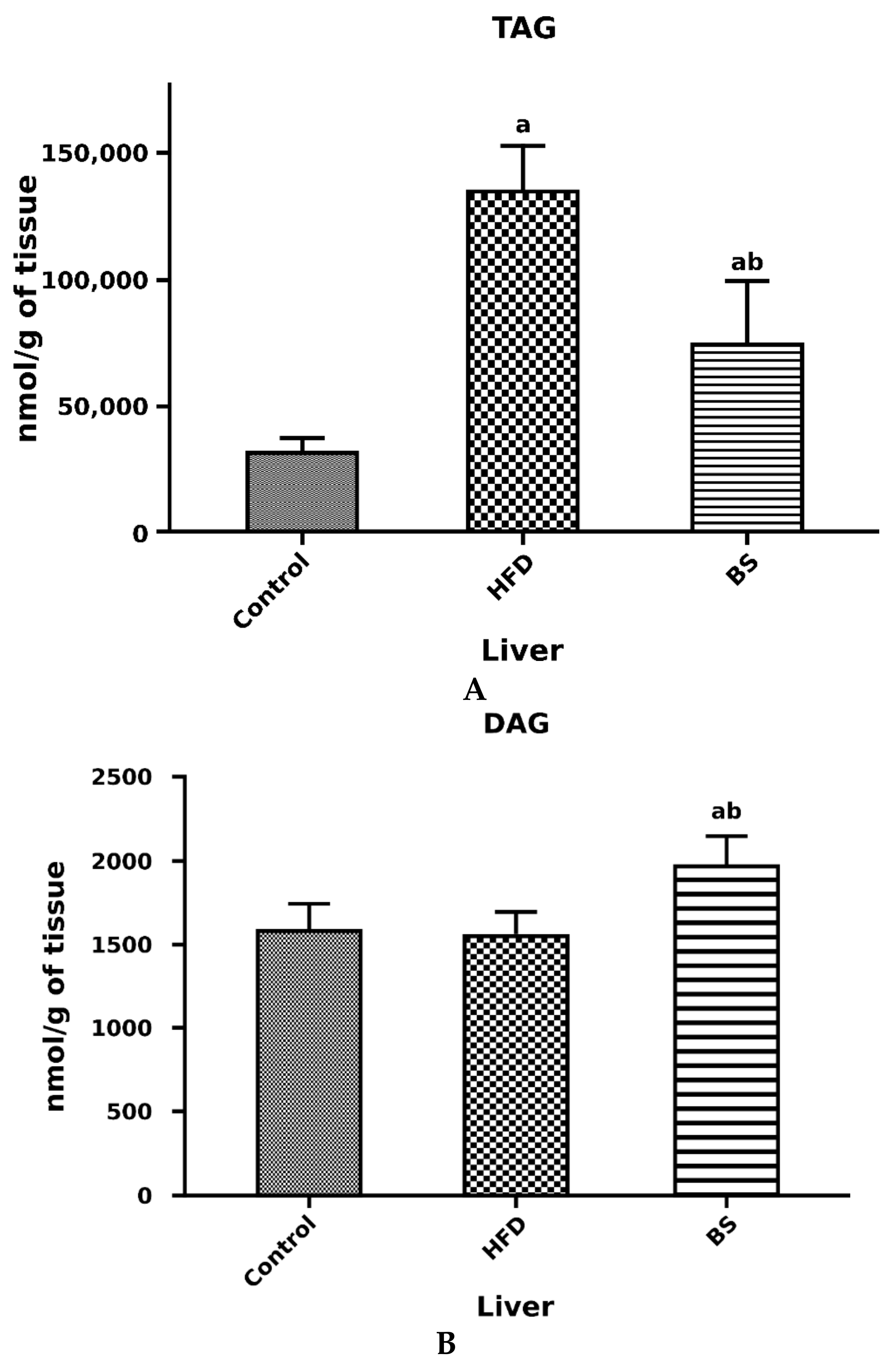
3.2. Liver Total FFA, TAG and DAG Concentrations
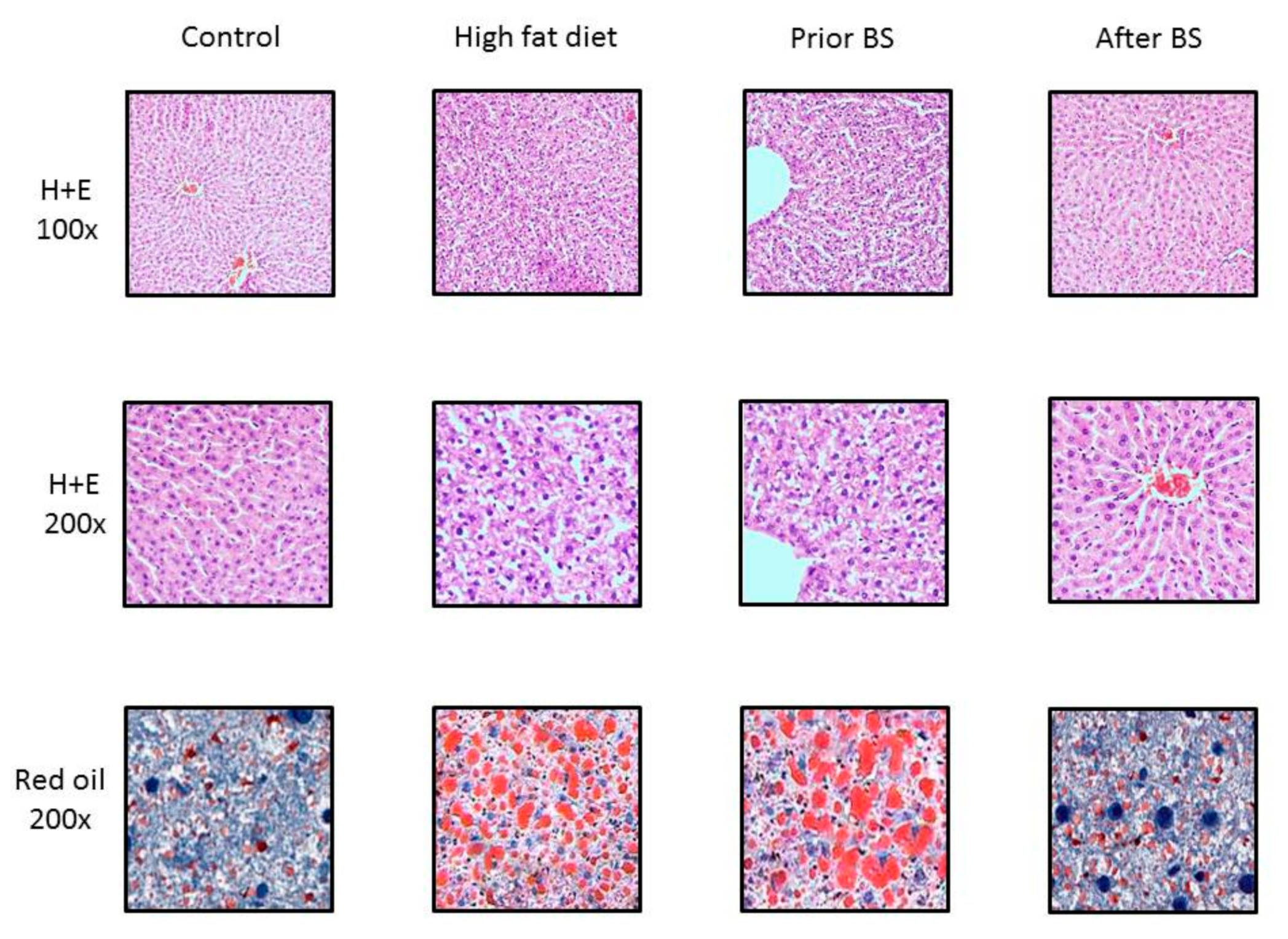
3.3. Histopathological Evaluation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Lancet Diabetes Endocrinology. Obesity and sustainability-in transition. Lancet Diabetes Endocrinol. 2019, 7, 161. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Christian, N.J.; Belle, S.H.; Berk, P.D.; Flum, D.R.; Garcia, L.; Horlick, M.; Kalarchian, M.A.; King, W.C.; Mitchell, J.E.; et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013, 310, 2416–2425. [Google Scholar] [CrossRef]
- Yamaoka, K.; Tango, T. Efficacy of lifestyle education to prevent type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2005, 28, 2780–2786. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q.; Li, H.; Li, H.; Jiang, Y.; An, Y.; et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef]
- Uusitupa, M.; Peltonen, M.; Lindström, J.; Aunola, S.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Valle, T.T.; Eriksson, J.G.; Tuomilehto, J. Ten-Year Mortality and Cardiovascular Morbidity in the Finnish Diabetes Prevention Study—Secondary Analysis of the Randomized Trial. PLoS ONE 2009, 4, e5656. [Google Scholar] [CrossRef]
- Nissen, S.E.; Nicholls, S.J.; Wolski, K.; Rodes-Cabau, J.; Cannon, C.P.; Deanfield, J.E.; Despres, J.P.; Kastelein, J.J.; Steinhubl, S.R.; Kapadia, S.; et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: The STRADIVARIUS randomized controlled trial. JAMA 2008, 299, 1547–1560. [Google Scholar] [CrossRef]
- James, W.P.T.; Caterson, I.D.; Coutinho, W.; Finer, N.; Van Gaal, L.F.; Maggioni, A.P.; Torp-Pedersen, C.; Sharma, A.M.; Shepherd, G.M.; Rode, R.A.; et al. Effect of Sibutramine on Cardiovascular Outcomes in Overweight and Obese Subjects. N. Engl. J. Med. 2010, 363, 905–917. [Google Scholar] [CrossRef]
- Mofidi, R.; Duff, M.D.; Wigmore, S.J.; Madhavan, K.K.; Garden, O.J.; Parks, R.W.; Wigmore, S. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br. J. Surg. 2006, 93, 738–744. [Google Scholar] [CrossRef]
- Ren, C.J.; Patterson, E.; Gagner, M. Early Results of Laparoscopic Biliopancreatic Diversion with Duodenal Switch: A Case Series of 40 Consecutive Patients. Obes. Surg. 2000, 10, 514–523. [Google Scholar] [CrossRef]
- Tucker, O.N.; Szomstein, S.; Rosenthal, R.J. Indications for Sleeve Gastrectomy as a Primary Procedure for Weight Loss in the Morbidly Obese. J. Gastrointest. Surg. 2008, 12, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Stefater, M.A.; Wilson-Pérez, H.E.; Chambers, A.P.; Sandoval, D.A.; Seeley, R.J. All Bariatric Surgeries Are Not Created Equal: Insights from Mechanistic Comparisons. Endocr. Rev. 2012, 33, 595–622. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Cornier, M.A.; Mazzone, T.; Stiles, S.; Cummings, S.; Klein, S.; McCullough, P.A.; Ren Fielding, C.; Franklin, B.A. Bariatric surgery and cardiovascular risk factors: A scientific statement from the American Heart Association. Circulation 2011, 123, 1683–1701. [Google Scholar] [CrossRef] [PubMed]
- Reames, B.N.; Finks, J.F.; Bacal, D.; Carlin, A.M.; Dimick, J.B. Changes in bariatric surgery procedure use in Michigan, 2006–2013. JAMA 2014, 312, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Heffron, S.P.; Parikh, A.; Volodarskiy, A.; Ren-Fielding, C.; Schwartzbard, A.; Nicholson, J.; Bangalore, S. Changes in Lipid Profile of Obese Patients following Contemporary Bariatric Surgery: A Meta-Analysis. Am. J. Med. 2016, 129, 952–959. [Google Scholar] [CrossRef]
- Theocharidou, E.; Papademetriou, M.; Reklou, A.; Sachinidis, A.; Boutari, C.; Giouleme, O. The Role of PCSK9 in the Pathogenesis of Non-alcoholic Fatty Liver Disease and the Effect of PCSK9 Inhibitors. Curr. Pharm. Des. 2018, 24, 3654–3657. [Google Scholar] [CrossRef] [PubMed]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, Clinical, and Population Relevance of 95 Loci for Blood Lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabes, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Seidah, N.G.; Prat, A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012, 11, 367–383. [Google Scholar] [CrossRef]
- Horton, J.D.; Cohen, J.C.; Hobbs, H.H. PCSK9: A convertase that coordinates LDL catabolism. J. Lipid Res. 2009, 50, S172–S177. [Google Scholar] [CrossRef]
- Leander, K.; Malarstig, A.; Van’t Hooft, F.M.; Hyde, C.; Hellenius, M.L.; Troutt, J.S.; Konrad, R.J.; Ohrvik, J.; Hamsten, A.; de Faire, U. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. Circulation 2016, 133, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Manthena, P.V.; Haas, M.E.; Ling, A.V.; Shin, D.J.; Graham, M.J.; Crooke, R.M.; Liu, J.; Biddinger, S.B. Role of Insulin in the Regulation of Proprotein Convertase Subtilisin/Kexin Type 9. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Costet, P.; Cariou, B.; Lambert, G.; Lalanne, F.; Lardeux, B.; Jarnoux, A.-L.; Grefhorst, A.; Staels, B.; Krempf, M. Hepatic PCSK9 Expression Is Regulated by Nutritional Status via Insulin and Sterol Regulatory Element-binding Protein 1c. J. Biol. Chem. 2006, 281, 6211–6218. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Horton, J.D. Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J. Lipid Res. 2010, 51, 3359–3363. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.P.; Knight, B.L.; Soutar, A.K. Regulation of low-density-lipoprotein-receptor mRNA by insulin in human hepatoma Hep G2 cells. Eur. J. Biochem. 1989, 181, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, B.J.; Pelletier-Beaumont, E.; Alméras, N.; Tremblay, A.; Poirier, P.; Bergeron, J.; Després, J.-P. PCSK9 levels in abdominally obese men: Association with cardiometabolic risk profile and effects of a one-year lifestyle modification program. Atherosclerosis 2014, 236, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Piché, M.-E.; Auclair, A.; Grenier-Larouche, T.; Biertho, L.; Marceau, S.; Hould, F.-S.; Biron, S.; Lebel, S.; Lescelleur, O.; et al. Acute and Chronic Impact of Bariatric Surgery on Plasma LDL Cholesterol and PCSK9 Levels in Patients with Severe Obesity. J. Clin. Endocrinol. Metab. 2017, 102, 4023–4030. [Google Scholar] [CrossRef]
- Pasze dla gryzoni (eng. Rodent Fodder). Available online: http://www.agropolmotycz.pl/gryzonie.html (accessed on 15 May 2019).
- Research Diets Inc. Available online: https://www.researchdiets.com/ (accessed on 15 May 2019).
- Glaser, C.; Demmelmair, H.; Koletzko, B. High-Throughput Analysis of Total Plasma Fatty Acid Composition with Direct In Situ Transesterification. PLoS ONE 2010, 5, e12045. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Błachnio-Zabielska, A.; Grycel, S.; Chacinska, M.; Zabielski, P. The role of adipose tissue and excess of fatty acids in the induction of insulin resistance in skeletal muscle. Postępy Higieny i Medycyny Doświadczalnej 2016, 70, 1142–1149. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.-X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of Hepatic Insulin Resistance in Non-alcoholic Fatty Liver Disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef] [PubMed]
- Dettlaff-Pokora, A.; Sucajtys-Szulc, E.; Sledzinski, T. Up-regulation of PCSK9 gene expression and diminished level of LDL-receptor in rat liver as a potential cause of post-lipectomy hypercholesterolemia. Mol. Cell Biochem. 2019, 455, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Dettlaff-Pokora, A.; Sledzinski, T.; Swierczynski, J. Up-Regulation Mttp and Apob Gene Expression in Rat Liver is Related to Post-Lipectomy Hypertriglyceridemia. Cell. Physiol. Biochem. 2015, 36, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Ferri, N.; Macchi, C.; Meroni, M.; Lanti, C.; Ricci, C.; Maggioni, M.; Fracanzani, A.L.; Badiali, S.; Fargion, S.; et al. Liver fat accumulation is associated with circulating PCSK9. Ann. Med. 2016, 48, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Abuaysheh, S.; Monte, S.; Dandona, P. Effect of Restricted Caloric Intake and Bariatric Surgery on PCSK9 Concentrations in Plasma. Diabetes 2018, 67 (Suppl. 1), 2093. [Google Scholar] [CrossRef]
- Baass, A.; Dubuc, G.; Tremblay, M.; Delvin, E.E.; Levy, E.; Davignon, J.; Lambert, M.; O’Loughlin, J. Plasma PCSK9 Is Associated with Age, Sex, and Multiple Metabolic Markers in a Population-Based Sample of Children and Adolescents. Clin. Chem. 2009, 55, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Soutar, A.K. Unexpected roles for PCSK9 in lipid metabolism. Curr. Opin. Lipidol. 2011, 22, 192–196. [Google Scholar] [CrossRef]
- Lakoski, S.G.; Lagace, T.A.; Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Genetic and Metabolic Determinants of Plasma PCSK9 Levels. J. Clin. Endocrinol. Metab. 2009, 94, 2537–2543. [Google Scholar] [CrossRef]
- Chan, D.C.; Hamilton, S.J.; Rye, K.A.; Chew, G.T.; Jenkins, A.J.; Lambert, G.; Watts, G.F.; Hamilton, S. Fenofibrate concomitantly decreases serum proprotein convertase subtilisin/kexin type 9 and very-low-density lipoprotein particle concentrations in statin-treated type 2 diabetic patients. Diabetes Obes. Metab. 2010, 12, 752–756. [Google Scholar] [CrossRef]
- Le May, C.; Kourimate, S.; Langhi, C.; Chetiveaux, M.; Jarry, A.; Comera, C.; Collet, X.; Kuipers, F.; Krempf, M.; Cariou, B.; et al. Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 684–690. [Google Scholar] [CrossRef]
- Lambert, G.; Jarnoux, A.-L.; Pineau, T.; Pape, O.; Chetiveaux, M.; Laboisse, C.; Krempf, M.; Costet, P. Fasting Induces Hyperlipidemia in Mice Overexpressing Proprotein Convertase Subtilisin Kexin Type 9: Lack of Modulation of Very-Low-Density Lipoprotein Hepatic Output by the Low-Density Lipoprotein Receptor. Endocrinology 2006, 147, 4985–4995. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Ohta, M.; Hirashita, T.; Masuda, T.; Inomata, M.; Kitano, S. Effects of Sleeve Gastrectomy on Lipid Metabolism in an Obese Diabetic Rat Model. Obes. Surg. 2013, 23, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Zabielski, P.; Błachnio-Zabielska, A.U.; Wójcik, B.; Chabowski, A.; Gorski, J. Effect of plasma free fatty acid supply on the rate of ceramide synthesis in different muscle types in the rat. PLoS ONE 2017, 12, e0187136. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | Control |
|---|---|
| Protein, min (%) | 23 |
| Fat, min (%) | 3 |
| Ash, max (%) | 7.5 |
| Fiber, max (%) | 5 |
| Lysine, max (%) | 1.5 |
| Methionine + cysteine, min (%) | 0.8 |
| Calcium, min (%) | 1.1 |
| Phosphorus, min (%) | 0.7 |
| Sodium, max (%) | 0.2 |
| Vitamin A (mg/kg) | 8000 |
| Vitamin B3 (mg/kg) | 1000 |
| Vitamin E (mg/kg) | 50 |
| Nutrient Class | Ingredient | High-Fat Diet (gm %) |
|---|---|---|
| Protein | Casein | 25.8 |
| Cystein L | 0.4 | |
| Carbohydrate | Lodex 10 | 16.2 |
| Sucrose | 9.4 | |
| Corn Starch | 0 | |
| Maltodextrin | 0 | |
| Fiber | Solka Floc, FCC200 | 6.5 |
| Celulose | 0 | |
| Fat | Lard | 31.7 |
| Soybean oil, USP | 3.2 | |
| Mineral | S10026 | 6.5 |
| Vitamin | Choline bitartrate | 0.3 |
| Vitamin mix | 0.1 |
| Variable | Control | High-Fat Diet (HFD) | Bariatric Surgery (BS) |
|---|---|---|---|
| Initial body weight (g) | 227.7 ± 14.8 | 227.7 ± 14.8 | 227.7 ± 14.8 |
| Body weight prior to BS (g) | 286.3 ± 14.7 | 392.8 ± 28.3 * | 380.2 ± 32.6 * |
| Body weight after BS (g) | 302.4 ± 16.8 | 405.6 ± 49.4 * | 258.4 ± 19.3 * |
| Daily food intake prior to BS (g) | 13.7 ± 3.5 | 16.3 ± 5.6 | 15.7 ± 4.3 |
| Daily food intake after BS (g) | NA | NA | 9.6 ± 3.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzegorczyk, E.; Książek, M.; Kurek, K.; Łukaszuk, B.; Rosołowski, M.; Paszko, A.; Krzyżak, M.; Żendzian-Piotrowska, M. Effect of Sleeve Gastrectomy on Proprotein Convertase Subtilisin/Kexin Type 9 (Pcsk9) Content and Lipid Metabolism in the Blood Plasma and Liver of Obese Wistar Rats. Nutrients 2019, 11, 2174. https://doi.org/10.3390/nu11092174
Grzegorczyk E, Książek M, Kurek K, Łukaszuk B, Rosołowski M, Paszko A, Krzyżak M, Żendzian-Piotrowska M. Effect of Sleeve Gastrectomy on Proprotein Convertase Subtilisin/Kexin Type 9 (Pcsk9) Content and Lipid Metabolism in the Blood Plasma and Liver of Obese Wistar Rats. Nutrients. 2019; 11(9):2174. https://doi.org/10.3390/nu11092174
Chicago/Turabian StyleGrzegorczyk, Ewa, Monika Książek, Krzysztof Kurek, Bartłomiej Łukaszuk, Mariusz Rosołowski, Agnieszka Paszko, Michalina Krzyżak, and Małgorzata Żendzian-Piotrowska. 2019. "Effect of Sleeve Gastrectomy on Proprotein Convertase Subtilisin/Kexin Type 9 (Pcsk9) Content and Lipid Metabolism in the Blood Plasma and Liver of Obese Wistar Rats" Nutrients 11, no. 9: 2174. https://doi.org/10.3390/nu11092174
APA StyleGrzegorczyk, E., Książek, M., Kurek, K., Łukaszuk, B., Rosołowski, M., Paszko, A., Krzyżak, M., & Żendzian-Piotrowska, M. (2019). Effect of Sleeve Gastrectomy on Proprotein Convertase Subtilisin/Kexin Type 9 (Pcsk9) Content and Lipid Metabolism in the Blood Plasma and Liver of Obese Wistar Rats. Nutrients, 11(9), 2174. https://doi.org/10.3390/nu11092174





