Dietary Fiber and Gut Microbiota in Renal Diets
Abstract
1. Introduction
2. Fiber Definition and Recommendation
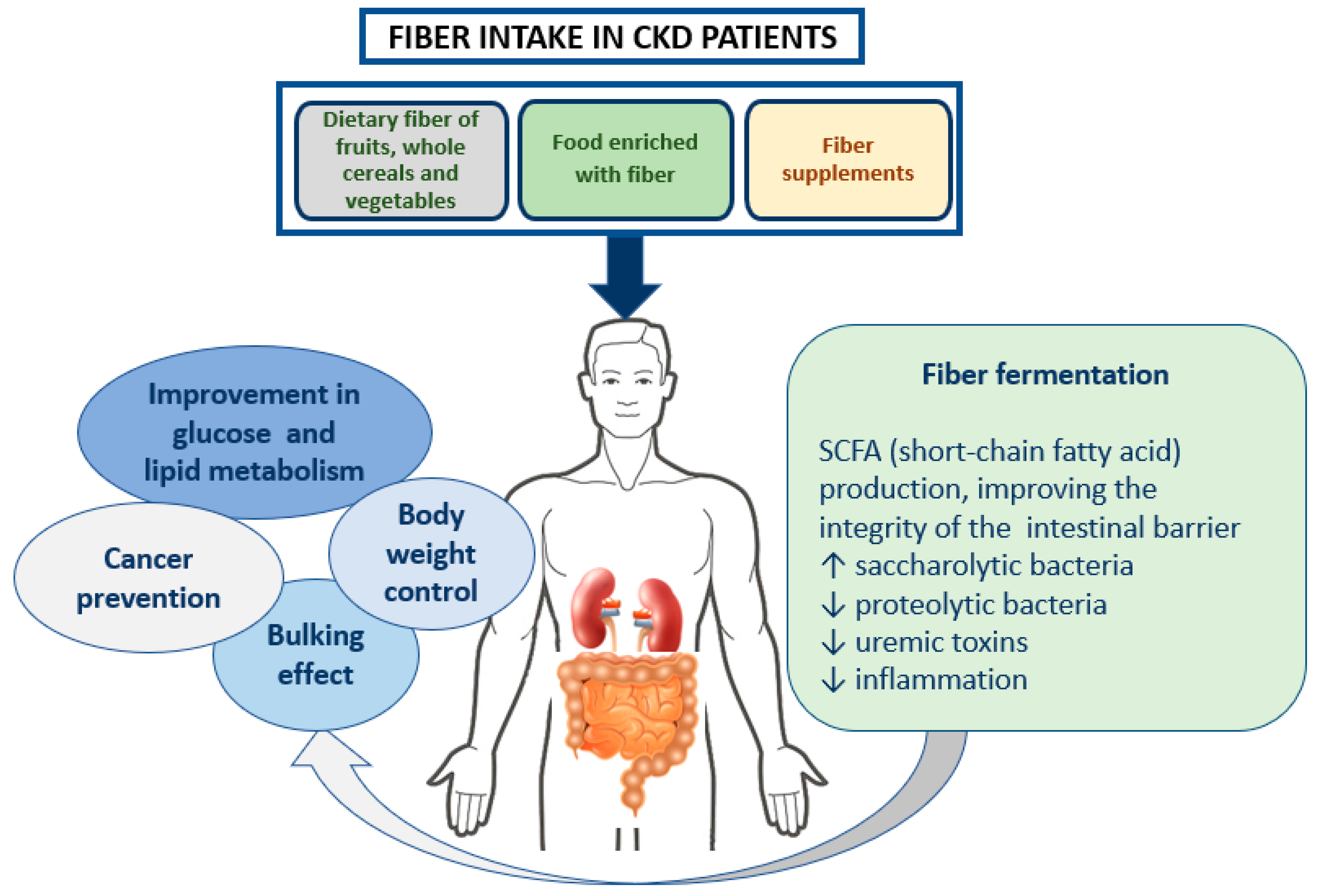
3. Dietary Fiber and Chronic Kidney Disease
3.1. Role in Intestinal Transit
3.2. Role in Weight Control
3.3. Role in Cancer Prevention
3.4. Role in Glucose and Lipid Metabolism
3.5. Role in the Gut Microbiome
3.6. Role in Biomarkers of Renal Function
4. Renal Diets
5. The Fiber in Renal Diets
6. Fiber in Dialysis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hall, M.E.; do Carmo, J.M.; da Silva, A.A.; Juncos, L.A.; Wang, Z.; Hall, J.E. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Cupisti, A.; Locatelli, F.; Bolasco, P.; Brunori, G.; Cancarini, G.; Caria, S.; De Nicola, L.; Di Iorio, B.R.; Di Micco, L.; et al. Low-protein diets for chronic kidney disease patients: The Italian experience. BMC Nephrol. 2016, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; European Uraemic Toxin Work Group (EUTox); et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transpl. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.W.; Hsu, K.H.; Lee, C.C.; Sun, C.Y.; Hsu, H.J.; Tsai, C.J.; Tzen, C.Y.; Wang, Y.C.; Lin, C.Y.; Wu, M.S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transpl. 2011, 26, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.W.; Hsu, K.H.; Hsu, H.J.; Lee, C.C.; Sun, C.Y.; Tsai, C.J.; Wu, M.S. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients–a prospective cohort study. Nephrol. Dial. Transpl. 2012, 27, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Salmean, Y.A.; Segal, M.S.; Palii, S.P.; Dahl, W.J. Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J. Ren. Nutr. 2015, 25, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Marlett, J.A.; McBurney, M.I.; Slavin, J.L.; American Dietetic Association. Position of the American Dietetic Association: Health implications of dietary fiber. J. Am. Diet. Assoc. 2002, 102, 993–1000. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. 2), S76–S99. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services and U.S. Department of Agriculture: Washington, DC, USA, 2015.
- EFSA. Scientific Opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI™ clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am. J. Kidney Dis. 2007, 49 (Suppl. 2), S1–S180. [Google Scholar] [CrossRef]
- Molitch, M.E.; Adler, A.I.; Flyvbjerg, A.; Nelson, R.G.; So, W.Y.; Wanner, C.; Kasiske, B.L.; Wheeler, D.C.; de Zeeuw, D.; Mogensen, C.E. Diabetic kidney disease: A clinical update from Kidney Disease: Improving global outcomes. Kidney Int. 2015, 87, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Vennegoor, M.; ter Wee, P.; Wanner, C.; Basci, A.; Canaud, B.; Haage, P.; Konner, K.; Kooman, J.; Martin-Malo, A.; et al. EBPG guideline on nutrition. Nephrol. Dial. Transpl. 2007, 22 (Suppl. 2), ii45–ii87. [Google Scholar] [CrossRef]
- Beto, J.A.; Ramirez, W.E.; Bansal, V.K. Medical nutrition therapy in adults with chronic kidney disease: Integrating evidence and consensus into practice for the generalist registered dietitian nutritionist. J. Acad. Nutr. Diet. 2014, 114, 1077–1087. [Google Scholar] [CrossRef]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health implications of dietary fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Mann, J.I.; Cummings, J.H. Possible implications for health of the different definitions of dietary fibre. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 226–229. [Google Scholar] [CrossRef]
- Mann, J.; Cummings, J.H.; Englyst, H.N.; Key, T.; Liu, S.; Riccardi, G.; Summerbell, C.; Uauy, R.; van Dam, R.M.; Venn, B.; et al. FAO/WHO scientific update on carbohydrates in human nutrition: Conclusions. Eur. J. Clin. Nutr. 2007, 61 (Suppl. 1), S132–S137. [Google Scholar] [CrossRef]
- Lupton, J.R.; Betteridge, V.A.; Pijls, L.T.J. Codex final definition of dietary fibre: Issues of implementation. Qual. Assur. Saf. Crops Food 2009, 1, 206–212. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 90/496/EEC of 24 September 1990 on Nutrition Labelling for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31990L0496 (accessed on 17 June 2019).
- EFSA. Scientific Opinion on the substantiation of health claims related to dietary fibre (ID 744, 745, 746, 748, 749, 753, 803, 810, 855, 1415, 1416, 4308, 4330) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1735. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review article: Dietary fibre in the era of microbiome science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924 (accessed on 17 June 2019).
- Klurfeld, D.M.; Davis, C.D.; Karp, R.W.; Allen-Vercoe, E.; Chang, E.B.; Chassaing, B.; Fahey, G.C., Jr.; Hamaker, B.R.; Holscher, H.D.; Lampe, J.W.; et al. Considerations for best practices in studies of fiber or other dietary components and the intestinal microbiome. Am. J. Physiol. Endocrinol. Metab. 2018, 315, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.E.; Subar, A.F.; Loria, C.M.; Reedy, J.L.; Baranowski, T. Need for technological innovation in dietary assessment. J. Am. Diet. Assoc. 2010, 110, 48–51. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Matsushita, K.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and incident CKD. J. Am. Soc. Nephrol. 2017, 28, 1248–1258. [Google Scholar] [CrossRef]
- Cano, A.E.; Neil, A.K.; Kang, J.Y.; Barnabas, A.; Eastwood, J.B.; Nelson, S.R.; Hartley, I.; Maxwell, D. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am. J. Gastroenterol. 2007, 102, 1990–1997. [Google Scholar] [CrossRef]
- Cupisti, A.; Gallieni, M.; Rizzo, M.A.; Caria, S.; Meola, M.; Bolasco, P. Phosphate control in dialysis. Int. J. Nephrol. Renovasc. Dis. 2013, 6, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, G.; Shibata, K.; Takizawa, T.; Ikeda, Y.; Tokita, Y.; Umemura, S.; Tochikubo, O. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am. J. Kidney Dis. 2002, 39, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J. The impact of nutrition on the human microbiome. Nutr. Rev. 2012, 70 (Suppl. 1), S10–S13. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; van der A, D.L.; Boshuizen, H.C.; Forouhi, N.G.; Wareham, N.J.; Halkjaer, J.; Tjønneland, A.; Overvad, K.; Jakobsen, M.U.; Boeing, H.; et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am. J. Clin. Nutr. 2010, 91, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Salmean, Y.A.; Zello, G.A.; Dahl, W.J. Foods with added fiber improve stool frequency in individuals with chronic kidney disease with no impact on appetite or overall quality of life. BMC Res. Notes 2013, 6, 510. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Goggins, W.B.; Yip, B.H.K.; Fung, F.D.H.; Leung, C.; Fang, Y.; Wong, S.Y.S.; Ng, C.F. Incidence and mortality of kidney cancer: Temporal patterns and global trends in 39 countries. Sci. Rep. 2017, 16, 15698. [Google Scholar] [CrossRef]
- Huang, T.B.; Ding, P.P.; Chen, J.F.; Yan, Y.; Zhang, L.; Liu, H.; Liu, P.C.; Che, J.P.; Zheng, J.H.; Yao, X.D. Dietary fiber intake and risk of renal cell carcinoma: Evidence from a meta-analysis. Med. Oncol. 2014, 31, 125. [Google Scholar] [CrossRef]
- World Cancer Research Found, Continuous Update Project, Third Expert Report. May 2018. Available online: https://www.wcrf.org/int/continuous-update-project (accessed on 4 June 2019).
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef]
- Carvalho, C.M.; Gross, L.A.; de Azevedo, M.J.; Viana, L.V. Dietary fiber intake (supplemental or dietary pattern rich in fiber) and diabetic kidney disease: A systematic review of clinical trials. Nutrients 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Fouque, D. Gut microbiota and inflammation in chronic kidney disease patients. Clin. Kidney J. 2015, 8, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H. Short chain fatty acids in the human colon. Gut 1981, 22, 763–779. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, L.; Guo, H.; Xu, Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 2017, 68, 20–30. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Cosola, C.; Gesualdo, L.; Fiaccadori, E. Intestinal microbiota in type 2 diabetes and chronic kidney disease. Curr. Diab. Rep. 2017, 17, 16. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am. J. Physiol.-Ren. Physiol. 2019, 316, F1211–F1217. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Soulage, C.O. The role of gut microbiota and diet on uremic retention solutes production in the context of chronic kidney disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Bergen, W.G.; Wu, G. Intestinal nitrogen recycling and utilization in health and disease. J. Nutr. 2009, 139, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Yuzbashian, E.; Asghari, G.; Sarverzadeh, S.; Azizi, F. Dietary fibre intake in relation to the risk of incident chronic kidney disease. Br. J. Nutr. 2018, 119, 479–485. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. [Google Scholar] [CrossRef]
- Aronov, P.A.; Luo, F.J.; Plummer, N.S. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol. 2011, 22, 1769–1776. [Google Scholar] [CrossRef]
- Lau, W.L.; Vaziri, N.D. the leaky gut and altered microbiome in chronic kidney disease. J. Ren. Nutr. 2017, 27, 458–461. [Google Scholar] [CrossRef]
- Lopes, R.C.S.O.; Balbino, K.P.; Jorge, M.P.; Ribeiro, A.Q.; Martino, H.S.D.; Alfenas, R.C.G. Modulation of intestinal microbiota, control of nitrogen products and inflammation by pre/probiotics in chronic kidney disease: A systematic review. Nutr. Hosp. 2018, 35, 722–730. [Google Scholar] [CrossRef]
- McFarlane, C.; Ramos, C.I.; Johnson, D.W.; Campbell, K.L. Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: A systematic review and meta-analysis. J. Ren. Nutr. 2019, 29, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Tu, K.H.; Lin, M.S.; Chang, S.W.; Fan, P.C.; Hsiao, C.C.; Chen, C.Y.; Hsu, H.H.; Tian, Y.C.; Chang, C.H. Does a supplemental low-protein diet decrease mortality and adverse events after commencing dialysis? A nationwide cohort study. Nutrients 2018, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Chiodini, P.; Cupisti, A.; Viola, B.F.; Pezzotta, M.; De Nicola, L.; Minutolo, R.; Barsotti, G.; Piccoli, G.B.; Di Iorio, B. Very low-protein diet plus ketoacids in chronic kidney disease and risk of death during end-stage renal disease: A historical cohort controlled study. Nephrol. Dial. Transpl. 2015, 30, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Norris, K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am. J. Nephrol. 2013, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gallieni, M.; Cupisti, A. DASH and Mediterranean diets as nutritional interventions for CKD patients. Am. J. Kidney Dis. 2016, 68, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Fumagalli, G.; Vigo, V.; Meola, M.; Cianchi, C.; Egidi, M.F. Nutrition and physical activity in CKD patients. Kidney Blood Press. Res. 2014, 39, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Gesualdo, L.; Cosola, C.; Gallieni, M.; Egidi, M.F.; Fusaro, M. Non-traditional aspects of renal diets: Focus on fiber, alkali and vitamin K1 intake. Nutrients 2017, 9, 5. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Rossi, A.; Innocenti, M.; Ricchiuti, G.; Bozzoli, L.; Sbragia, G.; Meola, M.; Cupisti, A. Dietary protein restriction for renal patients: Don’t forget protein-free foods. J. Ren. Nutr. 2013, 23, 367–371. [Google Scholar] [CrossRef]
- Sabanis, D.; Lebesi, D.; Tzia, C. Development of fibre-enriched gluten-free bread: A response surface methodology study. Int. J. Food Sci. Nutr. 2009, 60, 174–190. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food. Sci. Nutr. 2009, 60, 240–257. [Google Scholar] [CrossRef]
- National Institute for Food and Nutrition Database. Available online: https://www.crea.gov.it/it (accessed on 18 June 2019).
- Cosola, C.; Sabatino, A.; di Bari, I.; Fiaccadori, E.; Gesualdo, L. Nutrients, nutraceuticals, and xenobiotics affecting renal health. Nutrients 2018, 10, 808. [Google Scholar] [CrossRef]
- Williams, C.; Ronco, C.; Kotanko, P. Whole grains in the renal diet—Is it time to reevaluate their role? Blood Purif. 2013, 36, 210–214. [Google Scholar] [CrossRef]
- Yang, C.Y.; Tarng, D.C. Diet, gut microbiome and indoxyl sulphate in chronic kidney disease patients. Nephrology 2018, 23, 16–20. [Google Scholar] [CrossRef]
- Xu, H.; Huang, X.; Risérus, U.; Krishnamurthy, V.M.; Cederholm, T.; Arnlöv, J.; Lindholm, B.; Sjögren, P.; Carrero, J.J. Dietary fiber, kidney function, inflammation, and mortality risk. Clin. J. Am. Soc. Nephrol. 2014, 9, 2104–2110. [Google Scholar] [CrossRef]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef]
- Sirich, T.L. Dietary protein and fiber in end stage renal disease. Semin. Dial. 2015, 28, 75–80. [Google Scholar] [CrossRef]
- Kopple, J.D. The National Kidney Foundation K/DOQI clinical practice guidelines for dietary protein intake for chronic dialysis patients. Am. J. Kidney Dis. 2001, 38, S68–S73. [Google Scholar] [CrossRef]
- Meijers, B.K.; De Preter, V.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transpl. 2010, 25, 219–224. [Google Scholar] [CrossRef]
- Nordgaard, I.; Mortensen, P.B. Digestive processes in the human colon. Nutrition 1995, 11, 37–45. [Google Scholar]
- Xu, X.; Li, Z.; Chen, Y.; Liu, X.; Dong, J. Dietary fiber and mortality risk in patients on peritoneal dialysis. Br. J. Nutr. 2019, 1–19. [Google Scholar] [CrossRef]
- Wang, A.Y.; Sea, M.M.; Ng, K.; Wang, M.; Chan, I.H.; Lam, C.W.; Sanderson, J.E.; Woo, J. Dietary fiber intake, myocardial injury, and major adverse cardiovascular events among end-stage kidney disease patients: A prospective cohort study. Kidney Int. Rep. 2019, 4, 814–823. [Google Scholar] [CrossRef]
| Subgroup | Class of Poly, Oligosaccharides | Main Sources | Water Solubility | Viscosity | Fermentability |
|---|---|---|---|---|---|
| No Starch Polysaccharides, MU ≥ 10 | Cellulose | Outer layers of cereals. | - | - | + |
| Hemicellulose | Starchy endosperm and outer layers of cereals, fruits, and vegetable cell walls. | - (soluble after alkaline extraction) | - | - | |
| Seed (psyllium). | ++ | +++ | + | ||
| Cereal grains (β-glucans) | + | ++ | ++ | ||
| Mannans | Seeds, green coffee beans, aloe vera. | + | - | ++ | |
| Heteromannans | Grain legumes, guar gum (galactomannans), Konjac (glucomannans). | +++ | +++ | ++ | |
| Pectins | Fruit peel, beetroot, rice endosperm, legumes. | ++ | ++ | ++ | |
| Inulin and fructans | Chicory root, Jerusalem artichoke, onion, cereal grains (2–3% w/w). | ++ | - | ++ | |
| Resistant oligosaccharides, MU 3-9 | α-galactosides | Polymers derived by hydrolysis from polysaccharides. | +++ | - | +++ |
| β fructooligosaccharides (FOS) | |||||
| α-galactooligosaccharides (GOS) | |||||
| β-galactooligosaccharides (TOS) | |||||
| Xylo-oligosaccharides (XOS) | |||||
| Arabino-xylooligosaccharides (AXOS) | |||||
| Polydextrose | |||||
| Resistant starch, MU ≥ 10 | Type 1 - physically inaccessible starch | Type1- whole grains and legumes | - | ++ | |
| Type 2 - granular starches | Type 2- Green banana | ++ | |||
| Type 3 - gelatinized and retrograded starch | Type 3- Cooled starches in cooked starchy food | ++ | |||
| Type 4 - chemically modified | Type 4- synthetized | + | |||
| Associated substances | Lignin, waxes, chitins | Cell walls of plants, red algae, fungi. | - | waxes ++ | - |
| MU = monomeric units |
| Water Solubility | Viscosity | Fermentability | ||||
|---|---|---|---|---|---|---|
| YES | NO | YES | NO | YES | NO | |
| Beta-glucan, Gums, Pectins, Mucilage | ||||||
| Lignans | ||||||
| Fructo-oligosaccharides, Galacto-oligosaccharides | ||||||
| Inulin | ||||||
| Psyllium | ||||||
| Resistant starch, Cellulose | ||||||
| Fiber Content (g/100 g) | Type of Fibers | ||
|---|---|---|---|
| Median (IQ) | Min–Max | ||
| Bread | 12.5 (7.5–13) | 5.0–13 | Cellulose, psyllium, apple extract, deglutinated wheat fiber |
| Bread substitutes | 7.4 (6.7–9.0) | 4.0–15 | |
| Pasta | 4.8 (2.8–5.7) | 3.0–7.3 | Cellulose, inulin |
| Biscuits and cakes | 3.2 (2.0–3.6) | 0.5–8.5 | Bamboo fiber, pectin, |
| Flour (for bread) | 3.0 (2.8–4.0) | 2.7–5.4 | Cellulose, psyllium, apple extract, deglutinated wheat fiber |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camerotto, C.; Cupisti, A.; D’Alessandro, C.; Muzio, F.; Gallieni, M. Dietary Fiber and Gut Microbiota in Renal Diets. Nutrients 2019, 11, 2149. https://doi.org/10.3390/nu11092149
Camerotto C, Cupisti A, D’Alessandro C, Muzio F, Gallieni M. Dietary Fiber and Gut Microbiota in Renal Diets. Nutrients. 2019; 11(9):2149. https://doi.org/10.3390/nu11092149
Chicago/Turabian StyleCamerotto, Carla, Adamasco Cupisti, Claudia D’Alessandro, Fulvio Muzio, and Maurizio Gallieni. 2019. "Dietary Fiber and Gut Microbiota in Renal Diets" Nutrients 11, no. 9: 2149. https://doi.org/10.3390/nu11092149
APA StyleCamerotto, C., Cupisti, A., D’Alessandro, C., Muzio, F., & Gallieni, M. (2019). Dietary Fiber and Gut Microbiota in Renal Diets. Nutrients, 11(9), 2149. https://doi.org/10.3390/nu11092149





