In Utero Dexamethasone Exposure Exacerbates Hepatic Steatosis in Rats That Consume Fructose During Adulthood
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diet
2.2. Analysis of Blood Parameters
2.3. Analysis of Tissue Parameters
2.4. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.5. Immunoblotting
2.6. Intraperitoneal Glucose Tolerance Test (ip-GTT)
2.7. Intraperitoneal Pyruvate Tolerance Test (ip-PTT)
2.8. VLDL Production Assay
2.9. Statistical Analysis
3. Results
3.1. Effect of In Utero DEX Exposure on Body Weight Gain and Fructose Intake
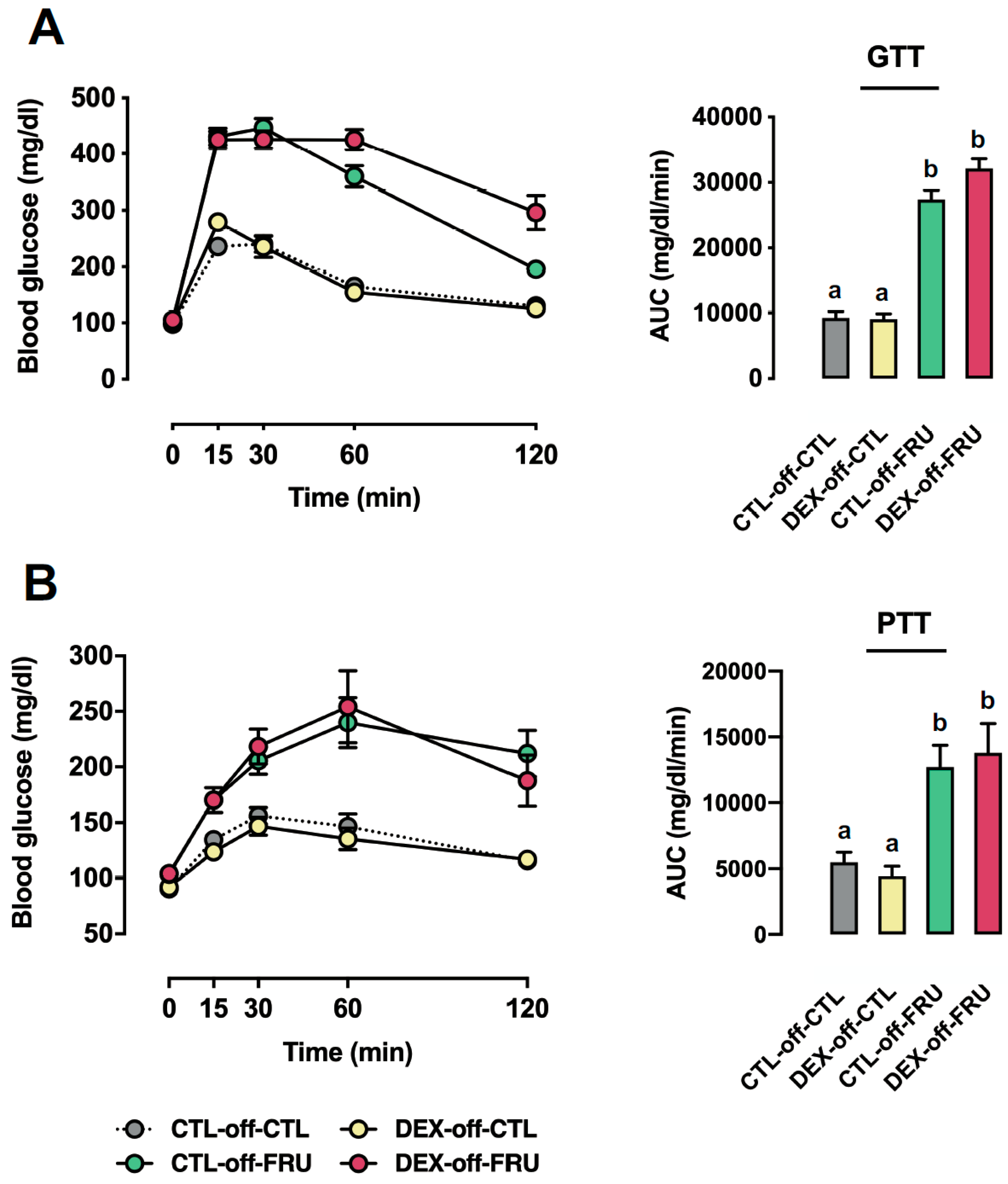
3.2. Effect of Fructose Consumption on Glucose Tolerance and Gluconeogenesis in Offspring Born to CTL and DEX-Treated Mothers
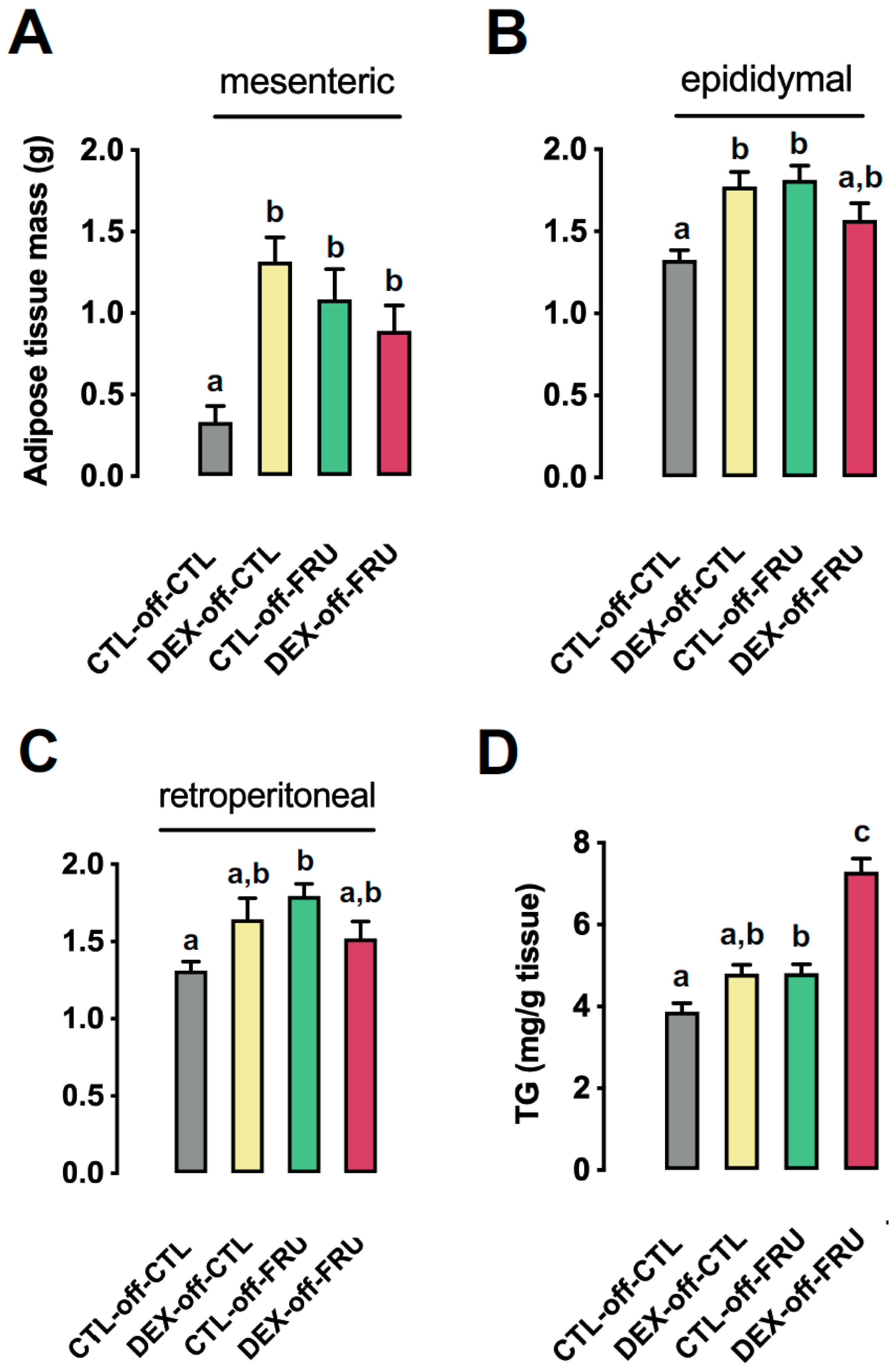
3.3. Effect of Fructose Consumption on Adiposity and Hepatic Triglyceride Content in Offspring Born to CTL and DEX-Treated Mothers
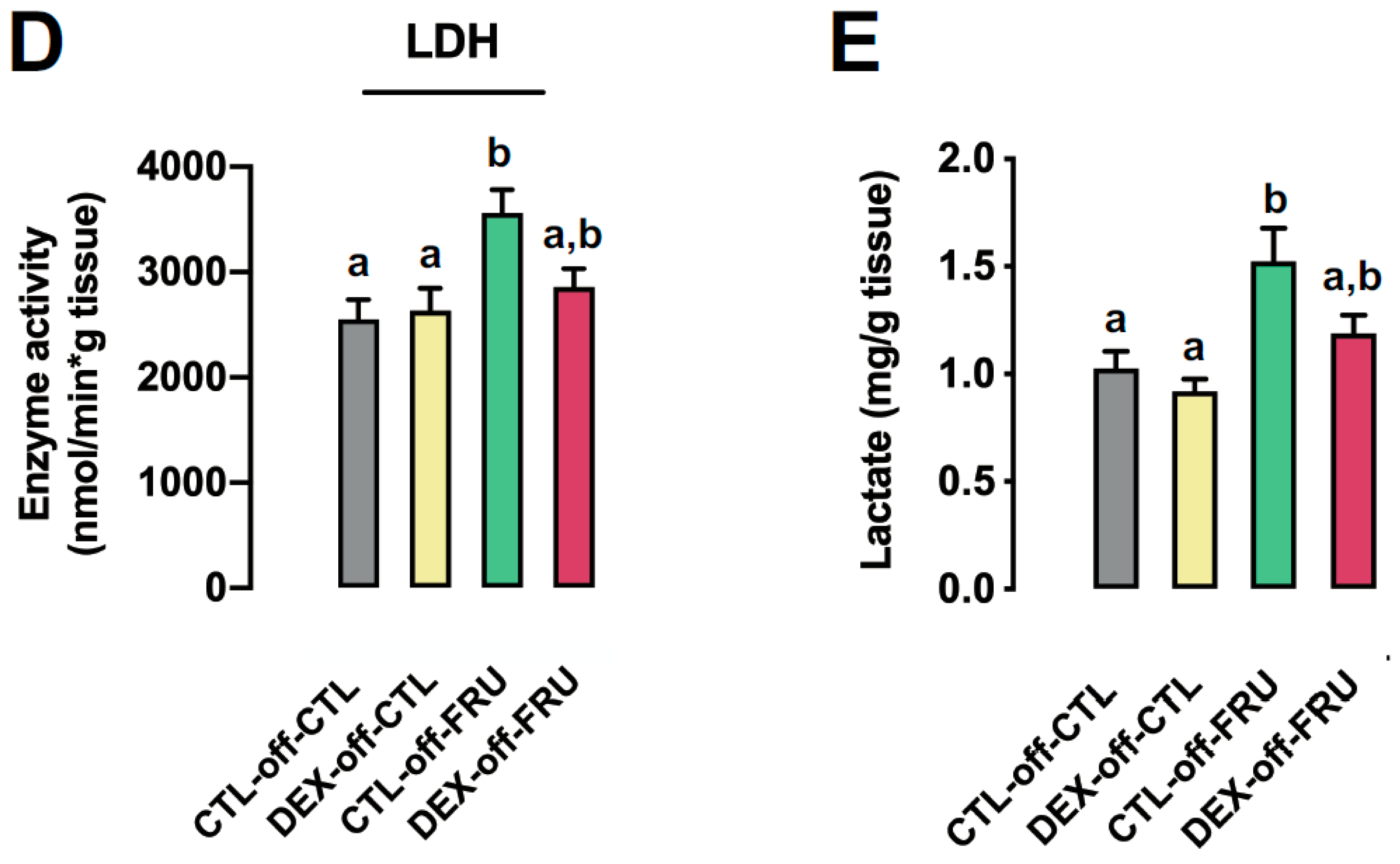
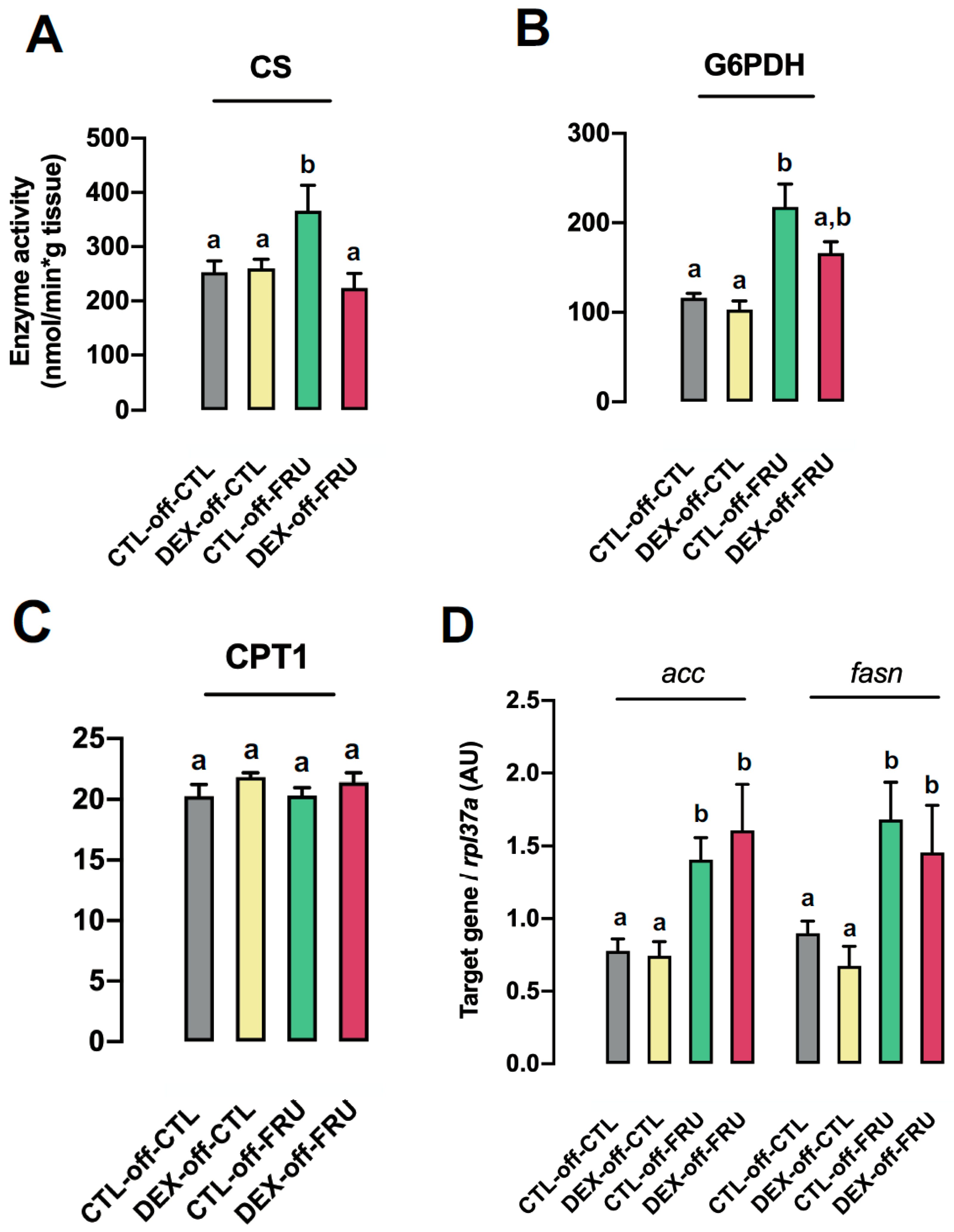
3.4. Changes in Hepatic Metabolism Cannot Account for the Augmented Liver Triglyceride Content Induced by Fructose Treatment in Offspring Born to DEX-Treated Mothers
3.5. Fructose-Induced Accumulation of Hepatic Triglycerides is Accompanied by Impaired VLDL Production in the Livers of Rats Born to DEX-Treated Mothers
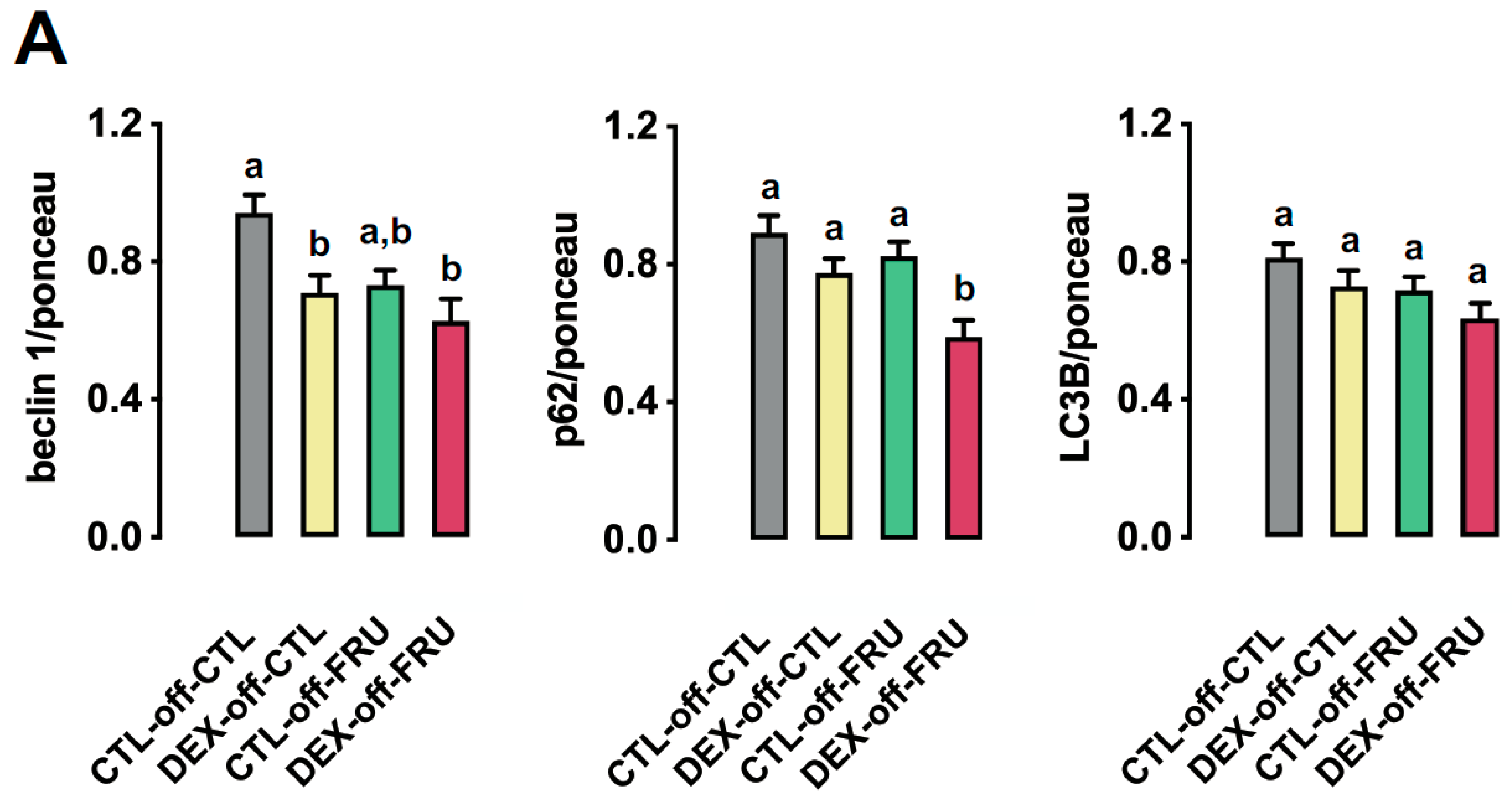
3.6. Effect of Fructose on the Expression of Autophagy Markers in the Livers of the Offspring Born to DEX-Treated Mothers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, I.S.; Ho, H.; Hoffman, B.B.; Reaven, G.M. Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987, 10, 512–516. [Google Scholar] [CrossRef]
- Schonfeld, G.; Pfleger, B. Utilization of exogenous free fatty acids for the production of very low density lipoprotein triglyceride by livers of carbohydrate-fed rats. J. Lipid Res. 1971, 12, 614–621. [Google Scholar]
- Mamo, J.C.; Hirano, T.; James, L.; Szeto, L.; Steiner, G. Partial characterization of the fructose-induced defect in very-low-density lipoprotein triglyceride metabolism. Metabolism 1991, 40, 888–893. [Google Scholar] [CrossRef]
- Wiggins, D.; Hems, R.; Gibbons, G.F. Decreased sensitivity to the inhibitory effect of insulin on the secretion of very-low-density lipoprotein in cultured hepatocytes from fructose-fed rats. Metabolism 1995, 44, 841–847. [Google Scholar] [CrossRef]
- Park, J.; Lemieux, S.; Lewis, G.F.; Kuksis, A.; Steiner, G. Chronic exogenous insulin and chronic carbohydrate supplementation increase de novo VLDL triglyceride fatty acid production in rats. J. Lipid Res. 1997, 38, 2529–2536. [Google Scholar]
- Rebollo, A.; Roglans, N.; Baena, M.; Padrosa, A.; Sánchez, R.M.; Merlos, M.; Alegret, M.; Laguna, J.C. Liquid fructose down-regulates liver insulin receptor substrate 2 and gluconeogenic enzymes by modifying nutrient sensing factors in rats. J. Nutr. Biochem. 2014, 25, 250–258. [Google Scholar] [CrossRef]
- Rattanavichit, Y.; Chukijrungroat, N.; Saengsirisuwan, V. Sex differences in the metabolic dysfunction and insulin resistance of skeletal muscle glucose transport following high fructose ingestion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1200–R1212. [Google Scholar] [CrossRef]
- Faria, J.A.; de Araújo, T.M.; Razolli, D.S.; Ignácio-Souza, L.M.; Souza, D.N.; Bordin, S.; Anhê, G.F. Metabolic Impact of Light Phase-Restricted Fructose Consumption Is Linked to Changes in Hypothalamic AMPK Phosphorylation and Melatonin Production in Rats. Nutrients 2017, 9, 332. [Google Scholar] [CrossRef]
- Rippe, J.M.; Angelopoulos, T.J. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients 2016, 8, 697. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Lowndes, J.; Sinnett, S.; Pardo, S.; Nguyen, V.T.; Melanson, K.J.; Yu, Z.; Lowther, B.E.; Rippe, J.M. The effect of normally consumed amounts of sucrose or high fructose corn syrup on lipid profiles, body composition and related parameters in overweight/obese subjects. Nutrients 2014, 6, 1128–1144. [Google Scholar] [CrossRef]
- Lowndes, J.; Sinnett, S.; Yu, Z.; Rippe, J. The effects of fructose-containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients 2014, 6, 3153–3168. [Google Scholar] [CrossRef]
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef]
- Ravelli, G.P.; Stein, Z.A.; Susser, M.W. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 1976, 295, 349–353. [Google Scholar] [CrossRef]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Drake, A.J.; Raubenheimer, P.J.; Kerrigan, D.; McInnes, K.J.; Seckl, J.R.; Walker, B.R. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology 2010, 151, 1581–1587. [Google Scholar] [CrossRef]
- Carbone, D.L.; Zuloaga, D.G.; Hiroi, R.; Foradori, C.D.; Legare, M.E.; Handa, R.J. Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology 2012, 153, 295–306. [Google Scholar] [CrossRef]
- Pantaleão, L.C.; Murata, G.; Teixeira, C.J.; Payolla, T.B.; Santos-Silva, J.C.; Duque-Guimaraes, D.E.; Sodré, F.S.; Lellis-Santos, C.; Vieira, J.C.; de Souza, D.N.; et al. Prolonged fasting elicits increased hepatic triglyceride accumulation in rats born to dexamethasone-treated mothers. Sci. Rep. 2017, 7, 10367. [Google Scholar] [CrossRef]
- Crowley, P.; Chalmers, I.; Keirse, M.J. The effects of corticosteroid administration before preterm delivery: An overview of the evidence from controlled trials. Br. J. Obstet. Gynaecol. 1990, 97, 11–25. [Google Scholar] [CrossRef]
- Fain, J.N. Effects of Dexamethasone and 2-deoxy-D-glucose on fructose and gluctose metabolism by incubated adipose tissue. J. Biol. Chem. 1964, 239, 958–962. [Google Scholar]
- Exton, J.H.; Miller, T.B.; Harper, S.C.; Park, C.R. Carbohydrate metabolism in perfused livers of adrenalectomized and steroid-replaced rats. Am. J. Physiol. 1976, 230, 163–170. [Google Scholar] [CrossRef]
- Lebenthal, E.; Sunshine, P.; Kretchmer, N. Effect of carbohydrate and corticosteroids on activity of α-glucosidases in intestine of the infant rat. J. Clin. Investig. 1972, 51, 1244–1250. [Google Scholar] [CrossRef]
- Drozdowski, L.A.; Iordache, C.; Clandinin, M.T.; Todd, Z.S.; Gonnet, M.; Wild, G.; Uwiera, R.R.; Thomson, A.B. Dexamethasone and GLP-2 administered to rat dams during pregnancy and lactation have late effects on intestinal sugar transport in their postweaning offspring. J. Nutr. Biochem. 2008, 19, 49–60. [Google Scholar] [CrossRef]
- Douard, V.; Cui, X.L.; Soteropoulos, P.; Ferraris, R.P. Dexamethasone sensitizes the neonatal intestine to fructose induction of intestinal fructose transporter (Slc2A5) function. Endocrinology 2008, 149, 409–423. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Pantaleão, L.C.; Nogueira, T.C.; Gomes, P.R.; Albuquerque, G.G.; Nachbar, R.T.; Torres-Leal, F.L.; Caperuto, L.C.; Lellis-Santos, C.; Anhê, G.F.; et al. Selective regulation of hepatic lipid metabolism by the AMP-activated protein kinase pathway in late-pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1146–R1156. [Google Scholar] [CrossRef][Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Zammit, V.A.; Newsholme, E.A. The maximum activities of hexokinase, phosphorylase, phosphofructokinase, glycerol phosphate dehydrogenases, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxykinase, nucleoside diphosphatekinase, glutamate-oxaloacetate transaminase and arginine kinase in relation to carbohydrate utilization in muscles from marine invertebrates. Biochem. J. 1976, 160, 447–462. [Google Scholar]
- Opie, L.H.; Newsholme, E.A. The activities of fructose 1,6-diphosphatase, phosphofructokinase and phosphoenolpyruvate carboxykinase in white muscle and red muscle. Biochem. J. 1967, 103, 391–399. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Verlag Chemie: Weinheim, Germany, 1963. [Google Scholar]
- Alp, P.R.; Newsholme, E.A.; Zammit, V.A. Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem. J. 1976, 154, 689–700. [Google Scholar] [CrossRef]
- Bieber, L.L.; Abraham, T.; Helmrath, T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal. Biochem. 1972, 50, 509–518. [Google Scholar] [CrossRef]
- Geidl-Flueck, B.; Gerber, P.A. Insights into the Hexose Liver Metabolism-Glucose versus Fructose. Nutrients 2017, 9, 1026. [Google Scholar] [CrossRef]
- Low, W.S.; Cornfield, T.; Charlton, C.A.; Tomlinson, J.W.; Hodson, L. Sex Differences in Hepatic De Novo Lipogenesis with Acute Fructose Feeding. Nutrients 2018, 10, 1263. [Google Scholar] [CrossRef]
- Barzilai, N.; Rossetti, L. Age-related changes in body composition are associated with hepatic insulin resistance in conscious rats. Am. J. Physiol. 1996, 270, E930–E936. [Google Scholar] [CrossRef]
- Teutsch, H.F.; Rieder, H. NADP-dependent dehydrogenases in rat liver parenchyma. II. Comparison of qualitative and quantitative G6PDH distribution patterns with particular reference to sex differences. Histochemistry 1979, 60, 43–52. [Google Scholar] [CrossRef]
- Grefhorst, A.; Hoekstra, J.; Derks, T.G.; Ouwens, D.M.; Baller, J.F.; Havinga, R.; Havekes, L.M.; Romijn, J.A.; Kuipers, F. Acute hepatic steatosis in mice by blocking beta-oxidation does not reduce insulin sensitivity of very-low-density lipoprotein production. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G592–G598. [Google Scholar] [CrossRef]
- Siddiqi, S.; Mani, A.M.; Siddiqi, S.A. The identification of the SNARE complex required for the fusion of VLDL-transport vesicle with hepatic cis-Golgi. Biochem. J. 2010, 429, 391–401. [Google Scholar] [CrossRef]
- Rozema, D.B.; Lewis, D.L.; Wakefield, D.H.; Wong, S.C.; Klein, J.J.; Roesch, P.L.; Bertin, S.L.; Reppen, T.W.; Chu, Q.; Blokhin, A.V.; et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 12982–12987. [Google Scholar] [CrossRef]
- Tadin-Strapps, M.; Peterson, L.B.; Cumiskey, A.M.; Rosa, R.L.; Mendoza, V.H.; Castro-Perez, J.; Puig, O.; Zhang, L.; Strapps, W.R.; Yendluri, S.; et al. siRNA-induced liver ApoB knockdown lowers serum LDL-cholesterol in a mouse model with human-like serum lipids. J. Lipid Res. 2011, 52, 1084–1097. [Google Scholar] [CrossRef]
- Ason, B.; Castro-Perez, J.; Tep, S.; Stefanni, A.; Tadin-Strapps, M.; Roddy, T.; Hankemeier, T.; Hubbard, B.; Sachs, A.B.; Michael Flanagan, W.; et al. ApoB siRNA-induced liver steatosis is resistant to clearance by the loss of fatty acid transport protein 5 (Fatp5). Lipids 2011, 46, 991–1003. [Google Scholar] [CrossRef]
- Chen, Z.; Fitzgerald, R.L.; Li, G.; Davidson, N.O.; Schonfeld, G. Hepatic secretion of apoB-100 is impaired in hypobetalipoproteinemic mice with an apoB-38.9-specifying allele. J. Lipid Res. 2004, 45, 155–163. [Google Scholar] [CrossRef]
- Minehira, K.; Young, S.G.; Villanueva, C.J.; Yetukuri, L.; Oresic, M.; Hellerstein, M.K.; Farese, R.V., Jr.; Horton, J.D.; Preitner, F.; Thorens, B.; et al. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 2008, 49, 2038–2044. [Google Scholar] [CrossRef]
- Sugden, M.C.; Langdown, M.L.; Munns, M.J.; Holness, M.J. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur. J. Endocrinol. 2001, 145, 529–539. [Google Scholar] [CrossRef]
- Zulkafli, I.S.; Waddell, B.J.; Mark, P.J. Postnatal dietary omega-3 fatty acid supplementation rescues glucocorticoid-programmed adiposity, hypertension, and hyperlipidemia in male rat offspring raised on a high-fat diet. Endocrinology 2013, 154, 3110–3117. [Google Scholar] [CrossRef]
- Wyrwoll, C.S.; Mark, P.J.; Mori, T.A.; Puddey, I.B.; Waddell, B.J. Prevention of programmed hyperleptinemia and hypertension by postnatal dietary omega-3 fatty acids. Endocrinology 2006, 147, 599–606. [Google Scholar] [CrossRef]
- Kimura, T.; Jia, J.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; Farzam, F.; et al. Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017, 36, 42–60. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015, 17, 759–770. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Field, J.; Bell, D.R.; Gonzalez, F.J.; Robertson, G.R. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Investig. 2000, 105, 1067–1075. [Google Scholar] [CrossRef]
- Sahai, A.; Pan, X.; Paul, R.; Malladi, P.; Kohli, R.; Whitington, P.F. Roles of phosphatidylinositol 3-kinase and osteopontin in steatosis and aminotransferase release by hepatocytes treated with methionine-choline-deficient medium. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G55–G62. [Google Scholar] [CrossRef]
- Rautou, P.E.; Mansouri, A.; Lebrec, D.; Durand, F.; Valla, D.; Moreau, R. Autophagy in liver diseases. J. Hepatol. 2010, 53, 1123–1134. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Skop, V.; Cahová, M.; Papáčková, Z.; Páleníčková, E.; Daňková, H.; Baranowski, M.; Zabielski, P.; Zdychová, J.; Zídková, J.; Kazdová, L. Autophagy-lysosomal pathway is involved in lipid degradation in rat liver. Physiol. Res. 2012, 61, 287–297. [Google Scholar]
- González-Rodríguez, A.; Mayoral, R.; Agra, N.; Valdecantos, M.P.; Pardo, V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lo Iacono, O.; Corazzari, M.; Fimia, G.M.; et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014, 5, e1179. [Google Scholar] [CrossRef]
- Rodriguez, A.; Durán, A.; Selloum, M.; Champy, M.F.; Diez-Guerra, F.J.; Flores, J.M.; Serrano, M.; Auwerx, J.; Diaz-Meco, M.T.; Moscat, J. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab. 2006, 3, 211–222. [Google Scholar] [CrossRef]
- Akiyama, K.; Warabi, E.; Okada, K.; Yanagawa, T.; Ishii, T.; Kose, K.; Tokushige, K.; Ishige, K.; Mizokami, Y.; Yamagata, K.; et al. Deletion of both p62 and Nrf2 spontaneously results in the development of nonalcoholic steatohepatitis. Exp. Anim. 2018, 67, 201–218. [Google Scholar] [CrossRef]
- Nobili, V.; Cianfarani, S.; Agostoni, C. Programming, metabolic syndrome, and NAFLD: The challenge of transforming a vicious cycle into a virtuous cycle. J. Hepatol. 2010, 52, 788–790. [Google Scholar] [CrossRef]
- Cianfarani, S.; Agostoni, C.; Bedogni, G.; Berni Canani, R.; Brambilla, P.; Nobili, V.; Pietrobelli, A. Effect of intrauterine growth retardation on liver and long-term metabolic risk. Int. J. Obes. 2012, 36, 1270–1277. [Google Scholar] [CrossRef]




| Net protein (%) | 22.5 |
| Ether extract (Fat content) (%) | 4.5 |
| Carbohydrates (%) | 55.0 |
| Fibrous matter (%) | 8.0 |
| Ash matter (%) | 10.0 |
| Kcal/g | 3.5 |
| CTL-off-CTL | DEX-off-CTL | CTL-off-FRU | DEX-off-FRU | |
|---|---|---|---|---|
| Birth weight (g) | 7.6 ± 0.2 a(16) | 3.7 ± 0.3 b(18) | _ | _ |
| Body weight at the 3rd week of life (g) | 73.7 ± 6.1 a(16) | 39.7 ± 5.2 b(18) | _ | _ |
| Body weight at the 8th week of life (g) | 350.1 ± 6.8 a(16) | 314.2 ± 11.3 b(18) | _ | _ |
| Body weight at the 16th week of life (g) | 474.6 ± 18.0 a(8) | 370.3 ± 10.9 b(7) | 510.8 ± 17.2 a,c(8) | 439.7 ± 21.3 a,b(11) |
| Body weight gain during the 1st period (g) | 276.4 ± 6.6 a(16) | 274.5 ± 13.5 a(18) | _ | _ |
| Body weight gain during the 2nd period (g) | 119.2 ± 19.4 a(8) | 84.7 ± 8.3 b(7) | 166.0 ± 10.9 a(8) | 107.3 ± 13.7 c(11) |
| Solid caloric intake during the 2nd period (kcal) | 5220 ± 134 a(8) | 4863 ± 1123 a (7) | 3884 ± 168 b(8) | 4259 ± 28 b(11) |
| Liquid caloric intake during the 2nd period (Kcal) | _ | _ | 1759 ± 94 a(8) | 1350 ± 60 b(11) |
| Total caloric intake during the 2nd period (Kcal) | 5220 ± 134 a(8) | 4863 ± 113 a(7) | 5642 ± 234 b(8) | 5609 ± 293 b(11) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payolla, T.B.; Teixeira, C.J.; Sato, F.T.; Murata, G.M.; Zonta, G.A.; Sodré, F.S.; Campos, C.V.; Mesquita, F.N.; Anhê, G.F.; Bordin, S. In Utero Dexamethasone Exposure Exacerbates Hepatic Steatosis in Rats That Consume Fructose During Adulthood. Nutrients 2019, 11, 2114. https://doi.org/10.3390/nu11092114
Payolla TB, Teixeira CJ, Sato FT, Murata GM, Zonta GA, Sodré FS, Campos CV, Mesquita FN, Anhê GF, Bordin S. In Utero Dexamethasone Exposure Exacerbates Hepatic Steatosis in Rats That Consume Fructose During Adulthood. Nutrients. 2019; 11(9):2114. https://doi.org/10.3390/nu11092114
Chicago/Turabian StylePayolla, Tanyara B., Caio J. Teixeira, Fabio T. Sato, Gilson M. Murata, Gizela A. Zonta, Frhancielly S. Sodré, Carolina V. Campos, Filiphe N. Mesquita, Gabriel F. Anhê, and Silvana Bordin. 2019. "In Utero Dexamethasone Exposure Exacerbates Hepatic Steatosis in Rats That Consume Fructose During Adulthood" Nutrients 11, no. 9: 2114. https://doi.org/10.3390/nu11092114
APA StylePayolla, T. B., Teixeira, C. J., Sato, F. T., Murata, G. M., Zonta, G. A., Sodré, F. S., Campos, C. V., Mesquita, F. N., Anhê, G. F., & Bordin, S. (2019). In Utero Dexamethasone Exposure Exacerbates Hepatic Steatosis in Rats That Consume Fructose During Adulthood. Nutrients, 11(9), 2114. https://doi.org/10.3390/nu11092114






